-
土壤有机质(soil organic matter, SOM)是指存留于土壤中的动植物残体、微生物体、根系分泌物和一些高度稳定的腐殖质等多种有机组分。SOM可通过保持基本的营养成分和水分以及改变土壤中关键的物理、化学和生物过程等途径维持植物、动物和微生物的生命,并在不断变化的环境中维持土壤生态系统的稳定性[1-2]。SOM矿化可直接提供植物生长所需的氮、磷等营养元素;另一方面,SOM可为土壤中的固氮菌提供其固氮所需要的能量,间接影响植物生长所需元素的可利用性[3-4]。SOM可改变土壤的物理条件、缓冲能力和离子交换能力,SOM损失会使土壤出现硬化、紧实和结块等现象,进而影响土壤的通气性、持水量和渗透能力;土壤缓冲能力和交换能力会随着腐殖质的增加而逐渐增强,研究发现[5]:土壤20%~70%的交换能力都是由土壤中的胶状腐殖质维持的。此外,SOM作为异养微生物所需的能量或底物来源,直接影响土壤微生物的数量和活性[6-7]。因此,SOM在土壤的物理、化学和生物转化过程中扮演着重要的角色。土壤是陆地生态系统最大的碳库,土壤有机碳总量达1 550亿t,作为土壤有机碳的最大来源,SOM在恢复和建立碳平衡、土壤碳和氮元素循环、环境可持续性和气候条件等方面起着至关重要的作用[8-9]。SOM积累和流失的速度直接影响大气中二氧化碳浓度,进而影响全球气候变化。SOM中碳的积累和流失通常通过2种方法进行估算,一种是直接通过测定有机质含量的变化计算,另外一种是根据放射性碳测定的有机物年龄推断,但对SOM中碳的估算高度依赖观测时间的范围[10]。在数月到数年内,新鲜植物凋落物分解过程中的质量损失速率接近于凋落物添加到土壤中的速率,因此,凋落物分解是土壤碳损失的主要途径;而在数千年的时间尺度上,碳储量的变化不能被直接观测到,通常是通过计算基岩,相似成土因素(基岩物质、气候和植被)下的风化时间来估算,因此土壤碳的数量和年龄受与风化有关的矿物变化控制。据估算,在过去的12 000 a里,人类的土地利用导致全球表层2 m土层的土壤流失了133 Pg碳[10-12]。认识控制SOM中碳稳定和释放的机制,探明近几十年到几个世纪内土壤有机碳储量的变化,对于预测全球气候变化的影响和制定提高土壤碳固存的管理策略具有重要意义[11, 13]。
然而,长期以来,解析SOM的化学成分十分困难,因为SOM是复杂的混合物,并在土壤中经历了短至数天、长至数千年的转化,化学成分十分复杂[14]。根据对生物降解的敏感性,SOM可分为易降解成分和腐殖质成分。前者主要是指仍能够判定出其前体(多糖、蛋白质和脂质)化学特征的成分,可用水解、浸提的方法进行测定;后者则是指复杂的有机聚合体,主要包括多酚、蛋白质、活性酶、脂质、多聚糖等及其衍生物,具有呈黄色或黑色、高分子量和难以降解的特征,靠常规方法往往难以测定[15-16]。近年来,热裂解气质联用(pyrolysis-gas chromatography/mass spectrometry,Py-GC/MS)技术被广泛用来测定SOM的化学成分和化学组成[17-19]。Py-GC/MS技术通过高温将SOM中的大分子降解成小分子和片段,然后通过气相色谱进行分离,是一种快速、有效、易操作、易重现的技术。SOM的稳定性主要取决于输入物质的化学性质[20]和分解过程[21]以及环境条件[22],所有这些因素都会在SOM组成上留下化学指纹。Py-GC/MS技术通过对SOM化学成分的解析,可提供SOM的“指纹图谱”(fingerprint),实现对SOM的定性分析,同时,通过测定热裂解产生的各种化学分子的相对丰度,可实现对SOM化学成分的定量分析[22-24]。
关于SOM化学组成研究进展已有一些报道。LEINWEBER等[25]从有机质前体物质和组成以及特定有机成分的功能方面进行了论述。KÖGEL-KNABNER等[26]总结认为Py-GC/MS可以有效追踪SOM成分的来源。LÜTZOW等[27]对Py-GC/MS在SOM稳定性方面的应用进行了分析,指出微生物能够分解自然界中的所有有机质,并认为微生物优先分解SOM中的易降解物质和选择性保留难降解物质这一机制只有在特定的环境下才成立。MEHRABANIAN[28]对Py-GC/MS表征SOM的优点进行总结,同时更新了脂肪酸、碳水化合物、木质素、芳香烃和含氮化合物组成的数据。DERENNE等[29]较为完整地总结了Py-GC/MS、热解场电离质谱法、热裂解质谱技术在SOM研究中的应用进展,从不同热裂解技术的优缺点,热裂解对SOM产物的鉴定,在土壤生态过程中的应用和研究SOM化学组成对环境变化的响应等4个方面进行了总结。MA等[30]对Py-GC/MS在不同生态系统中的应用和SOM化学组成及其影响因素进行了总结。目前,Py-GC/MS除用于表征不同环境下SOM化学组成之外,还从分子层面对SOM影响土壤元素循环的内在机制方面进行了一些研究。本研究在对SOM的来源和组成以及Py-GC/MS技术综述的基础上,重点阐述基于Py-GC/MS技术研究SOM化学的进展,为深入研究SOM在生态系统过程的作用提供参考。
HTML
-
最初研究者对土壤中一类黑色物质化学的组成和结构进行分析,并将其命名为土壤腐殖质[31],这类腐殖质包括非腐殖物质和腐殖物质。前者是指类似于生物质残体的普通有机化合物,如糖、蛋白质和木质素等,后者是普遍存在于土壤中的特殊化合物,如胡敏酸、富里酸和胡敏素。也有学者将存在于土壤中的动植物及微生物残体在不同分解和合成阶段形成的各种产物等不同形态和状态的含碳有机化合物统称为SOM,而多数研究者认为,SOM是指微生物作用下形成的腐殖质、动植物残体和微生物体[32-33]。
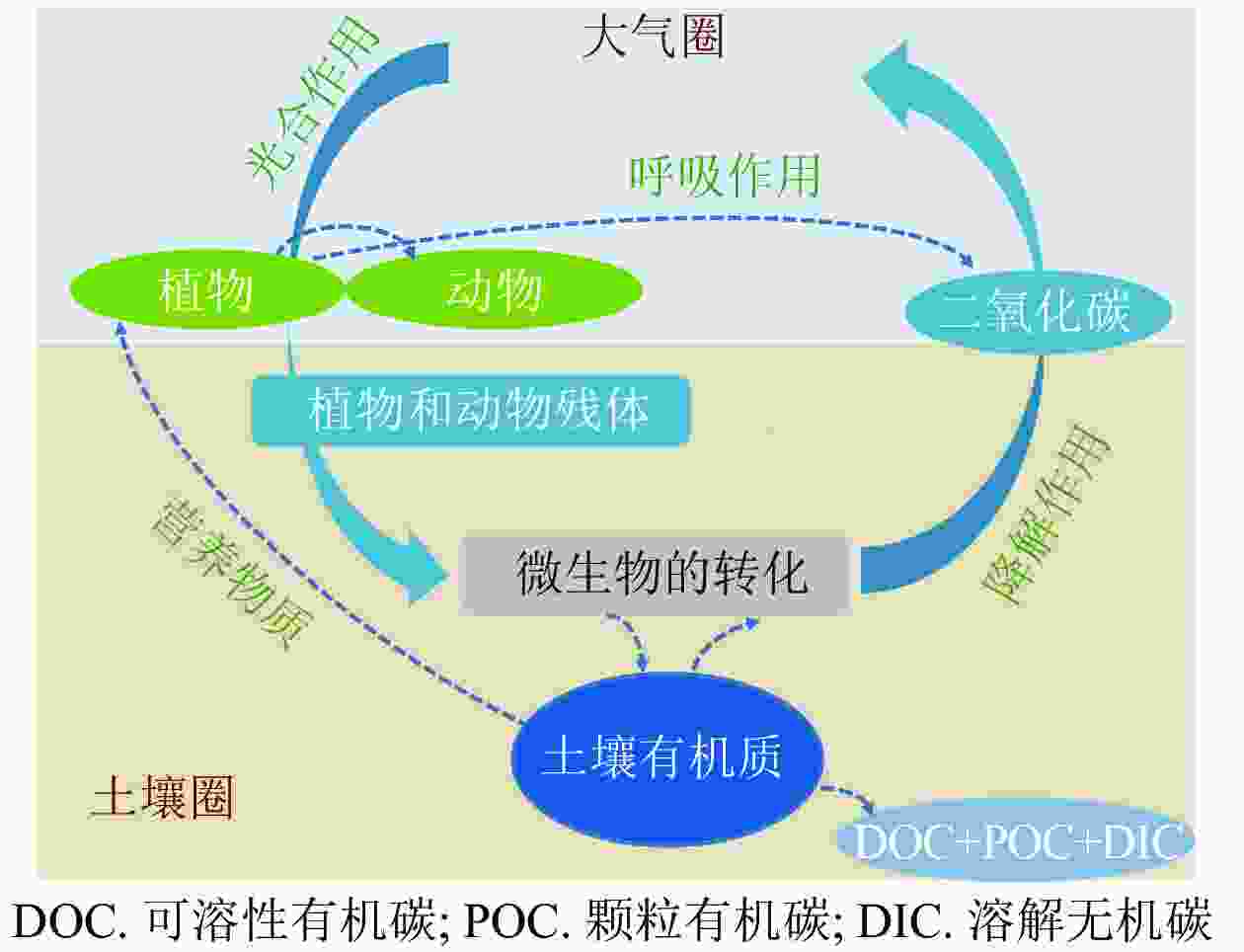
SOM主要来源于微生物和酶等对动、植物残体及分泌物的分解,通常以有机、无机复合体态存在于土壤中,少部分以机械混合态、生命体态、溶液态存在[34]。植物有机质通过凋落物和根系分泌物等方式进入土壤,是SOM形成的基础,植物有机化学成分的质量以及气候条件共同决定SOM的含量和组成,植物养分在分解过程中被释放到土壤,显著提高SOM含量[35]。植物根系分泌物和一些其他代谢产物也是SOM的重要来源之一,如根系在生长发育期间会以分泌物形式将有机质释放到土壤中,包括低分子量的糖、氨基酸、有机酸、酚类和酶等物质;土壤微生物死亡后残体进入土壤形成SOM,或者在微生物和酶的作用下转化成SOM (图1)[36]。
-
现有研究SOM的技术中,固态13C核磁共振波谱技术可以基于含碳原子的官能团定量和定性分析土壤有机质中烷基碳、烷氧基碳、芳香基碳和羰基碳组分的化学结构[37]。热解原子发射检测技术可以用于分析含氮化合物;热裂解衍生试剂法可以检测SOM特定的某一成分;热解质谱法可对SOM化学成分进行定量分析,但质谱图较为复杂;离线色谱质谱技术可以有效识别不同类别化学成分,分析过程中会丢失部分易挥发成分;红外光谱可以研究土壤腐殖酸的结构和组分,但这些组分仍然是混合物质,而非单一成分[29]。稳定13C同位素示踪技术被用于研究输入土壤的有机质分解过程及土壤有机碳的动态变化过程[38]。然而,这些技术均无法较为简便和完整地鉴别出土壤样品中有机质的化学成分。热裂解气质联用(Py-GC/MS)技术可以相对高效地解析SOM化学分子结构和化学组成,并可定性和半定量解析SOM中具体化合物的含量和质量。
-
根据Py-GC/MS获取的SOM化学组成中化学分子结构和性质,SOM热裂解产物可以归类为烷烃、烯烃、多糖、木质素、含氮化合物、酚类、芳香烃、多环芳烃、脂肪酸和萜烯类等[29]。其中烷烃和烯烃主要来源于植物生物聚合物和微生物,以植物为主。多糖是大部分陆地生态系统SOM中相对含量最高(29.4%~55.3%)的成分,是最容易被微生物分解利用的物质,可产生呋喃衍生物和左旋葡萄糖,角质和软木脂可通过其单体(主要是脂肪族羟基酸和二酸)识别。木质素是可以长时间储存在土壤中的相对稳定的物质,约占腐殖物质的34%~68%,木质素标记物包括4-乙烯基苯酚、2-甲氧基苯酚(愈创木酚)和2,6-二甲氧基苯酚(丁香酚),少部分丁香酸(二甲氧基-4-羟基苯甲酸)可能来源于单宁。含氮化合物主要来源于蛋白质和氨基酸,也有部分是微生物在氨气(NH3)存在条件下对木质素或其他酚类代谢的产物,主要包括吡啶、吡咯、吲哚和氨基酸等[39-40],通常情况下,有机质中含氮化合物的大量存在与微生物对有机质的高度分解密切相关。苯酚和3/4-甲基苯酚是SOM中常见的酚类化合物,苯酚是一种具有多种来源(蛋白质、木质素)的热解产物。芳香烃类是来自蛋白质、单宁酸和其他炭类物质的热裂解产物,主要包括苯、甲苯、乙烯苯。多环芳烃主要是有机质燃烧的产物,在SOM化学组成中以萘和二甲基萘为主[41]。SOM中脂肪酸包括植物源性和微生物源性物质。萜烯类以角鲨烯为主,角鲨烯在微生物适应高海拔、低温和低氧气浓度的环境条件下生长起到重要作用[42]。
目前,利用Py-GC/MS技术对SOM化学的研究主要集中在4个方面:①SOM化学组成和其来源的前体物质研究。植物残体是SOM的重要前体物质,通过Py-GC/MS技术可以发现植物残体中的一些特定的标记分子,用来揭示其在土壤中的转化过程。在氮肥添加试验中,植物木质素的生物标记分子2-甲氧基-4-乙烯苯酚在对照样方中进入有机质的轻组分中;而在添加氮肥的样方中,植物木质素直接进入固化过程,形成稳定的重组分有机质[43]。②SOM化学对气候变化、土地利用方式和土壤管理方式的响应研究。草地转化为耕地会造成有机质中木质素衍生物,包括2-甲氧基苯酚、2-甲氧基-4-甲基苯酚等化合物相对丰度的降低[1];而土地免耕会造成SOM轻组分中含氮化合物、呋喃、酚类、烯烃类相对丰度的增加[2]。③特殊SOM化学成分,包括未知氮(SOM中未能鉴定和不能水解的氮组分)、可溶性有机质等的研究。通过Py-GC/MS技术,SCHULTEN等[44]鉴定出土壤未知有机氮的27组化合物成分,并构建了土壤有机氮的三维分子结构;PROKUSHKIN等[45]通过Py-GC/MS技术鉴定出了土壤可溶性有机质中33种化学成分,并揭示了其从土壤溶液向溪流迁移的规律。④SOM化学成分和化学组成对土壤过程和功能的影响研究。在阿拉斯加的黑云杉Picea mariana林,SOM的多糖衍生物和甲苯、苯酚、4-甲基苯等未知来源的衍生物能够解释SOM降解的程度和方式[20]。
-
以往研究发现,SOM中化合物会被微生物选择性分解和保留,或者和土壤中金属元素形成有机-无机结合体[27]。在这2种机制作用下,SOM在土壤中具有一定的稳定性。哪些成分易被微生物利用,哪些成分容易形成有机-无机结合体,哪些成分会长期储存于土壤中?这是基于SOM化学组成从分子水平评估SOM稳定性的内在机制。在研究火山灰农业土壤腐殖质化学组成时发现,有机质的矿化可能主要来源于木质素和黑炭,芳香烃的贡献较小[46]。关于富含黑炭的崩积土壤中有机质化学组分对高锰酸钾和重铬酸盐的敏感性的研究表明,高锰酸钾促进碳水化合物的氧化及脂肪族和芳香结构的相对富集,重铬酸钾氧化后的土壤中包含黑炭、含氮化合物和脂肪族[47]。DAI等[16]通过实验室培养比较培养前后SOM化学成分发现,4 ℃时,多糖和酚类较脂质类更易被降解;25 ℃时,多糖和木质素较脂质易被降解。此外,新鲜有机质进入土壤后可能与矿物质、黏粒物质结合形成稳定的团聚体并保存在土壤中[2, 14, 48]。LEINWEBER[25]利用脱机热解和直接热解-质谱分析技术研究SOM稳定性发现,烷基化合物和木质素二聚体与土壤的铝和铁氧化物以及脂肪族化合物结合成黏土矿物。对青藏高原的高寒草地研究发现,土壤轻组有机质中植物源化合物(木质素、长链烷基化合物、多糖和酚类化合物)比微生物源化合物(短链烷基化合物、氮类化合物和几丁质)和芳香族化合物(芳香族化合物和多环芳香族化合物)对土壤水分的增加更为敏感[49]。综上所述,SOM中多糖、木质素、长链烷烃更易被微生物分解,脂肪族和芳香族化合物较稳定,但这种特性只是相对的。
-
SOM作为大气二氧化碳(CO2)的重要物质来源,其化学组成影响土壤碳循环过程。在北极苔原的研究中,利用Py-GC/MS技术建立培养条件下土壤呼吸速率的预测模型,结合实测数据证实,初始多糖的丰度较好代表了土壤累积呼吸量[16, 50]。与之类似,马书琴等[51]研究藏北高寒草地SOM化学组成表明,多糖相对丰度与土壤CO2累积排放量也存在较好的拟合关系。关于SOM化学组成在土壤氮的矿化过程中的作用方面的研究较少。但是,SOM是微生物降解氮的主要能量提供者,在氮转化过程中的作用不容忽视。HEUMANN等[52]利用热解场电离质谱分析法,通过长期实验室培养(>200 d)德国下萨克森沙质耕地土壤发现,甾醇化合物相对丰度和土壤氮矿化速率显著相关。
基于Py-GC/MS技术可以分析微生物和酶在不同有机质化学成分的降解过程中的调控作用。DIJKSTRA等[21]研究松针凋落物的化学性质及其降解过程发现,新鲜针叶的主要成分二萜酸被微生物迅速利用,针叶中愈创木基木质素和多糖成分保存在土壤中,依据土壤剖面中己糖/戊糖值可知土壤中白腐真菌优先分解戊糖。GRANDY等[23]在研究SOM化学组成和酶活性关系时发现,β-1,4-N-乙酰葡糖胺糖苷酶活性和多糖相对丰度负相关,和木质素相对丰度正相关,磷酸酶、β-1,4-葡糖苷酶、β-D-1,4-纤维二糖酶、亮氨酸氨基肽酶、β-1,4-木糖苷酶的活性和木质素、多糖的相对丰度之间相关性不显著,这可能反映了SOM化学成分降解和酶动力学在时间尺度上存在差异。MASCIANDARO等[53]利用热裂解技术分析脲酶、磷酸酶、蛋白酶和β-葡萄糖苷酶的活性,比较不同耕作方式下土壤的化学和生物性质发现,即使在腐殖化程度较高的集约耕作土壤中仍能保持同等生化能量水平的腐殖质酶库,表明SOM化学组成比含量更加有效地反映土壤生物过程的变化特征。
-
热裂解可以表征植被演替和土壤发育初期的SOM化学组成和特征。植被的演替和变化对SOM的化学组成的影响主要表现在物种入侵、火灾后植物演替和耕地造林3个方面。①植物入侵。在西班牙的欧洲山毛榉Fagus sylvatica林人工种植苏格兰松Pinus sylvestris后,苏格兰松林下SOM中多糖含量较高,这很可能与SOM中复杂成分的迁移有关;欧洲山毛榉林土壤剖面中奇数碳长链烷烃的丰度高于苏格兰松土壤剖面,表明林地SOM具有更强的稳定性,并且来自于自然植被欧洲山毛榉的SOM标记化学成分长链正构烷烃C31~C33在苏格兰松人工种植林中仍能存在100 a[54]。SANTANA等[55]研究巴西南部外来森林种植园发现,SOM中来源于新物种的长链烷类、脂质物质增加,而来源于本土物质的木质素成分被降解。MIN等[56]发现虎杖Polygonum cuspidatum入侵草地生态系统后,凋落物中难降解化合物成分增加,入侵草地的SOM中的木质素和几丁质含量明显增加。LI等[57]在黄河三角洲的研究发现,互花米草Spartina alterniflora短期入侵盐沼地会提高SOM的稳定性,而入侵淡水沼泽则会降低SOM的稳定性。植物入侵对SOM的影响主要体现在原先有机质的降解和新物种有机质的积累,这可能是因为地上植物发生变化时,土壤微生物对新入侵的植物产生的有机质比较陌生,仍保持对原先SOM的降解模式。②火灾后植物演替或植物的自然演替。YASSIR等[39]研究林火发生后,白茅属Imperata不同阶段的次生演替、原生林和9年生的马占相思Acacia mangium人工林下土壤发现,相比草地土壤,林下SOM中植物源化合物的含量较低,微生物产物的贡献较高。在研究南极灰壤的潜在母质有机物时发现,新生长的植被对表土有机质的影响大于母质所产生的有机质[58]。草地生态系统在自然演替过程中,演替初期以植物来源的多糖和木质素为主,随着时间推移,土壤中易降解有机质逐渐被分解,而较为稳定的物质(酚类,脂肪类和木质素类)被保存下来[58]。③耕地造林。研究耕地造林后SOM化学组成变化特征发现,随着造林时间的推移,土壤中作物源有机质的相对丰度逐渐减少,木质源有机质的相对丰度逐渐增加[59]。具体为,造林5 a后土壤中含有短链和中链脂肪族物质,造林25 a后土壤中包含丰富的木质素物质,这种影响可能仅发生在土壤表层(0~2 cm),较深(2~10 cm)的土壤中仍保留着演替初期的一些较稳定的化学成分[60]。
3.1. SOM化学组成
3.2. 基于SOM化学组成评估SOM的稳定性
3.3. 基于SOM化学组成研究土壤物质循环
3.4. 基于SOM化学组成揭示土壤演替过程
-
SOM化学组成与其结构和外界环境条件密切相关,是多个因素综合影响的结果。影响SOM含量和动态过程的最重要因子是气候,其次是土壤母质、植被类型、土地利用、耕作方式、人类活动和野火等。
-
气候条件及其介导的植被分布和微生物活性差异是影响SOM化学组成的重要因素。对苔原、泰加林、草原、温带森林和热带雨林的18个表土样品的热解产物进行因子分析,发现不同气候区域植被下土壤腐殖质化学组成存在较大差异[24]。JIMÉNEZ-GONZÁLEZ等[22]对西班牙33个不同区域不同植被条件下SOM化学分析表明,SOM分子组成随环境因子的变化而变化,环境因子的影响程度依次为气候、植被和母质。对意大利阿尔卑斯山的森林生态系统土壤研究表明,海拔越高,SOM腐殖化程度越高,芳烃和脂肪酸含量越高,而土壤温度是解释海拔对土壤微生物群落结构、细菌和真菌丰度、有机质的化学组成的影响的重要因子[61]。在马扎罗山研究不同海拔和植被类型下SOM化学组成发现,有机质的输入速度和组成依次由气候和植被类型控制,有机质的分解效率受制于土壤pH、温度和水分,在特定地理位置呈现特定甾醇、木质素衍生物和多环芳香族化合物的相对丰度和比值[62]。对阿拉斯加黑云杉林土壤研究发现,温度和湿度的变化对原位SOM分解能产生加和效应,在最适宜的温度和湿度条件下,多糖衍生物和不能确定来源的化合物(甲苯、苯酚、4-甲基苯酚和一些无法识别的化合物)的相对丰度变化可以解释所有地点的矿化变化的44%[20]。因此,气候条件可能通过影响植物凋落物含量,影响微生物活性及对有机质的选择性利用和保留,进而决定SOM化学组成。
温度和降水是影响SOM化学组成的主要气候环境因子。有研究表明,降水促进土壤中木质素的降解和脂肪族化合物积累,土壤温度升高会加速SOM的降解,降低土壤团聚体中SOM含量 [63]。XU等[64]通过研究北极冻土带SOM化学组成发现,随着气温上升,永冻层的融化和活土层的加深,冻融层有机质可能参与到北极的生物地球化学循环。在研究水稻Oryza sativa SOM化学组成时发现,升温导致土壤脂肪酸和酚类物质减少14%[65]。针对不同组分有机质化学组成对气候变化的响应的研究[49]也得到类似结论,即温度升高和降雨增加导致轻组有机质中微生物衍生化合物增加和植物源化合物(木质素、长链烷基化合物、多糖和酚类)减少。极地和高纬度地区对气候变化的响应更敏感,全球升温趋势下,永久冻土融化导致了土壤碳释放,会进一步加剧极端天气[8]。在利用Py-GC/MS技术对5种极地土壤的室内实验中发现,4 ℃条件下,SOM多糖含量与CO2演替表现为显著相关,25 ℃条件下SOM中多糖和木质素的含量降低,表明多糖含量较高的土壤产出CO2较多[16]。综上所述,升温和降水促进了SOM中多糖的降解及木质素和脂肪族化合物的聚集。
土壤中CO2浓度变化和大气氮沉降可能影响SOM化学组成。有研究发现,CO2浓度的升高导致水稻根际土SOM中杂环氮化合物、酚酸和酚类化合物的相对丰度分别增加了37%、65%和26%[65]。研究施氮肥对不同粒径(>250,53~250和<53 μm)有机质化学组成的影响发现,氮处理后土壤微粒组分中苯并呋喃物质减少,多糖相对丰度增加[66]。GRANDY等[67]在密歇根州曼尼斯蒂国家森林的糖枫Acer saccharum林和黑橡树Quercus velutina林开展了6 a的氮沉降实验,发现氮沉降导致糖枫林SOM中多糖的主要裂解产物糠醛相对丰度从15.9%下降到5.0%,在黑橡树林SOM中从8.8%增加到24.0%;氮沉降还使糖枫林生态系统粒径>250 μm组分SOM中总木质素衍生物与多糖的比值从0.9提高到3.3,而其他粒径级SOM和黑橡树林生态系统中的该比值没有变化;氮沉降还增加了2种生态系统中粒径为63~250 μm和>250 μm组分木质素衍生物与含氮化合物的比值,而粒径<63 μm组分木质素衍生物与含氮化合物的比值未发生显著变化。不同形态的氮对SOM化学组成的影响也不同。对中国北方森林的研究发现,硝态氮的添加增加了土壤SOM轻组分乙酸和甲苯的相对丰度,降低了苯酚的相对丰度,而铵态氮的添加增加了苯和吡咯的比例以及重馏分中的矿化指数(吡咯/酚),促进有机质矿化[68]。总体而言,土壤CO2浓度增加导致SOM中杂环氮和酚类物质相对丰度增加,氮沉降促进多糖成分降解和有机氮矿化。
-
植被类型对SOM的化学组成具有重要影响作用,这是由于不同植物源的化合物在土壤中的积累过程和初始凋落物的化物成分直接影响着SOM化学组成[69],不同生态系统的植被类型则是影响凋落物数量和组成的重要因素[58],土壤微生物对不同有机物 (多糖、木质素)的选择性分解和残留也可能导致不同植被下SOM化学组成存在差异[70-71]。目前,关于植被类型对SOM化学组成的研究主要集中于森林、农田和草地生态系统中[72]。
森林生态系统SOM主要来源于植被枯枝落叶、死亡根茎和根系分泌物以及动物残体[73]。与草地等其他生态系统类型相比,森林生态系统SOM中含有较丰富木质素和含氮化合物[74]。有研究发现:森林土壤枯枝落叶层有机质主要是初级木质素和多糖类物质,腐殖质层则是经过微生物作用的芳香烃类和含氮化合物[71]。底层土壤存在大量易降解的植物质来源的有机化合物(长链脂肪酸和多糖物质),这些物质主要来源于植物的根系[75]。SUÁREZ-ABELENDA等[76]在西班牙巴斯克地区研究不同含量有机质-铝复合物的酸性森林土壤中有机质化学组成时发现,铝含量较高的土壤中植物来源的有机质(木质素、长链烷烃和烯烃、甲基酮和脂肪酸)贡献较小,而微生物来源的有机质(微生物糖和含氮化合物)含量较高。这是由于新鲜有机质分解过程中,初级有机物释放的酸被矿物风化释放的碱缓冲,矿物中的铝和次级有机物结合形成微团聚体。
另外,森林中不同植物下的SOM中生物质信号源以及其发育状况存在一定差异。STEWART等[71]研究夏威夷森林芒萁属Dicranopteris和指叶木属Cheirodendron植物发现,2种植物下土壤中有机质化学组成在土壤团聚体中相似,但在不同土壤层中存在明显的差别。前者植物有机质以木质素为主,土壤中含有大量来源于木质素的芳香烃类;后者植物中有机质包括脂质、含氮化合物、多糖,土壤中则含有大量来自角质和软木质生物的脂质物质;2种植物共同存在时,土壤中有机质则大部分来自二者根系的物质,包括多糖、脂质和木质素。GRANDY等[74]研究阔叶林和针叶林土壤时发现前者有机质分子数多于后者,并且前者储藏着丰富的多糖类、木质素和芳香类化合物,而后者SOM含有来自于针叶树的代表性物质——树脂物质(双萜酸)[21]。这表明,森林生态系统中,地上植物的有机化学成分组成显著影响SOM化学组成。
草地生态系统土壤的凋落物层SOM以降解程度较低的木质纤维为主,腐殖层包含木质纤维素物质、微生物代谢产物和脂肪酸,矿质层中则以微生物转化植物多糖后的呋喃为主,几乎没有木质素[77]。YASSIR等[39]研究白茅属草原发现,SOM中碳水化合物相对丰度较高,含氮化合物的相对丰度较低。在对藏北高寒草地的研究发现,SOM化学组成以含氮化合物为主[51]。STURSOVA等[78]研究荒漠草原土壤时发现,酚类有机质很容易被降解,从而限制SOM的积累。这表明,草地SOM中主要化学成分在不同研究区域表现出明显差异,可能是不同研究区域之间地上植被有机物化学组成和土壤微生物对有机质的降解偏好不同造成。
相比草地和森林生态系统土壤,农田SOM主要由高分子重组有机质组成,芳香程度高、含量低且更稳定[79]。RUMPEL等[1]和VERDE等[17]研究发现,农田SOM中二甲氧基苯酚/愈创木酚均低于森林土壤和草地,说明农田SOM中植物质来源的新鲜有机质含量低而稳定好的有机质较为丰富。DONG等[80]对中国河套平原SOM研究结果显示,SOM轻组组分中木质素和烷烃及其衍生物的相对丰度较高,分别为19.89%和23.18%,含氮化合物相对丰度较低,仅为1.71%,而重组组分中烷烃及其衍生物和含氮化合物相对丰度较高,分别为15.06%和13.98%,木质素衍生的化合物相对丰度较低,仅为3.89%。总体上,农田生态系统土壤中有机质化学组成相对单一、稳定,而自然植被(森林、草地)覆盖下的土壤中有机质化学组成较为复杂且矿化程度低。因此,长期耕作可能会加剧SOM中顽固性化合物(木质素和角质)的降解[81]和土壤CO2的排放[82]。
-
不同土地利用方式下,有机质的来源和分解过程可能不同,导致SOM化学组成存在差异。基于热裂解技术研究森林、牧场和咖啡园土壤腐殖酸和富里酸组分发现,有机质化学结构和植物有机质的化学成分密切相关,牧场土壤富里酸中含有较丰富的芳香烃和碳水化合物,腐殖酸中含有较丰富的木质素衍生物乙烯基愈创木酚;咖啡园土壤富里酸中含有较丰富的芳香烃和多酚物质;森林土壤样品的腐殖酸和富里酸中含有丰富的含氮化合物[83]。对亚热带强淋溶土的研究发现,蚕豆Cajanus cajan和玉米Zea mays耕作系统土壤中木质素衍生物和脂肪类物质明显高于草地,表明微生物优先分解多糖等物质[84]。因此,SOM化学组成取决于地上植被的化学性质和微生物对有机物的分解。
另外,当土地利用方式发生变化时,对土壤生态系统产生扰动,凋落物输入的数量和质量可能改变,进而影响土壤养分状况、进入土壤的有机碎屑的数量和组成,从而影响SOM周转和组成。这在由耕地转化为人工林[59]、草地转变为耕地[1, 85]和牧场转化为耕地[86]以后SOM化学组成的变化特征一系列研究中得到证实。森林(或草地)转为耕地是一种常见的农业管理实践模式,通常会导致SOM含量下降。在法国Lusignan区域草地转化为耕地后,SOM中木质素衍生物相对丰度下降,但转化1 a后,耕地的SOM化学组成基本与未转化草地相似[1]。原始森林向牧场的转变会导致土壤碳储量增加,这归因于更高的肥力和更细密的草地根系统对土壤碳的巨大贡献。新西兰的松林转化为牧场后,来自草地根胚乳物质导致SOM成分中环庚烷类物质(中/短链亚甲基化合物)增加,来自黑麦草Lolium perenne和三叶草Trifolium repens角质层物质导致SOM成分中角质和角碳(长链脂肪族结构)和来自结构碳水化合物的纤维素标记物和多糖相对丰度增加[87]。ZHANG等[88]对美国弗洛里达州淡水湿地转化为甘蔗Saccharum officinarum农田后SOM化学进行研究,发现转化后SOM中芳香族化合物相对丰度从11.37%增加到12.30%,多环芳烃从0%增加到1.63%,正烷烃从8.90%增加到16.17%,正烯烃从25.10%增加到28.77%,脂肪族化合物从7.33%增加到11.77%,脂肪酸从1.93%增加到3.87%,同时木质素从12.87%下降到2.47%,酚类化合物从23.33%下降到16.13%,多糖从2.40%下降到1.03%,苯并呋喃从1.67%下降到0.27%,含氮化合物从5.07%下降到4.67%。在森林(或草地)和农田的转化中,既有森林砍伐后转化为农田,又有农田弃耕后的重新造林,前者会引起SOM中脂肪族化合物的减少和木质素衍生物的增加,而后者会导致短链和中链脂肪族化合物和碳水化合物增加[17, 59]。
-
在过去的20 a里,世界范围内严重的野火数量不断增加,火灾不仅导致植被烧毁,还会改变土壤有机层的组成,包括生物化学结构的转变,新形成物质的积累,以及不同耐热性物质(新鲜或烧焦的生物质)的输入和减少,这种改变取决于不同土壤深度的温度变化幅度和SOM各种化学成分在发生改变之前对热的耐受程度[32, 89]。火灾可能引起大量烧焦的物质渗入土壤,加之高温对土壤表层的影响,产生杂环氮化合物和顽固性有机质[90-93],导致SOM中主导化学成分由易降解的物质转变为稳定的化合物。通常,中度野火作用下有机质组成的变化可能是由于森林冠层中植物炭化物质的外部输入所致,高强度火灾导致新形成的组分发生热凝结和不稳定物质的降解[94]。DE LA ROSA等[92]研究严重火灾对森林SOM化学组成的影响,发现火烧土壤中多糖化合物、木质素衍生物和正构烷烃/烯烃等活性有机质相对丰度明显低于未烧土壤,而且难降解的含氮化合物(吡咯)的相对丰度增加。在阿拉斯加夏栎Quercus robur下土壤中也发现,燃烧的SOM中多糖相对丰度减少,而芳香族和脂肪族化合物增加[95]。野火的一个重要作用是产生木炭进入土壤,NOCENTINI等[96]研究不同粒径(<0.5、0.5~1.0、1.0~2.0和>2.0 mm)木炭的有机质化学组成发现,细木炭的芳香型物质相对丰度较低,富含氮化合物,而粗木炭主要由芳香物质组成。
有研究表明:火烧产生的有机质可能会长期储存在土壤中。对美国科罗拉多州的海曼火灾发生14 a后的土壤研究表明:土壤中有机质的芳香性随火烧程度和深度的增加而增加[97]。de LA ROSA等[98]对西班牙加那利松Pinus canariensis森林发生严重林火11 a后的SOM进行评价,发现短链正构烷烃在火灾过程中因热诱导激发而增加,火烧后呋喃占主导地位,C6多糖的丰度降低,芳香族化合物相对富集。JIMÉNEZ-MORILLO等[89]对典型的地中海橡树Quercus suber林火烧2 a后研究发现,未火烧SOM以多糖、蛋白质和木质素的化合物为主,而燃烧的SOM化学成分以木质素标记物为主,随后依次是蛋白质、烷基化烃和多环芳烃。FARIA等[99]对地中海桉树Eucalyptus globulus林火灾后25个月研究也发现,火烧导致芳香化合物、含氮化合物、木质素衍生化合物和多糖的富集,这主要是由于火烧导致了正烷烃的热裂解,表现为短链与长链化合物比例的增加和典型的奇偶碳优势指数的改变。因此,火烧移除了SOM中不耐热的生物质组分(多糖和长链烷烃),保留了木质素、芳香族和含氮化合物。
-
长期刈草和不同的耕作方式可能通过影响稳定组分和不稳定组分之间的比例影响SOM化学组成。LI等[100]对内蒙古锡林格勒半干旱草原研究发现,长期刈草导致SOM中不稳定碳(碳水化合物等)降解和稳定碳(芳香烃和烷烃类)增加。不同的土壤管理措施(轮作和耕作、作物不同品种、生产力)可能影响SOM化学组成。PARDO等[85]在非洲南部研究施肥对土壤腐殖酸结构的影响时发现,与未耕种的土壤相比,位于麦金多区域的不使用矿物肥料的土壤中烷基丰度增加,而在奇瓦卡区域的偶尔施用化肥的耕地土壤中烷基丰度降低。MASCIANDARO等[53]在地中海地区研究发现,集约耕作后,搁置可以使土壤自然恢复代谢活性和土壤肥力,即集约耕作下SOM的矿化度(吡咯/糠醛)明显高于未受干扰的土壤,而搁置条件下土壤矿化程度介于两者之间。SPACCINI等[101]研究氢氟酸处理后的土壤热裂解产物表明,与常规耕作相比,减少耕作的土壤会选择性保留较新鲜的木质素。DORADO等[102]研究常规耕作、低耕、免耕和非耕作土壤中腐殖酸的结构发现,保护性耕作(免耕)增加了土壤碳的含量,这部分碳物质主要来自木质素或微生物量等新鲜的有机质,可见免耕可能不利于有机质的积累。与此类似,BUURMAN等[2]比较免耕和常规耕作的SOM化学组成发现,免耕SOM中长链烷烃的丰富度较低,有机质分解较快。总体而言,长期耕作对SOM化学组成的影响主要表现为微生物源物质的增加、木质素相对丰度的降低以及地上向地下投入的变化(软木脂/角质值升高)[102-103]。
对于耕作条件下不同粒级土壤中有机质化学组成方面的研究也有不少报道。DIECKOW等[84]研究亚热带强淋溶土发现,蚕豆和玉米耕作系统土壤的颗粒有机物中苯乙烯、吲哚和脂肪类相对丰度明显高于草地、裸地和燕麦Avena strigosa/玉米系统,不同耕作方式对黏土粒的有机质组成没有影响;黏土中木质素衍生物丰富度低于颗粒有机物,呋喃高于颗粒有机质,说明多糖等不稳定的化合物可能受到黏土中有机物-矿物相互作用的保护。BOL等[14]研究耕地不同粒级组分中SOM组成发现,植物来源的有机物并未储存在土壤中,这些物质转化为微生物残留物质和有机矿物质相互作用而保留在土壤中,即颗粒有机物(粒径>63 μm)中木质素、碳水化合物和蛋白质占主导地位,粉砂和黏土组分中几乎没有木质素。BUURMAN等[2]研究土壤游离组分和闭蓄组分中有机质化学组成发现,闭蓄组分在“隔离”作用下,凋落物或其他物质(稀树草原中烧焦的物质)中较易分解的有机成分被较好保存下来,其烷烃、烯烃、芳香烃、脂肪酸、木质素、多环芳烃和酚类相对含量均明显高于游离组分。
此外,种植的农作物发生变化或者使用不同的施肥方法也影响SOM化学组成。由种植C3植物改变为C4植物23 a后SOM化学组成发现:土壤中含有丰富的来源于C4植物的苯酚化学成分和同时来源于玉米和小麦的蛋白质物质[104]。ARANDA等[105]研究发酵橄榄渣对碳化和硅化的土壤改良后的SOM化学组成发现:吡咯/酚值增加,糠醛/吡咯值降低,脂肪族/芳香族比值降低。ESHETU等[106]研究发现,堆肥处理下SOM中烷基酚和木质素单体的相对丰度增加,碳水化合物相对丰度降低。在研究荷兰海洋壤土沉积物发现,常规耕作和有机耕作的SOM化学组成差异很小[107]。
4.1. 气候条件
4.2. 植被类型
4.3. 土地利用变化
4.4. 火烧
4.5. 土壤管理
-
近几十年来,Py-GC/MS分析技术广泛应用于SOM研究中,包括实验室培养和大规模的野外试验,以提供SOM在分子水平的信息和揭示SOM化学成分的特征。Py-GC/MS技术在SOM化学组成中的研究主要体现在以下几方面:①通过对热裂解产物中生物化学标记分子的鉴定,可以用来比较不同生态系统和不同植被条件SOM化学特征;②对不同地区和不同环境条件下SOM化学组成特征的调查和比较,从化学分子层面研究SOM的化学特征及其对气候变化和人类活动的响应规律,探讨气候条件、土地利用变化、耕作模式和火灾扰动等不同因素对SOM化学组成的影响;③基于SOM化学组成研究土壤物质循环过程的主要影响因素,揭示SOM的化学转化过程及其在土壤生态过程中的作用机制。虽然通过Py-GC/MS技术研究SOM已经取得了一些进展,但是Py-GC/MS技术是一种定性和半定量的分析技术,从热裂解图谱中获得SOM化学分子绝对定量的数据仍然很困难。此外,基于Py-GC/MS技术研究主要停留在对SOM的解析层面上,尚未很好地利用分子组成对土壤物质的生物地球化学循环的内在机制方面进行深层次的剖析。未来可通过研究特定SOM化学成分和鉴定未知SOM的化合物成分,揭示SOM化学分子动力学对土壤微生物的消长和胞外酶的分泌的影响机制,探讨化学成分在某些特定生态过程中的重要地位,尝试通过Py-GC/MS技术从SOM本质特征,也就是SOM化学成分和化学组成角度揭示SOM在土壤中循环和转化的内在过程和影响机制,及其对气候变化和人类活动的响应机制。










 DownLoad:
DownLoad: