-
无性繁殖和克隆可塑性作为克隆植物特有的生活史性状[1],存在资源与能量的权衡制约[2-3]。生物量分配贯穿于克隆植物的整个生活史,并直观表达植物的生态对策,克隆表型及构件的生物量分配可对环境资源条件的变化做出可塑性响应,促使植物采取相应的适应策略以利用有限的资源,使生长与繁殖获得高度协调[4]。因此,克隆植物表型及生物量分配变化可反映植株在不同资源水平的生态适应性、生长与繁殖及资源储存策略。植物克隆性主要体现在多种类型的营养繁殖习性中,克隆分株的输出数量直接表征克隆繁殖能力的大小,而地下芽库的动态也与此密切相关[5]。研究发现:克隆植物地上器官的生产力和种群的更新主要依赖地下芽库[6],如芦苇Phragmites australis分株均由根茎芽萌发形成,根茎芽对分株的贡献率达100%[7]。光强和氮素是植物生长发育的重要限制因子,一定程度上影响植物的自然分布[8-10]。同时,植物亦会采取不同的策略适应光照和氮素的变化,其中,形态生长指标和生物量分配格局的改变是生态适应策略之一。如杉木Cunninghamia lanceolata幼苗通过形态可塑性适应不同光环境,提高生存适合度[11];而披针叶茴香Illicium lanceolatum分配较多的生物量于枝条和树干,以应对光强的降低[12];草珊瑚Sarcandra glabra通过改变营养器官的形态特征,依靠调节生物量的积累及分配来适应光强及氮素的变化[13]。芒萁Dicranopteris dichotoma生活史世代中以孢子体占绝对优势地位,借助根状茎完成克隆繁殖[14-15]。作为亚热带低山丘陵区退化植被的标志种之一,芒萁能够适应从暖性针叶群落下层遮光生境到次生裸地、从植被退化后土壤资源剩余到资源流失等多种生境,在马尾松Pinus massoniana、杉木等单优群落下层及杜鹃Rhododendron simsii灌草丛下层形成单优草本层片[16-17]。本研究在不同光照和氮素水平下,分析了芒萁克隆繁殖及各构件生物量分配的影响,以期为解释芒萁单优层片形成机制提供依据。
HTML
-
在浙江省杭州市临安区青山湖国家森林公园玲珑山景区(30°13′05″N,119°40′23″E),选取芒萁克隆分株为材料。采用盆栽法(以下盆栽芒萁简称芒萁),选择带芽且长势基本一致的芒萁克隆分株苗1株,保留10 cm左右的根状茎,移栽至40 cm×21 cm×17 cm的塑料盆内。栽植基质为马尾松单优群落下层表土,并置于2层遮光棚内缓苗1个月。
-
试验设置3个遮光棚,分别覆盖3层(L3)、2层(L2)、1层(L1)黑色尼龙遮阳网。选择3个晴天,每隔2 h,利用光量子仪测定光量子通量密度,每个点测定30个数据,最后计算与无遮阳网相比的透光率分别为4.75%(L3)、13.00%(L2)和35.96%(L1)。将盆栽苗随机分成6组,每组10盆,分别置于3个遮光棚内,每棚2组,对2组进行施氮(N1)和不施氮(N0)处理。施氮量依据彭扬等[18]对草本植物的研究确定。每盆施0.6 g纯氮,将3 g硫酸铵[(NH4)2SO4]分析纯溶解于200 mL纯水中,均匀喷入盆内土壤表面,未施氮处理组喷入等量的纯水。
-
当芒萁克隆分株几乎不再出土时,利用标尺测定各处理组分株植株高度、羽叶长和羽叶宽。记录克隆分株数量,克隆分株的辨别标准为株高至少距地面10 cm,羽叶完全展开不卷曲。将芒萁整盆倒出,用水洗净并记录根状茎的芽数,以得到芽库的大小数据。分别称取羽叶、茎、根状茎及细根的质量,细根的判别标准为除根状茎以外的所有不定根。然后放入烘箱105 ℃杀青30 min,80 ℃烘干至恒量,分别测定其干质量。每个处理重复测定5次。
各器官含水量为:含水量=(鲜质量−干质量)/鲜质量×100%;生物量分配比为:各器官生物量分配比=某器官生物量干质量/总生物量干质量×100%
-
所有数据利用Excel 2010、SPSS 19.0进行处理,使用Origin Pro 8.0制图。采用单因素方差分析(one-way ANOVA)和最小显著差异法(LSD)比较各处理(L1N0、L1N1、L2N0、L2N1、L3N0、L3N1)在显著性水平0.05下的差异性,采用双因素方差分析检验光照、氮素及其交互作用在显著性水平0.001、0.01和0.05水平对繁殖特性、分株形态生长指标及生物量分配影响的显著性。
1.1. 材料
1.2. 试验设计
1.3. 指标测定方法
1.4. 数据处理
-
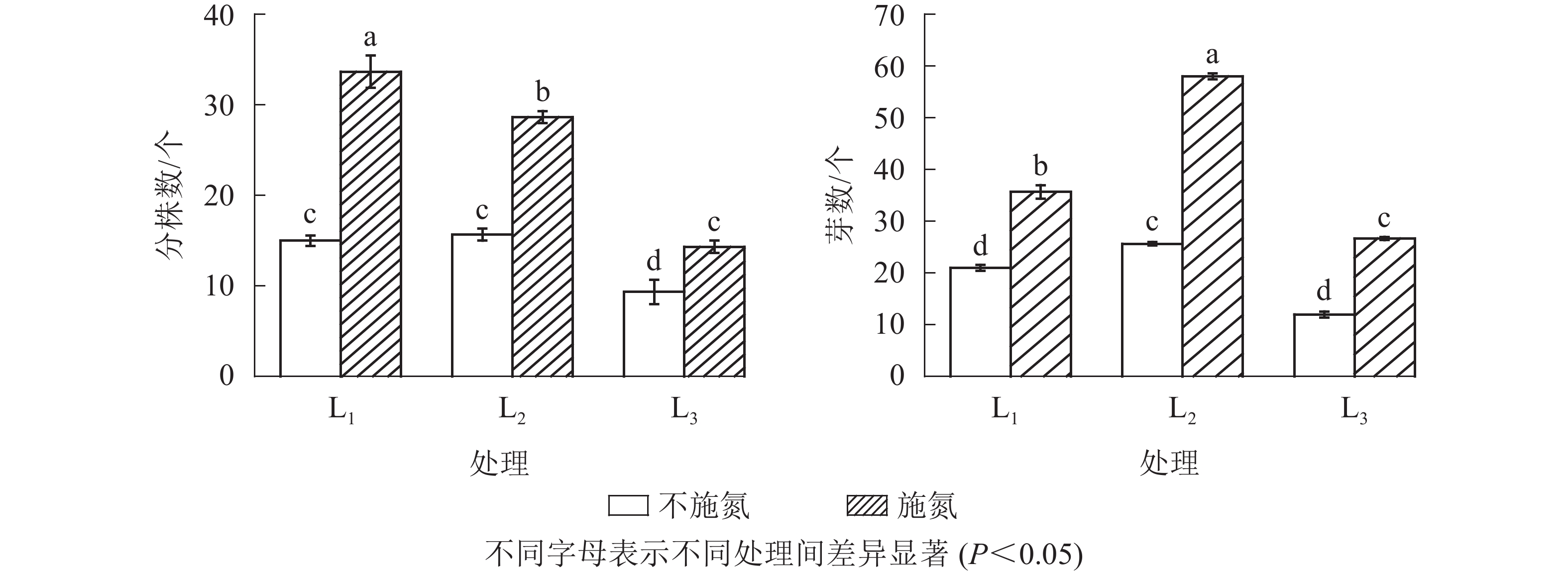
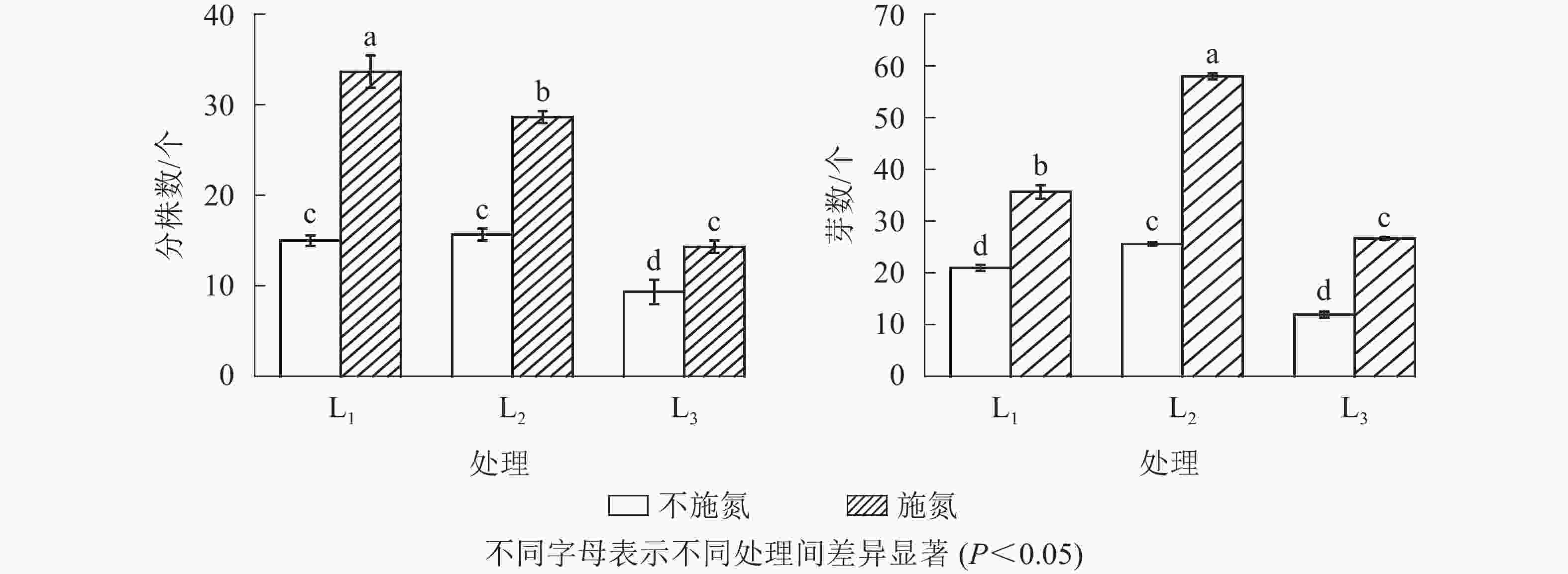
图1显示:随遮光程度增加,N0水平下克隆分株数在L1与L2处理间无显著差异(P>0.05),但显著高于L3处理(P<0.05),说明强度遮光降低芒萁克隆分株数量。N1与N0相比,各遮光处理下克隆分株数均显著增加(P<0.05),分别增加了124.7%(L1)、82.8%(L2)和53.8%(L3),3个遮光处理的克隆分株数从大到小依次为L1、L2、L3,且3个遮光处理间差异显著(P<0.05)。
无论施氮与否,根状茎芽数均在L2处理出现最大值,芽数由大到小依次为L2、L1、L3。N0水平下,根状茎芽数在L1与L3处理间差异不显著(P>0.05);N1水平下的根状茎芽数显著高于N0(P<0.05)。
-
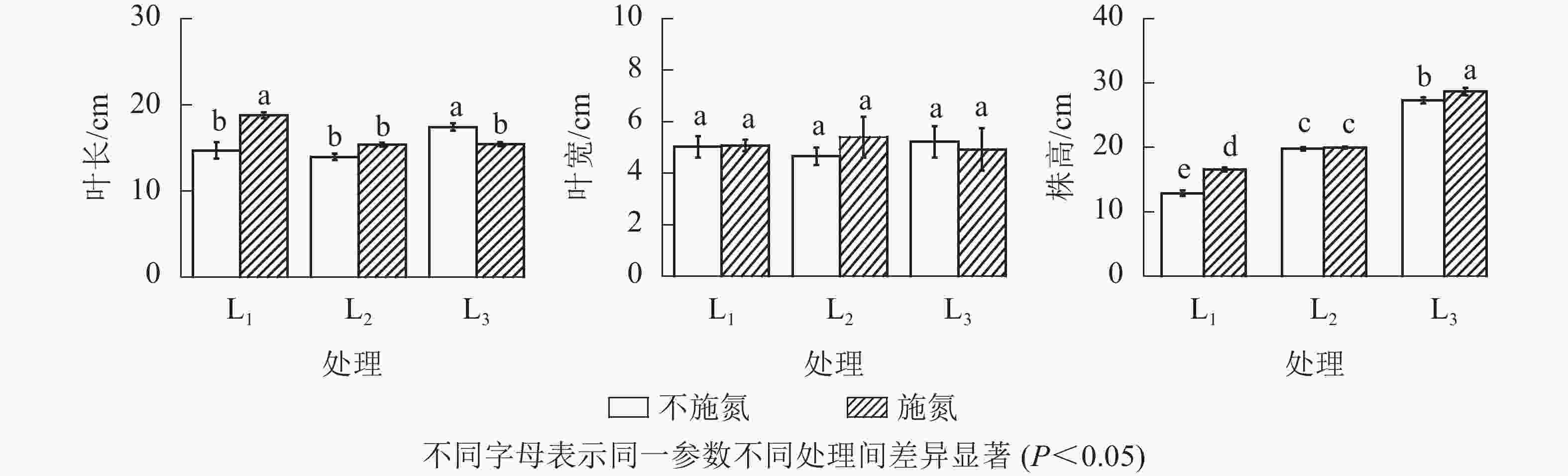
由图2可知:分株株高随遮光程度增加呈不断增加的趋势,其中L1和L3处理下N1显著高于N0(P<0.05),L2处理下2种氮素水平之间差异不显著(P>0.05)。
在N0水平下,分株羽叶长在L1与L2处理间无显著差异(P>0.05),L3呈显著增大(P<0.05)的变化特征。N1水平下,分株羽叶长表现为L1显著高于L2和L3(P<0.05),且L2与L3的羽叶长几乎接近。N1与N0相比,L1分株羽叶长显著增加(P<0.05),L3显著减小(P<0.05),而L2无显著性变化(P>0.05)。就芒萁克隆分株羽叶宽变化而言,同一遮光处理下N0与N1处理无显著差异(P>0.05)。因此,芒萁克隆分株叶片形态对光照和氮素的响应,主要表现在羽叶长度的变化。
-
从表1可见:N0水平下,分株羽叶、茎、根状茎和细根以及地上部分与地下部分的鲜质量生物量均在L2出现最大值,且显著高于L1和L3(P<0.05),L1、L2和L3的根状茎鲜质量间均差异显著(P<0.05)。在L1和L2处理下,分株鲜质量生物量分配于细根、根状茎、茎、羽叶的大小顺序表现为先升后降再上升的变化,分别在根状茎和羽叶出现最大值。L3则表现为不断增加,在羽叶出现最大值。3种遮光处理下地上部分与地下部分鲜质量生物量之比从大到小依次为L3、L2、L1,比值大小分别为2.66±0.11、1.36±0.06、0.94±0.05,且差异显著(P<0.05),说明随着遮光程度的增加,芒萁将更多的能量或光合产物投资地上部分,其中对羽叶的投资显著高于茎,遮光程度较低时,芒萁将更多的能量或光合产物积累于地下部分,特别是地下根状茎。
氮素 光照 羽叶/g 茎/g 根状茎/g 细根/g 地上部分鲜质量/g 地下部分鲜质量/g 地上鲜质量/地下鲜质量 N0 L1 7.47±0.62 d 2.36±0.09 c 8.58±0.12 d 1.85±0.09 d 9.84±0.69 cd 10.43±0.22 d 0.94±0.05 d L2 17.03±1.26 c 3.41±0.09 b 11.97±0.28 b 3.03±0.02 c 20.45±1.29 b 15.00±0.30 b 1.36±0.06 c L3 6.86±0.46 d 2.42±0.12 c 2.06±0.05 f 1.41±0.03 e 9.28±0.56 d 3.47±0.07 f 2.66±0.11 a N1 L1 19.58±0.71 b 5.03±0.23 a 14.47±0.15 a 9.58±0.28 a 24.61±0.56 a 24.04±0.36 a 1.03±0.03 d L2 21.86±0.88 a 3.73±0.23 b 10.54±0.18 e 3.68±0.11 b 25.59±1.07 a 14.22±0.10 c 1.80±0.09 b L3 8.80±0.25 d 3.37±0.10 b 7.38±0.20 e 1.48±0.02 de 12.17±0.21 c 8.86±0.23 e 1.38±0.06 c 说明:同列不同字母表示同一器官不同处理间差异显著(P<0.05) Table 1. Fresh weight of each organ of D. dichotoma under different treatments
N1水平下,芒萁地上部分鲜质量生物量显著增大(P<0.05),地下部分鲜质量生物量除L2显著降低外(P<0.05),其余均显著增加(P<0.05),L2地下部分鲜质量生物量的降低主要在于根状茎鲜质量生物量的降低。随着遮光程度的增大,细根、根状茎、茎、羽叶生物量的变化均表现为先升后降再上升的规律,且均在羽叶出现最大值。
由表2可知:在N0水平下,除茎生物量干质量L3超过L1和L2外,羽叶、根状茎和细根以及地上部分与地下部分的生物量干质量以L2最大,地上部分超过地下部分。在N1水平下,羽叶、茎、根状茎和细根以及地上部分与地下部分的生物量干质量从大到小依次为L1、L2、L3,且不同处理间差异显著(P<0.05),L1和L2的地上部分与地下部分比值显著增加(P<0.05),L3降低但差异不显著(P>0.05)。无论施氮与否,芒萁植株各器官生物量干质量分配格局基本与鲜质量一致,即地上生物量超过地下生物量,其中地上部分以羽叶生物量高于茎,地下部分以根状茎高于细根。
氮素 光照 羽叶/g 茎/g 根状茎/g 细根/g 地上部分干质量/g 地下部分干质量/g 地上干质量/地下干质量 N0 L1 3.53±0.05 d 1.25±0.00 d 3.04±0.00 d 1.08±0.03 e 4.78±0.05 d 4.12±0.02 c 1.16±0.01 d L2 6.77±0.35 c 1.60±0.06 c 4.33±0.15 b 1.53±0.06 c 8.37±0.29 c 5.85±0.10 b 1.43±0.06 c L3 5.95±0.39 c 2.01±0.06 b 1.91±0.11 e 1.11±0.05 e 7.97±0.45 c 3.03±0.07 d 2.63±0.11 a N1 L1 12.82±0.22 a 3.11±0.10 a 5.09±0.05 a 2.70±0.04 a 15.93±0.20 a 7.80±0.04 a 2.04±0.02 b L2 9.33±0.42 b 2.04±0.04 b 3.96±0.14 c 1.84±0.07 b 11.37±0.42 b 5.80±0.17 b 1.97±0.09 b L3 6.54±0.15 c 1.59±0.04 c 1.92±0.04 e 1.28±0.02 d 8.13±0.12 c 3.20±0.05 d 2.54±0.07 a 说明:同列不同字母表示同一器官不同处理间差异显著(P<0.05) Table 2. Dry weight of each organ of D. dichotoma under different treatments
-
表3表明:光照、氮素以及交互作用对芒萁鲜质量和干质量生物量分配均产生显著影响。光照除对茎鲜质量的影响达到显著外(P<0.05),其余器官均达到极显著水平(P<0.01)。光照和光照×氮素对分株数有显著影响(P<0.05),对株高具有极显著影响(P<0.001);氮素对分株数和株高无显著影响(P>0.05);芽库大小均受光照、氮素及其交互作用的极显著影响(P<0.001);光照、氮素及其交互作用对分株羽叶的长与宽均未达到显著程度(P>0.05)。
参数 光照 氮素 光照×氮素 分株数 5.5* 0.7 4.3* 芽数 513.0*** 1267.6*** 104.0*** 株高 53.1*** 2.2 38.2*** 叶长 1.0 0.8 1.8 叶宽 0.0 0.1 0.4 羽叶鲜质量 37.7*** 35.5*** 7.1*** 茎鲜质量 5.5* 37.2*** 10.2** 根状茎鲜质量 316.8*** 173.7*** 87.8*** 细根鲜质量 197.4*** 257.7*** 195.9*** 地上部分鲜质量 37.7*** 46.1*** 9.4** 地下部分鲜质量 434.4*** 362.4*** 168.5*** 羽叶干质量 17.1*** 103.2*** 38.0*** 茎干质量 33.0*** 60.8*** 68.7*** 根状茎干质量 115.1*** 18.9** 30.2*** 细根干质量 46.6*** 106.7*** 47.8*** 地上部分干质量 26.9*** 138.2*** 61.1*** 地下部分干质量 223.4*** 103.2*** 90.0*** 说明:*P<0.05;**P<0.01;***P<0.001 Table 3. F-value and significance analysis of light intensity and nitrogen level on each parameter of D. dichotoma
-
由表4可知:不同光照和氮素处理下芒萁各器官含水量不同。N0水平时,地上部羽叶和茎含水量均在L2最大,L3最小,且显著小于L1和L2(P<0.05);地下部分根状茎和细根同样表现为L3显著低于L1、L2(P<0.05)。N1与N0相比,地上部分器官含水量除L3显著升高外(P<0.05),其余均有所降低;根状茎含水量表现为L1、L2变化不显著(P>0.05),L3显著升高(P<0.05);细根含水量表现为L2无显著变化,L1显著升高(P<0.05),L3显著降低(P<0.05)。
氮素 光照 羽叶含水量/% 茎含水量/% 根状茎含水量/% 细根含水量/% N0 L1 51.58±0.04 b 47.01±0.02 ab 64.49±0.01 b 41.24±0.03 c L2 60.00±0.01 a 52.39±0.03 a 63.74±0.02 b 49.59±0.02 b L3 13.08±0.03 e 16.30±0.02 d 7.33±0.04 c 20.89±0.04 d N1 L1 34.35±0.01 c 37.55±0.03 c 64.76±0.01 b 71.61±0.01 a L2 57.35±0.00 ab 44.58±0.03 bc 62.46±0.01 b 49.98±0.02 b L3 25.53±0.01 d 52.82±0.02 a 73.89±0.01 a 13.54±0.00 e 说明:同列不同字母表示同一器官不同处理间差异显著(P<0.05) Table 4. Moisture content of each organ of D. dichotoma under different treatments
由表5可知:N0水平时,L3处理的羽叶和茎干生物量分配比例显著高于L1、L2处理(P<0.05),但根状茎和细根干生物量分配比例显著低于L1、L2(P<0.05)。
氮素 光照 羽叶分配比例/% 茎分配比例/% 根状茎分配比例/% 细根分配比例/% N0 L1 39.69±0.00 dA 14.00±0.00 bC 34.20±0.00 aB 12.12±0.00 aD L2 47.48±0.02 cA 11.29±0.01 cC 30.44±0.01 bB 10.79±0.01 abC L3 54.00±0.01 bA 18.37±0.00 aB 17.36±0.00 dB 10.31±0.01 cC N1 L1 54.00±0.00 bA 13.13±0.00 bC 21.47±0.00 cB 11.40±0.00 abD L2 54.27±0.01 bA 11.92±0.00 cC 23.12±0.01 cB 10.70±0.00 abC L3 57.71±0.01 aA 14.01±0.00 bC 17.00±0.00 dB 11.30±0.00 abD 说明:不同小写字母表示各器官干生物量分配比例在不同处理间差异显著(P<0.05);不同大写字母表示同一处理下各器官干生物量 分配比例差异显著(P<0.05) Table 5. Biomass distribution ratio of each organ of D. dichotoma under different treatments
N1与N0相比,各遮光处理下羽叶生物量分配比例显著增加;茎生物量分配比例除L3显著降低外(P<0.05),其余均变化不显著(P>0.05);L1、L2处理下根状茎生物量分配比例显著降低(P<0.05),细根无显著变化(P>0.05),L3根状茎生物量分配比例无显著变化(P>0.05),细根显著升高(P<0.05)。同一处理下,各器官的生物量干质量分配比例均表现为羽叶显著高于根状茎(P<0.05),根状茎显著高于茎和细根(P<0.05)。
2.1. 光照和氮素对芒萁繁殖特性的影响
2.2. 光照和氮素对芒萁形态生长特征的影响
2.3. 光照和氮素对芒萁生物量分配的影响
2.4. 光照、氮素以及光照×氮素对芒萁克隆繁殖能力、形态生长以及生物量分配的影响
2.5. 不同光照和氮素条件下芒萁各器官含水量及生物量分配的变化
-
资源异质性是生物生境普遍存在的基本属性[19]。异质生境中,克隆植物能够主动搜寻生长所需的资源,通过表型可塑性、调节生物量分配格局,缓解局部资源匮乏带来的压力并加速逃避不利生境,实现在异质生境中扩散定居的目的[20]。作为克隆植物特有的等级结构,克隆分株的数量能够直观呈现克隆植物的克隆繁殖能力[21],而克隆分株来源于克隆器官上的芽库,芽库的动态决定了克隆植物的潜在克隆性[22]。本研究中,光照及光照×氮素对芒萁克隆分株有显著影响(P<0.05),氮素对克隆分株无显著影响,光照、氮素及光照×氮素对根状茎芽数均有极显著影响(P<0.001)。说明光照是影响芒萁克隆分株输出的主要因素,无论是光照还是氮素,还是两者的复合因素均会对芽库动态产生影响,具体表现为L3处理的克隆分株输出量显著小于L1、L2(P<0.05),施氮显著提高各遮光处理的分株数(P<0.05),但增加比例随遮光程度增加而减小,分株数总体表现为随遮光程度的增加而显著降低(P<0.05)。表明强度遮光(L3)中光资源不足,在一定程度上抑制了克隆分株的输出和地下芽库的水平。
氮素输入在一定程度上提高分株数和芽库水平,但氮素对分株输出的提高同样受光资源的影响,即强度遮光下输出显著降低,对芽库水平的提高也只在适宜光照环境(L2)下获得显著效应。有研究发现:土壤氮素能够扩充芽库,促使分蘖节芽优先形成子株[23-24]。本研究结果也支持该结论。大量研究发现:光照不足条件下,植物可通过向上生长增加株高并扩大叶面积,来捕获更多的太阳辐射能,增强对光资源的竞争能力[25-27]。本研究中,芒萁株高、羽叶长随遮光程度的增加显著增加(P<0.05),这种形态变化也体现了芒萁适应弱光的生态策略。
-
植物体内含水量与植物生理生化作用能力密切相关。本研究中,同一氮素水平下,L2处理下的芒萁羽叶含水量均显著高于其他处理(P<0.05),说明L2处理下芒萁的光合生理代谢最旺盛,最有利于植物光合产物的合成;L3处理下的细根含水量显著低于其他处理(P<0.05),说明L3处理下芒萁对土壤水分的吸收能力都不及L1和L2。施氮处理导致L3处理下的芒萁羽叶代谢增强,根系吸水能力下降,同时根状茎的代谢增强,说明L3处理下,施氮有利于芒萁根状茎的生长以实现将克隆分株放置最优资源环境的可能。
-
生物量分配格局是植物适应环境的一种生存策略,是对生长、繁殖和维持等功能间进行资源分配的权衡,也反映了植物在生长过程中对环境的响应规律及能量分配策略[28-29]。根据最优分配理论(optimal partitioning theory,OPT),自然环境中,由于地上和地下资源空间上的分离,陆生植物需要调整地上和地下部分的生物量分配以平衡对资源的吸收[30-31],而克隆植物可借助其特殊的等级构件和地下储藏器官,完成养分传输和营养物质的储存,协调资源在时间上分布的不均匀性[32]。
针对芒萁各器官的分配比例,在光资源、养分充足的生境和光资源、养分贫乏的生境中,首先芒萁都倾向于将能量分配于羽叶片,其次是根状茎。这可能是芒萁将能量优先分配羽叶,以便合成更多的碳水化合物,最大限度地覆盖地表,有利于形成单优草本层片;其次将能量用于根状茎的分配,以占据有利生境和搜索土壤养分、水分资源,最终提高芒萁克隆分株在异质生境的生态适合度。与L1、L2处理相比,芒萁在L3处理下,更优先将能量分配给羽叶,而对根状茎的投资显著低于L1、L2(P<0.05)。
分析地上与地下生物量之比可知:地上生物量的投资随遮光程度的增大而增加。一般认为,弱光下克隆植物将生物量优先分配于地上部分,利于摄取更多的光资源[33-35]。本研究结果也符合前人研究的结论,但未表现出克隆植物的趋富专化特征[36]。有研究发现:根状茎类克隆植物七瓣莲Trientail europaea根茎生物量分配随土壤氮的增加而增加[37],而对扁秆荆三棱Bolboschoenus planiculmis的研究发现:根茎分配对养分并未发生可塑性反应[38]。本研究则发现:施氮反而造成芒萁根状茎生物量分配的降低,可能是氮素含量梯度设置不利于芒萁根状茎提高储藏功能,具体何种含量有利芒萁将能量投资根状茎的生长有待进一步探索。
3.1. 光照和氮素处理对芒萁克隆繁殖能力和形态生长的影响
3.2. 光照和氮素处理对芒萁克隆分株含水量的影响
3.3. 光照和氮素处理对芒萁克隆分株生物量分配的影响
-
综上所述,得出以下结论:①光照是影响芒萁克隆分株输出的主要因素,L2处理下分株输出和芽库最大;光照和氮素均对芽库动态有显著性影响,施氮对根状茎芽数有显著促进作用;②芒萁能够通过增加株高适应弱光环境,施氮一定程度有利于增加株高;③无论是光资源和养分充足生境还是光资源和养分贫乏生境,芒萁羽叶为光合产物优先分配中心,根状茎属于次优先分配中心。羽叶光合产物的积累有利于芒萁快速覆盖地表,根状茎生物量的积累有利于根状茎芽的形成和觅食行为的表达,对芒萁拓殖新的生境有一定促进作用。









 DownLoad:
DownLoad:
