-
阻燃剂是赋予易燃物质难燃特性的功能性助剂,在避免火灾发生、蔓延和保障人类生命财产安全中具有特殊作用[1]。随着溴系阻燃剂的逐步禁用,有机磷阻燃剂(OPFRs)成为溴系阻燃剂的合适替代品,被广泛用于建筑、纺织等行业[2]。其中氯代有机磷阻燃剂分子中含有阻燃元素磷及氯,其阻燃性比普通磷酸酯高得多。典型代表为磷酸三(2-氯乙基)酯(TCEP)、磷酸三(2-氯丙基)酯(TCPP)和磷酸三(1, 3-二氯丙基)酯(TDCPP)[3]。OPFRs主要以物理共混方式分散在聚合物中,其与聚合物本身及添加组分不发生化学反应,在物品生产、使用等过程中较易通过挥发、磨损暴露等方式释放至周围环境中[4]。目前在室内尘土[5]、空气、水体、土壤和生物体[6]中均有不同程度的检出。研究表明:OPFRs普遍具有神经毒性[7]和致癌性[8],对人体和环境生物造成危害。WOLSCHKE等[9]认为:TDCPP、TCPP和TCEP难以被光解,ZHONG等[10]报道TCPP难以降解。开展去除环境中OPFRs成为当前的关注点,其中吸附法仍是主流方法之一,寻找新型高效的去除OPFRs材料是研究热点。智能水凝胶是一类受到外界环境刺激后自身性质会发生明显变化的水凝胶[11]。温度敏感型水凝胶的研究备受关注,在药物控释[12-13]、物质分离[14]、固定化酶[15]等生物医学领域都有广泛的应用。分子印迹技术[16] (molecular imprinting technology,MIT)是将模板分子与功能单体相结合,在交联剂作用下共聚得到固体介质,再通过物化法将模板分子洗脱,获得与目标分子空间构型和功能基团排列相匹配的结合位点的分子印迹聚合物(molecular imprinting polymers,MIPs)。传统MIPs依靠高交联度来保持其印迹结构的刚性,但交联度过高时可能降低生物相容性和溶胀性,导致聚合物中模板分子吸附和脱附困难[17]。温敏水凝胶能够响应环境温度的变化,从而弥补传统印迹聚合物的缺陷。研究发现:温敏单体的加入可使分子印迹聚合物对模板分子的识别具有温度可控性[16],即在不同温度下体积发生收缩溶胀,使印迹空腔结合模板分子的亲和力发生变化[18]。同时,温敏特性的引入可改善高交联度MIPs的缺点,不仅能提高聚合物对目标分子的选择吸附能力,还能感知温度变化,实现对目标分子的自动识别或释放[19]。近年来,MIPs优良的选择吸附能力成为一种极具发展前景的吸附材料。然而,温敏型分子印迹聚合物用于有机磷阻燃剂的选择吸附研究还很少,其中,TCPP作为常用型有机磷化合物,将该技术用于水溶液中TCPP的吸附分离,可实现较好的效果。本研究以TCPP为模板分子,丙烯酸(AA)为功能单体,N-异丙基丙烯酰胺(NIPAm)为温敏单体,采用自由基聚合法制备了具有温敏特性的温敏型分子印迹水凝胶(T-MIHs)。获得的T-MIHs可在吸附去除水溶液TCPP的同时,达到高效选择识别目的。通过测定水凝胶在不同温度和不同底物溶液中的体积变化,研究T-MIHs对温度及模板分子的响应性能,并对模板分子的吸附机制进行探讨。
-
N-异丙基丙烯酰胺(分析纯,质量分数为98%),丙烯酸(分析纯,质量分数为99%),N,N′-亚甲基双丙烯酰胺(分析纯,质量分数为98%)购于上海阿拉丁试剂有限公司;过硫酸铵(化学纯,质量分数为98%),二甲基亚砜(分析纯,质量分数为99%)购于国药集团化学试剂有限公司;磷酸三(2-氯丙基)酯购于上海迈瑞尔化学技术有限公司。
UV-2550型紫外分光光度计(Shimadzu,日本);IRPrestige-21型傅立叶变换红外光谱仪(Shimadzu,日本);DF-101S型集热式磁力搅拌器(巩义市予华仪器有限公司,中国);AVANCE Ⅲ HD-400M型核磁共振波谱仪(Bruker,瑞士);Quanta 200FEG型环境扫描电子显微镜(FEI,美国);真空干燥箱(北京益康科学仪器有限公司,中国);SJIA-10N型冷冻干燥机(宁波市双嘉仪器有限公司,中国);DSC Q2000型差示扫描量热仪(TA,美国)。
-
参考文献[20]制备温敏水凝胶,并结合分子印迹技术制备T-MIHs。取0.48 mmol 模板分子TCPP、22.09 mmol温敏单体N-异丙基丙烯酰胺(NIPAm)、21.86 mmol功能单体丙烯酸(AA)、0.16 mmol 交联剂N,N′-亚甲基双丙烯酰胺(MBAA)于10 mL试管中,加入2.5 mL二甲基亚砜并超声溶解20 min,继续加入0.04 mmol引发剂过硫酸铵(APS),充分溶解混匀,试管浸入90 ℃油浴中反应10 min,得到8 g白色透明产物。反应完成后将所得水凝胶切成小块先用50 mL甲醇∶乙酸为9∶1(体积比)的溶液洗脱3 d (隔12 h换1次洗脱液),然后使用甲醇洗脱24 h,直到完全洗脱模板以及未反应的单体。将水凝胶60 ℃真空干燥,得到T-MIHs。作为对照制备温敏非印迹水凝胶(T-NIHs),按照T-MIHs的配比均匀混合制成AA/NIPAm前体液,然后加入一定量的过硫酸铵。除了不加模板分子TCPP,其他步骤和操作与T-MIHs相同。
-
采用傅里叶变换红外光谱仪进行分析,扫描范围为4 000~400 cm−1;将样品冷冻干燥,研磨粉碎,采用核磁共振仪测试其13C核磁共振波(NMR)谱,H/X双共振固体探头,4 mm 二氧化锆(ZrO2)转子,转速5 kHz,检测共振频率100.625 MHz,采样时间5.12 μs,循环延时6.50 μs,扫描次数4 096次;样品经冷冻干燥喷金后,用扫描电镜在真空条件下对其表面形貌进行观察。
-
为了探究温敏印迹水凝胶温度响应机理,采用称量法测定水凝胶在不同温度蒸馏水中的平衡溶胀率(swelling ratio,Rs)。称取7份干燥至恒量的T-MIHs及T-NIHs,在不同温度下(20、25、30、35、40、45、50 ℃)置于10 mL蒸馏水中浸置平衡24 h。温度条件采用恒温水浴锅控制,达溶胀平衡后,用滤纸拭干凝胶表面水分,记录水凝胶在各个温度的平衡质量m24h,均平行测定3次,计算平衡溶胀率Rs:
式(1)中:m24h是第24 小时水凝胶的质量,m0是初始时刻凝胶干燥至恒量时的质量。
-
通过差示扫描量热仪测定水凝胶的热性能。称取5~10 mg干燥样品,氮气氛围(流量:60 mL·min−1),升温速率10 ℃·min−1,升温范围10~120 ℃。采集样品在升温过程中的质量变化,得到样品的热失重曲线,分析产物热性能。
-
分别称取10 mg T-MIHs和T-NIHs置于10 mL离心管中,向离心管中加入 5 mL 1.5 mg·L−1的TCPP溶液,恒温吸附,分别于1、2、4、6、8、10、12、24、32、48、72 h时吸取上清液,用紫外分光光度计测其吸光度,求出吸附后TCPP的质量浓度。根据吸附前后溶液质量浓度变化,计算出水凝胶对模板分子的吸附量Q(mg·g−1):
式(2)中:C0、Ct分别代表溶液初始质量浓度(mg·L−1)和某一时间的溶液质量浓度(mg·L−1),V代表吸附溶液的体积(L),m代表凝胶的质量(g)。
选择准一级和准二级动力学模型对T-MIHs和T-NIHs的吸附特性进行线性拟合,表达式如下:
式(3)~(4)中:qe和qt分别为水凝胶吸附达到平衡时和t时对目标化合物的吸附量(mg·g−1),k1和k2分别为准一级、准二级吸附速率常数。
-
配置0.3、0.5、0.7、0.9、1.1、1.3、1.5、1.7、1.9 mg·L−1的TCPP溶液于10 mL离心管中,各加入10 mg T-MIHs和T-NIHs,恒温吸附24 h,使吸附达到平衡。取上清液用紫外分光光度计检测其吸光度,计算吸附后TCPP的质量浓度。按式(2)计算凝胶对模板分子的吸附量Q(mg·g−1)。采用Langmuir和Freundlich吸附模型对T-MIHs和T-NIHs的等温吸附特性进行线性拟合。等温吸附线性方程如下:
式(5)~(6)中:Qmax和Qe分别为水凝胶饱和与平衡时的吸附量(mg·g−1);Ce为静态吸附平衡质量浓度(mg·L−1);K和Kf分别为Langmuir和Freundlich吸附常数;n为特征常数(n越大,说明吸附强度越大,0<n<10。n>1,有利于吸附;n=1,线性吸附;n<1则不利于吸附)。
-
选取TDCPP、TCEP为结构类似物。分别称取3份10 mg T-MIHs和T-NIHs置于离心管中,加入质量浓度为1 mg·L−1的TCPP和结构类似物溶液4 mL,恒温振荡24 h,取上层清液,紫外分光光度计测其吸光度,并按式(2)计算出各自的吸附量。计算印迹因子(α)和选择因子(β),比较各自的印迹效果:
式(7)中:QT-MIHs代表印迹聚合物微球对底物的吸附量;QT-NIHs代表非印迹聚合物微球对底物的吸附量。
-
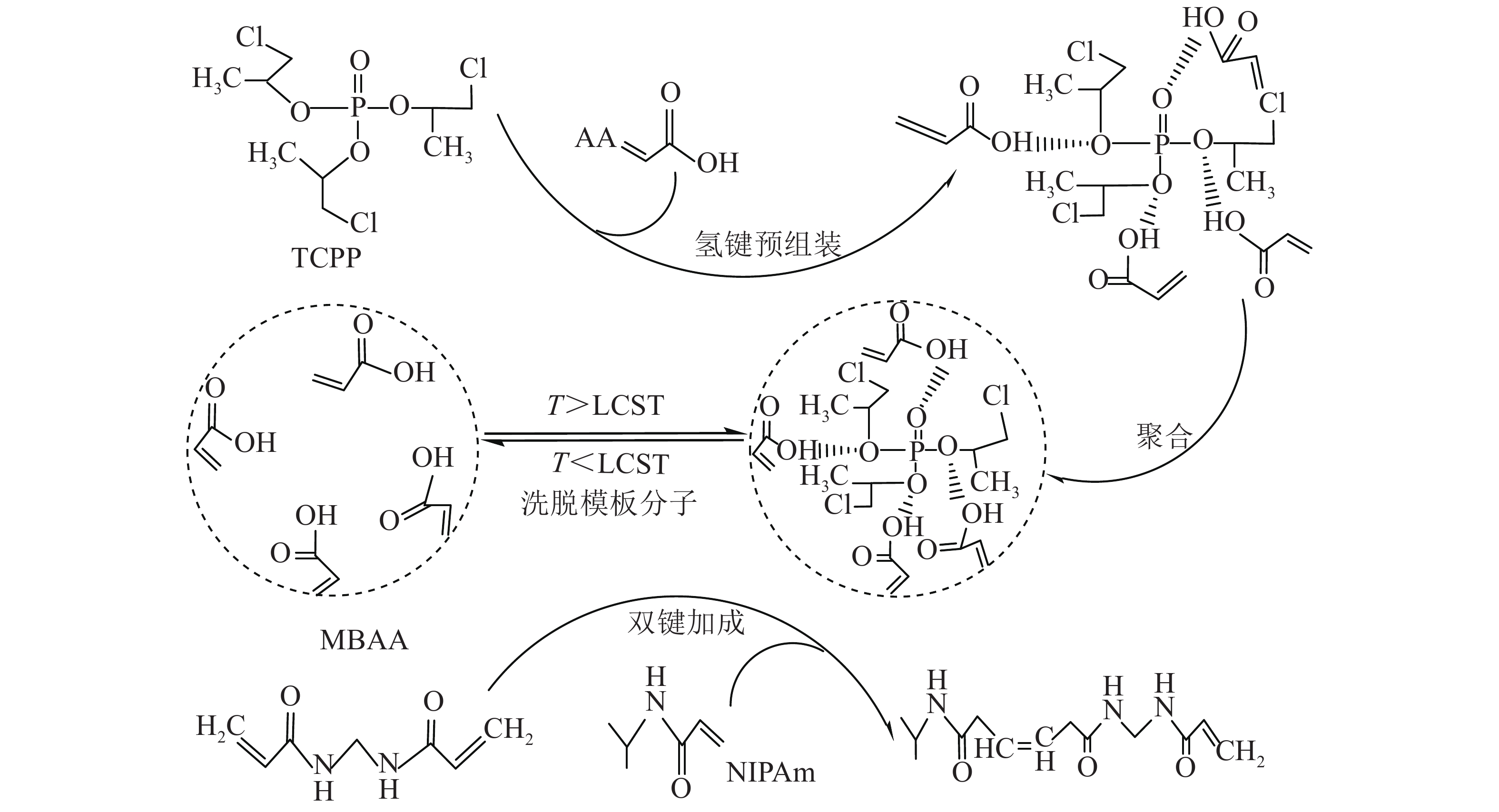
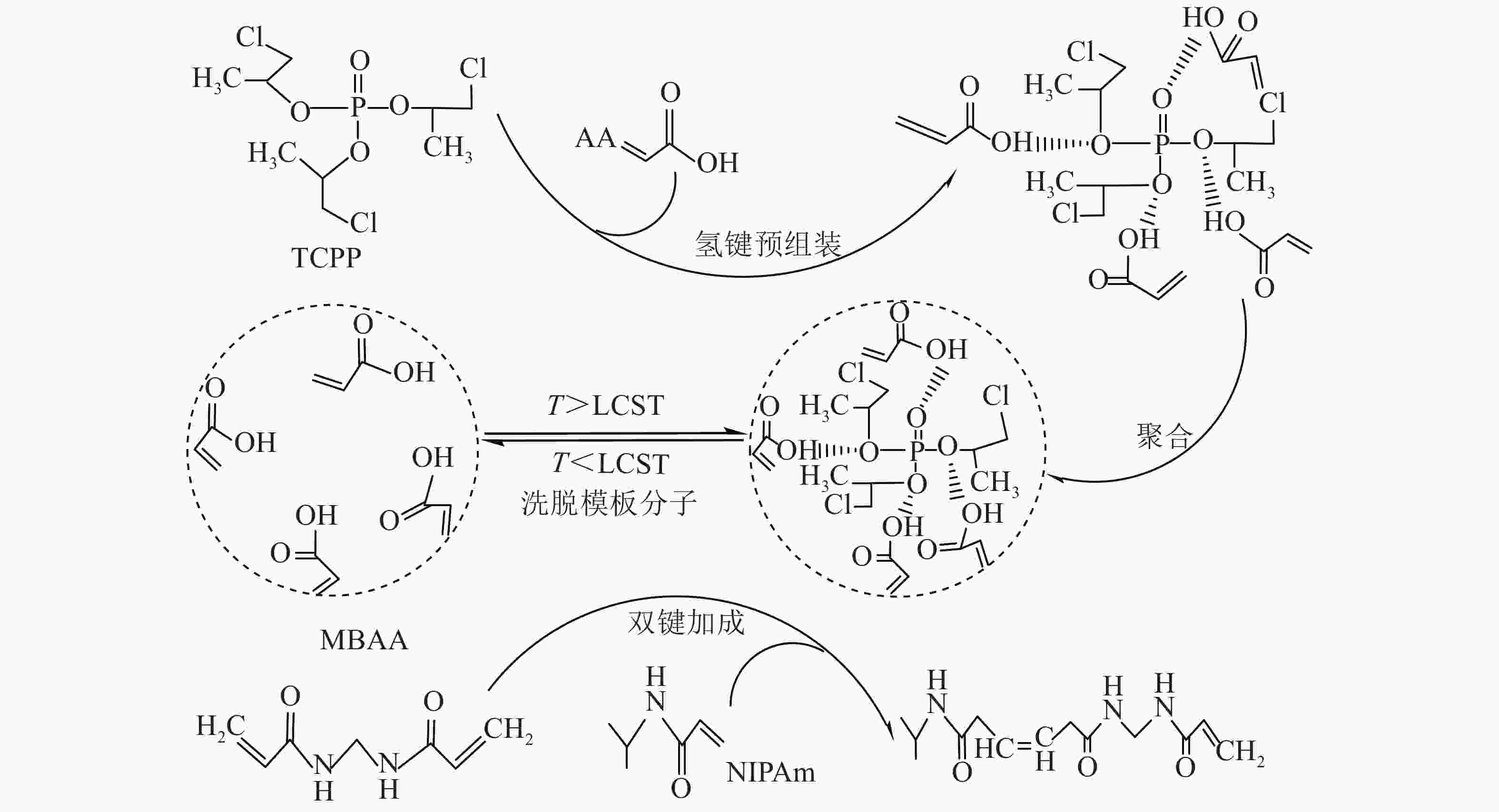
T-MIHs的合成反应式如图1。以TCPP为模板分子,NIPAm、AA分别为温敏单体和功能单体,通过MBAA交联制备T-MIHs。为使模板分子能固定在水凝胶内部,选择TCPP与AA的羟基发生氢键预组装,NIPAm与MBAA之间发生双键加成反应,在甲醇/乙酸混合溶液中洗脱模板分子,得到三维网络结构的T-MIHs。因模板分子被水凝胶聚合物印迹而留下独特的空间结构,可作为识别活性位点而表现出特异识别性能,同时由于NIPAm的温敏特性,随着温度变化,水凝胶表现出溶胀收缩性质。
-
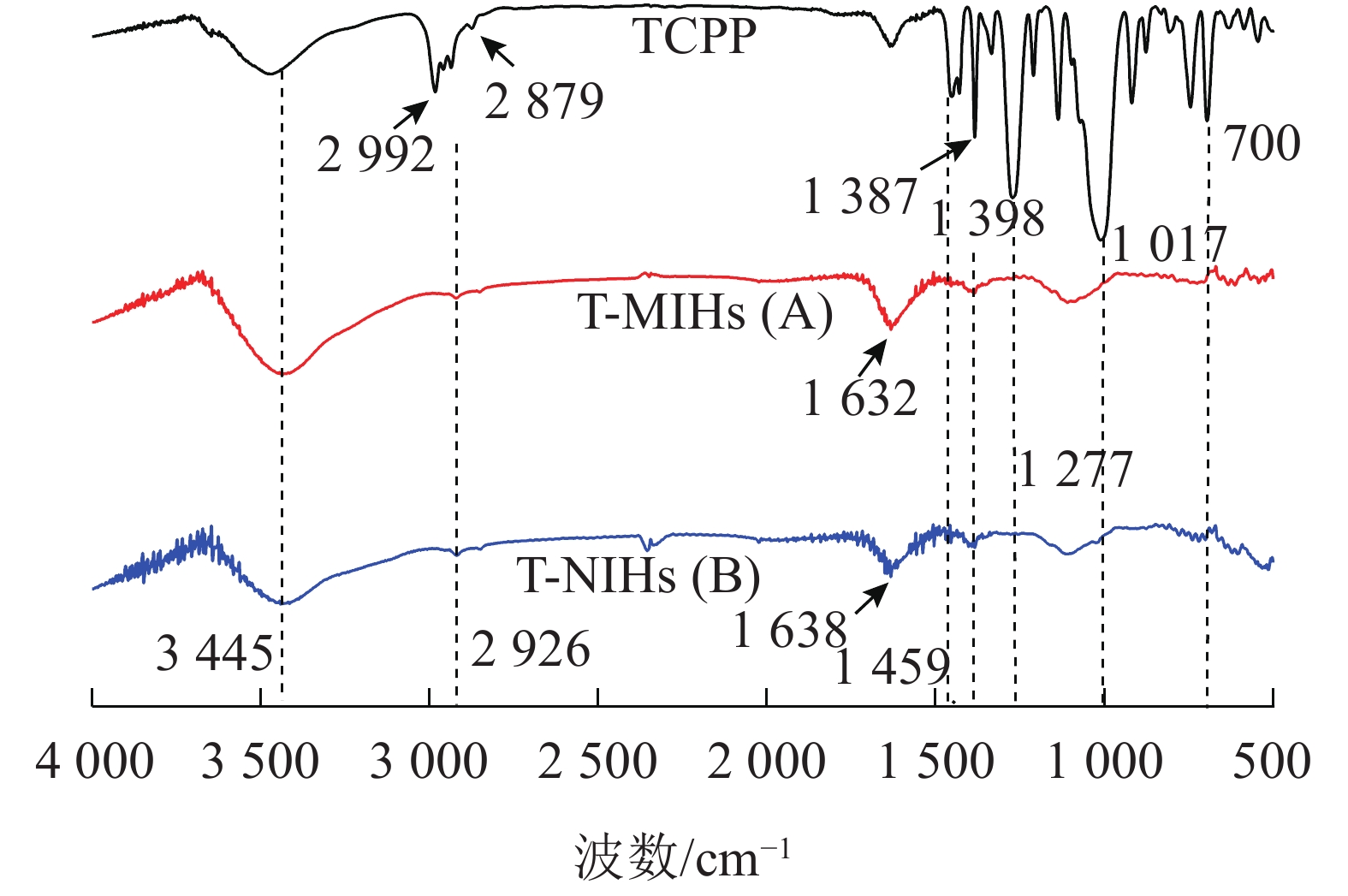
由图2可知:T-MIHs和T-NIHs分别在1 632和1 638 cm−1出现酰胺上的C=O伸缩振动吸收峰[21-22];1 398 cm−1出现异丙基的特征吸收峰,3 445 cm−1为—OH伸缩振动峰[23]及—NH伸缩振动峰的重叠峰[24];2 928及2 926 cm−1附近为MBAA上的—NH伸缩振动峰[19]。以上结果说明:单体AA、MBAA、NIPAm发生聚合,初步证明产物成功合成。同时TCPP在2 992、2 879 cm−1附近出现—CH3、—CH2的C—H对称伸缩振动吸收峰,1 459 cm−1附近为C—H的骨架振动,1 387 cm−1为—CH3的C—H弯曲振动,1 277 cm−1为P=O的伸缩振动特征吸收峰,1 017 cm−1附近出现P—O—C伸缩振动吸收峰,700 cm−1有较强吸收峰为C—Cl伸缩振动吸收峰[25]。这些具备TCPP官能团的特征吸收峰在T-MIHs和T-NIHs的红外光谱图中并未体现,说明T-MIHs成功制备,模板分子已被完全除去。
-
由图3可知:NIPAm的C6、AA的C1及MBAA的C1、C7(烯烃碳原子)峰强度减弱,而T-MIHs在13C谱化学位移(δ)为173处出现C4、C9和C11(羰基碳原子)的核磁波谱峰,δ=39处出现C5、C6、C7、C8、C10、C12、C13的碳骨架(亚甲基碳原子)核磁波谱峰,δ=19处为C1和C3(甲基碳原子)的核磁波谱峰。而C2处的次甲基碳原子因其含量较少,峰强度较低,故被甲基和亚甲基碳原子的波谱峰覆盖。结果表明:水凝胶骨架成功制备。
-
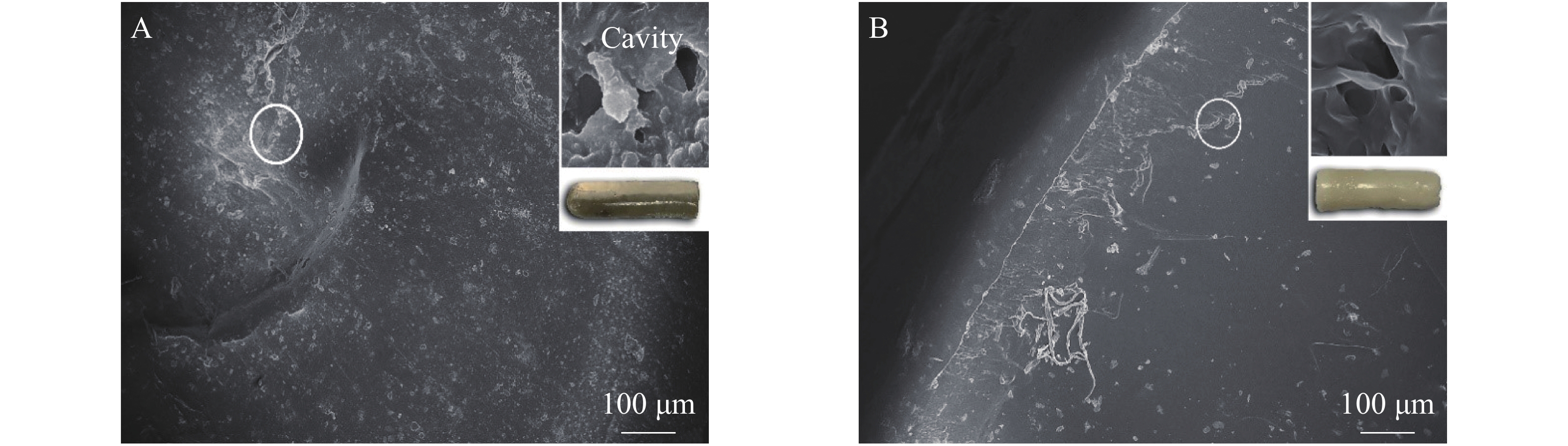
从图4A可以看出:T-MIHs表面粗糙不平整,这是由于模板分子洗脱过程中扩散造成的,水凝胶内部留有模板分子的识别位点孔状结构,TCPP通过这些孔穴发生印迹作用,从而表现出特异选择性。图4B中T-NIHs相较于T-MIHs表面平滑,无明显孔穴网络结构,易造成非特异性吸附[21]。结果表明:制备得到的T-MIHs具有特异识别位点。
-
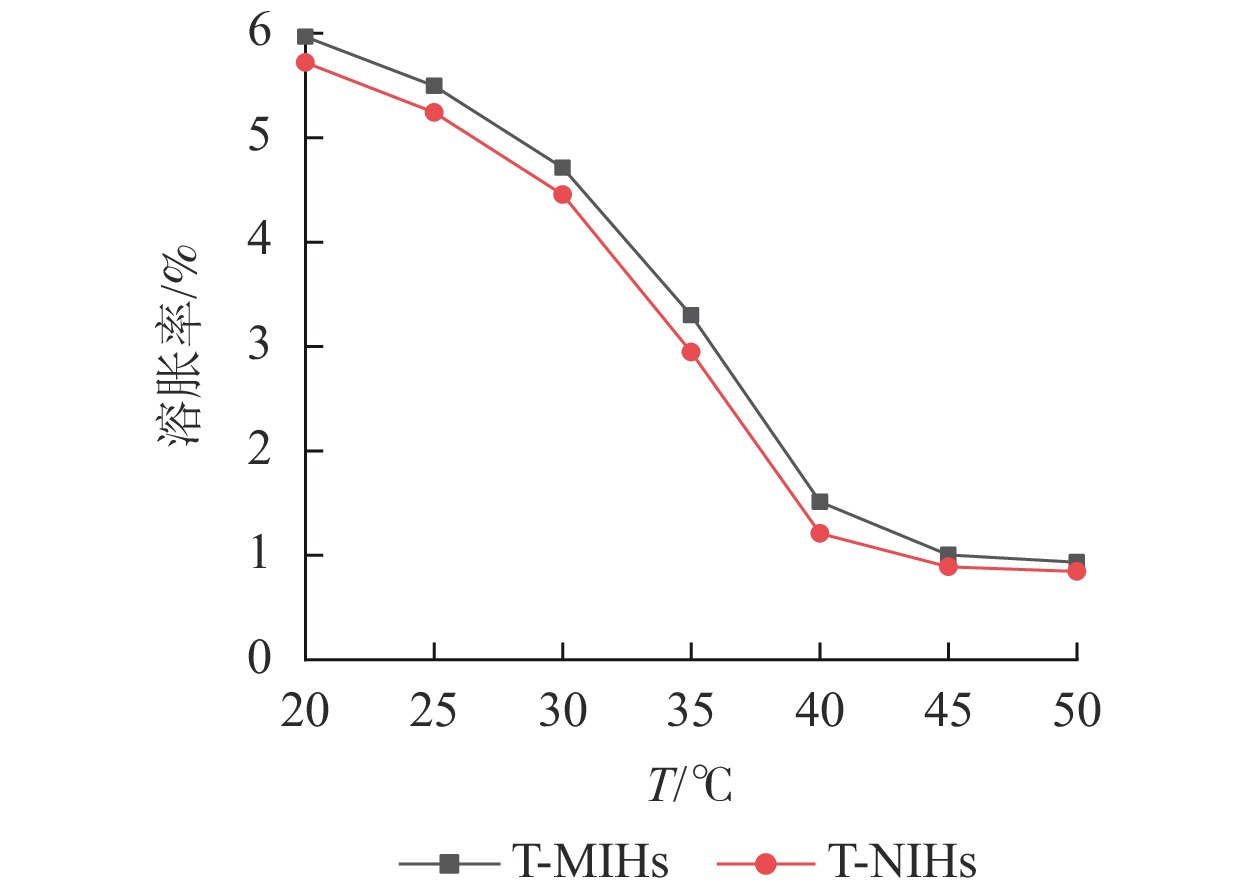
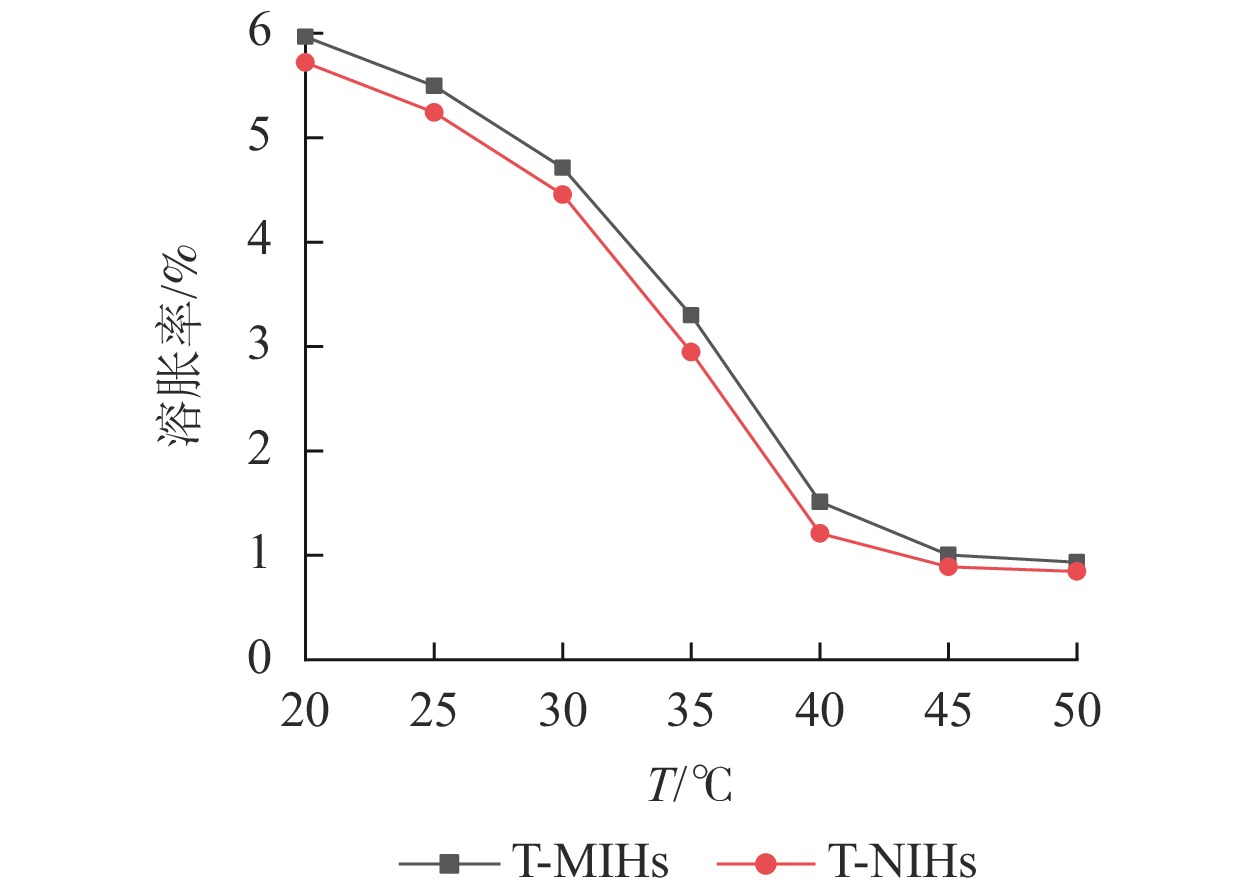
以NIPAm为功能单体制备的水凝胶,可以感应环境温度的刺激,在溶胀与缩水之间发生可逆性的体积变化。从图5可见:T-MIHs对温度变化具有明显的刺激响应性,在45~50 ℃时凝胶的平衡溶胀率变化不大,但在40 ℃附近,溶胀率曲线的斜率发生了突跃。由此可认为:40 ℃左右即为水凝胶的低临界溶液温度(LCST)值。T-NIHs在不同温度下的平衡溶胀率变化趋势与T-MIHs基本相似,说明模板分子(TCPP)的引入对水凝胶的相变温度影响较小。
-
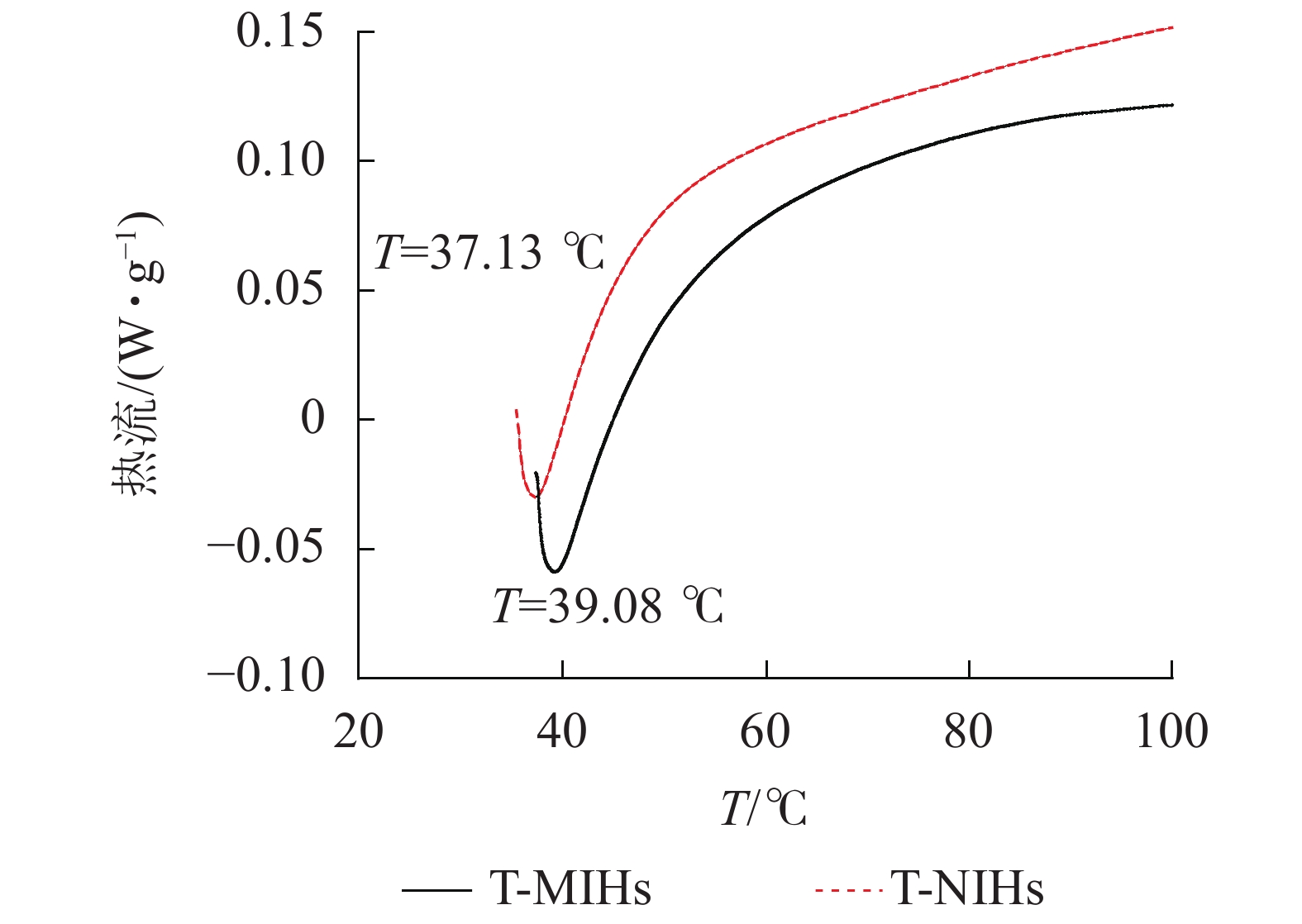
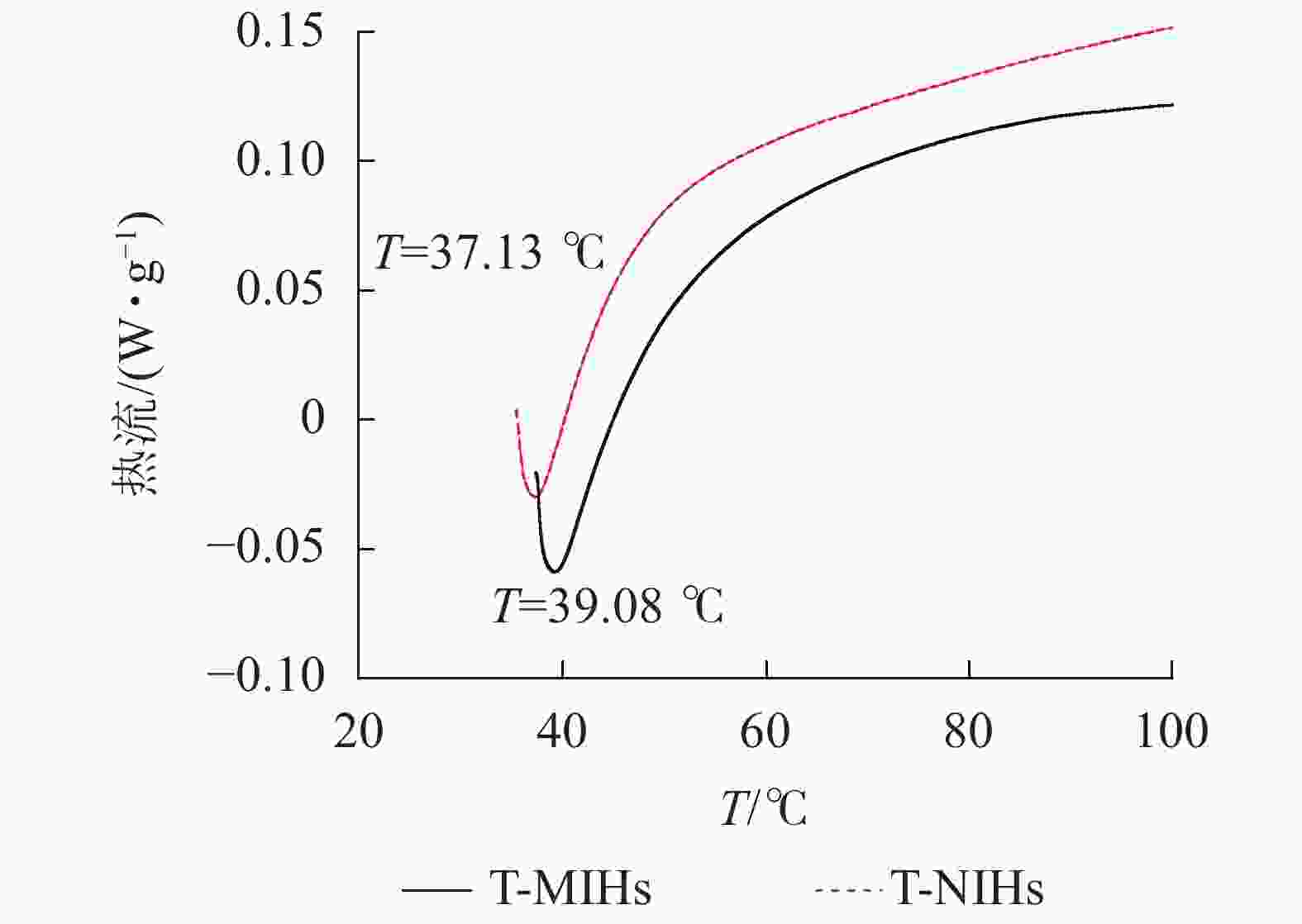
具有一定的温敏特性,能随外界温度变化而发生体积相变,是由于凝胶与水相分离而产生的热效应。从图6可以看出:升温过程中,T-MIHs在39 ℃左右出现吸热峰,T-NIHs的相变温度较低,在37 ℃左右出现吸热峰,表明随着温度的升高,水凝胶不断收缩。此结果与上述称量法测定结果基本保持一致,表明吸热峰越窄,去溶胀速率越高。结果证明制备的水凝胶保留PNIPAm的温敏特性。由于NIPAm单体在聚合过程中引入其他单体会改变共聚物的LCST值,疏水性共聚单体会使低临界溶液温度(LCST)值降低,亲水性共聚单体会使 LCST 值升高[26],因此T-MIHs、T-NIHs的LCST值均略高于PNIPAm的LCST 值。
-
配制0.1~1.5 mg·L−1的TCPP/TCEP/TDCPP标准溶液,利用紫外分光光度计测量其吸光度,并绘制标准曲线(表1)。为进一步研究T-MIHs对TCPP的选择识别和吸附能力,对其进行定量分析和理论模型构建,结果如图7和表2所示。对比2个动力学方程的拟合结果,T-MIHs和T-NIHs的准一级动力学拟合相关系数(分别为0.952 5和0.955 3)均高于准二级动力学的线性拟合系数(分别为0.827 5和0.921 2),这表明T-MIHs和T-NIHs的吸附过程更符合准一级动力学吸附特征,吸附速率分别为0.029 6和0.024 2 g·mg−1·min−1。
样品 方程 R2 标准差 抽样数 P TCPP y = 0.555 6x+0.001 5 0.999 2 0.015 6 6 <0.000 1 TCEP y = 0.600 4x−0.036 4 0.990 1 0.061 1 6 <0.000 1 TDCPP y = 0.561 3x−0.004 2 0.992 5 0.056 6 6 <0.000 1 说明:x为样品质量浓度(mg·L−1);y为待测样品的吸光度 Table 1. Standard curve equation of OPFRs in methanol
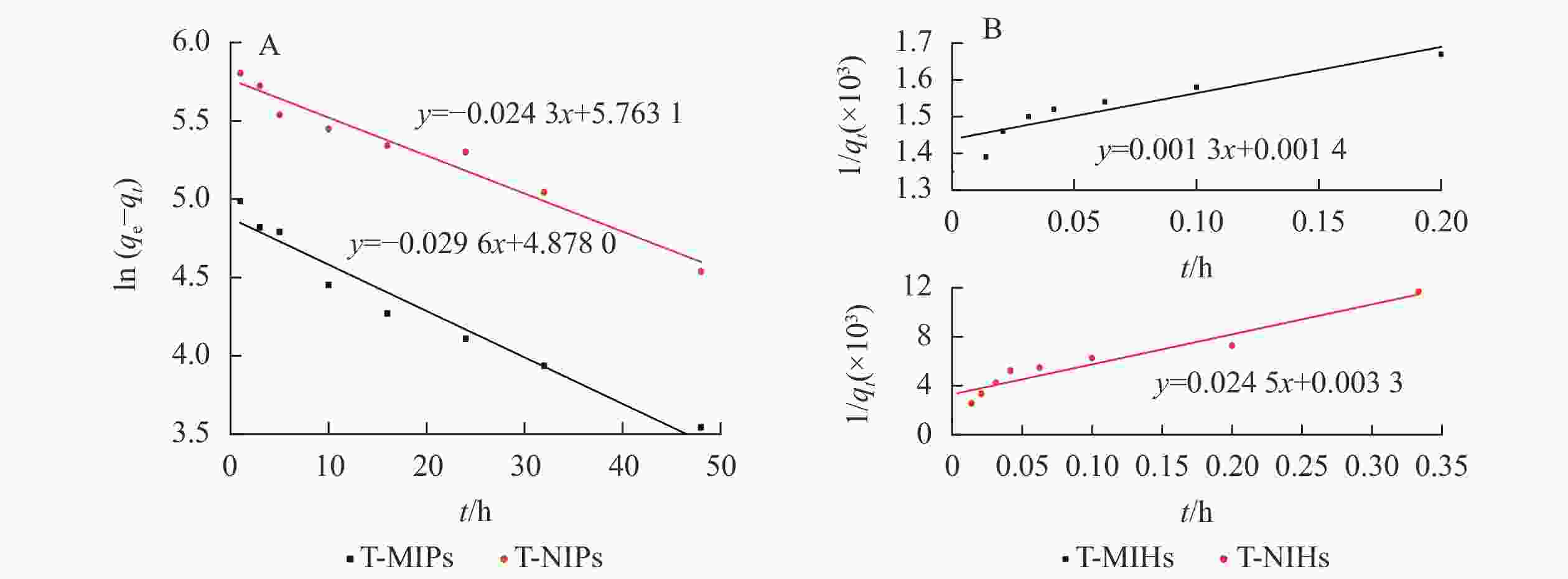
Figure 7. Fitting curve diagram of Pseudo-first-order equation (A) and Pseudo-second-order equation (B)
准一级动力学 准二级动力学 样品 方程 qe/(mg·g−1) K1 R2 方程 qe/(mg·g−1) K2 R2 T-MIHs y1 = −0.029 6x1 + 4.878 0 131.363 7 0.029 6 0.952 5 y2 = 0.001 3x2 + 0.001 4 714.285 7 0.001 6 0.827 5 T-NIHs y1 = −0.024 3x1 + 5.763 1 318.343 1 0.024 2 0.955 3 y2 = 0.024 5x2 + 0.003 3 303.951 4 0.008 7 0.921 2 说明:y1为 ln(qe−qt),x1为 t;y2为 1/qt,x2为 t Table 2. Adsorption dynamic model parameter
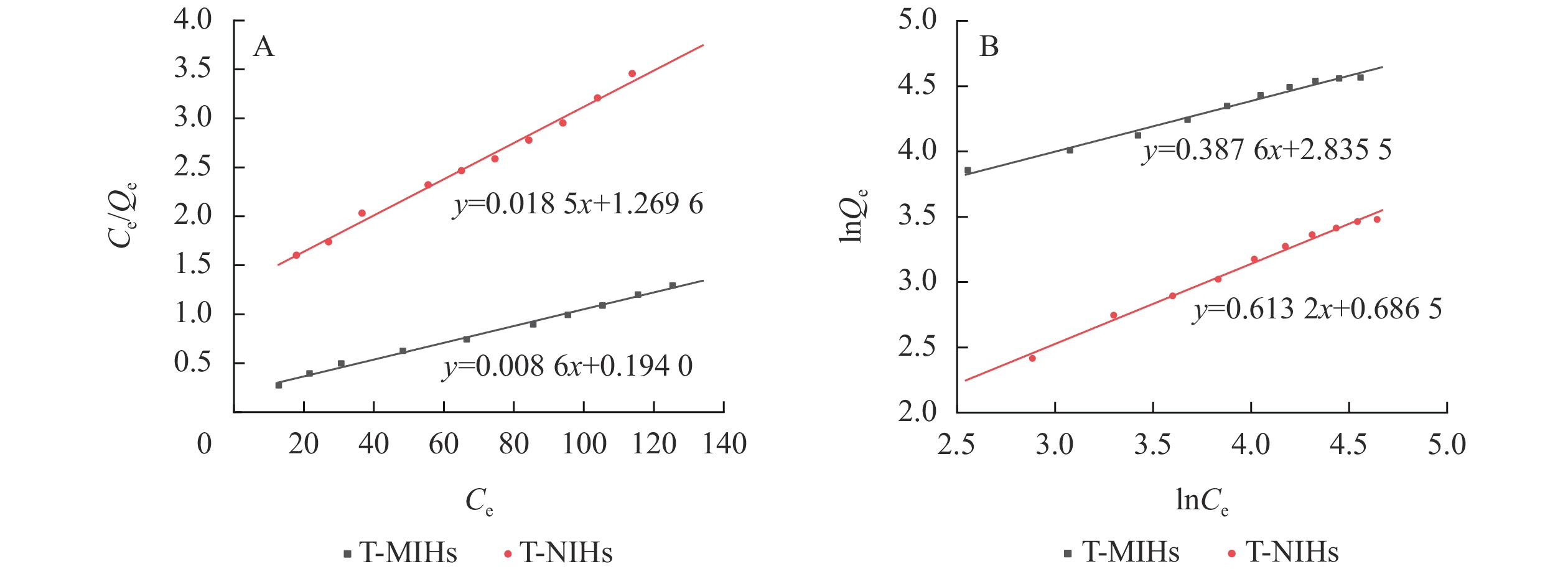
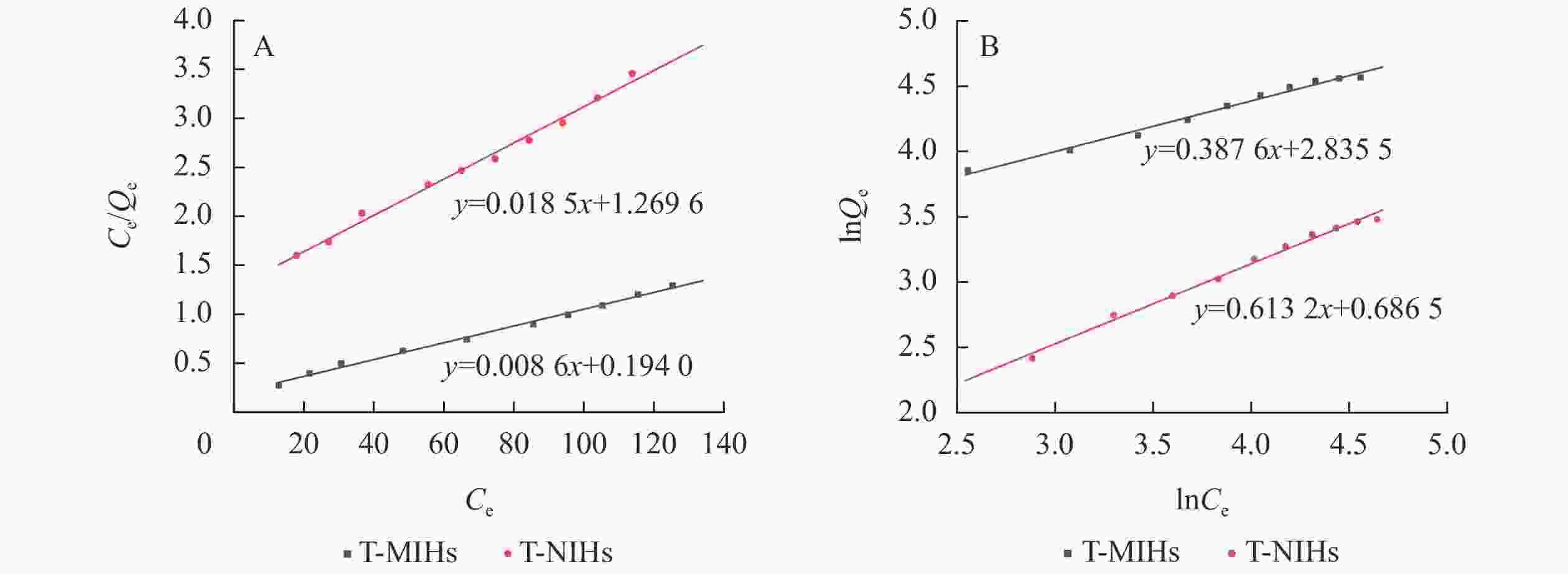
从T-MIHs及T-NIHs的Langmuir方程(R2分别为0.994 0和0.991 4)和Freundlich方程(R2分别为0.986 2和0.991 0)的拟合相关系数(图8和表3)可以看出:Langmuir吸附模型线性相关性高于Freundlich模型,故T-MIHs和T-NIHs对TCPP的等温吸附过程更符合Langmuir模型。Langmuir模型的建立是基于单分子层吸附以及吸附质在吸附剂表面具有等同吸附能的假设,可以初步判断T-MIHs及T-NIHs对TCPP的吸附均为化学吸附过程,其饱和吸附量分别为116.686 1和54.083 3 mg·g−1。此外,结合吸收剂对污染物吸附能力的实例(表4),结果表明T-MIHs对溶液中TCPP具有优异的吸附效果。
Langmuir吸附等温线 Freundlich吸附等温线 样品 方程 K Qmax/(mg·g−1) R2 方程 Kf n R2 T-MIHs y1 = 0.008 6x1+0.194 0 0.044 2 116.686 1 0.994 0 y2 = 0.387 6x2+2.835 9 17.045 0 2.580 3 0.986 2 T-NIHs y1 = 0.018 5x1+1.269 6 0.014 6 54.083 3 0.991 4 y2 = 0.613 2x2+0.686 5 1.986 8 1.630 8 0.991 0 说明:y1为 Ce/Qe,x1为 Ce;y2为 lnQe,x2为 lnCe Table 3. Adsorption isothermal equation parameters
Table 4. Comparison of adsorption capacity of TCPP on different absorbents
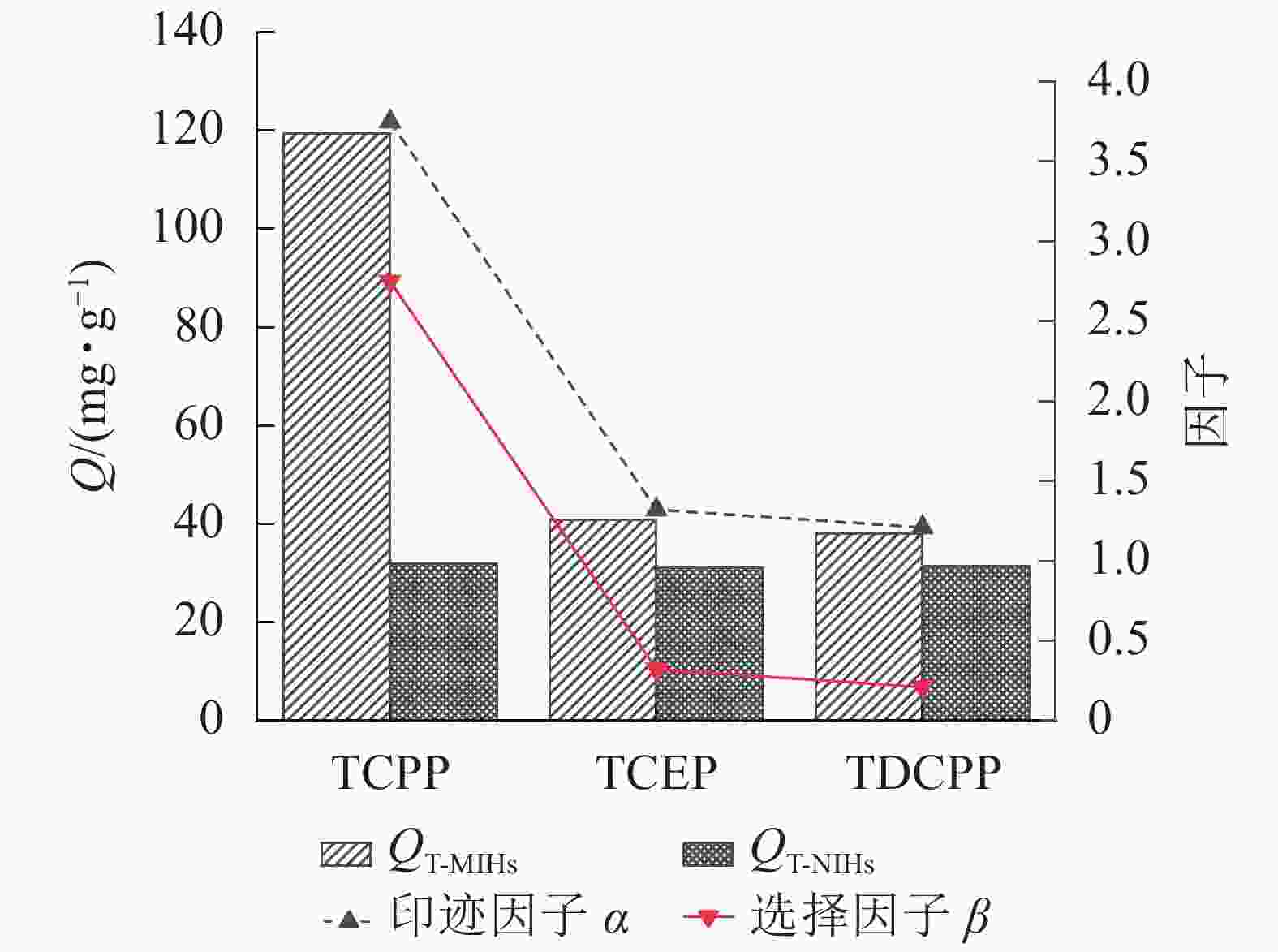
为探究T-MIHs的特异识别性,选择与TCPP结构类似的TCEP和TDCPP为吸附底物,通过平衡吸附实验测定T-MIHs对3种化合物的平衡吸附容量,计算印迹因子α和选择因子β。由图9可见:相比于结构类似物,T-MIHs对模板分子的吸附容量最大,达119.32 mg·g−1,印迹因子α和选择因子β分别为3.75和2.75。原因是水凝胶内部存在与模板分子匹配的孔穴结构,表现出对模板分子的特异识别性;同时,T-MIHs对2种结构类似物的吸附容量较小,分别为38.08和40.86 mg·g−1,其印迹因子分别为1.21和1.32,是因为TCPP与TCEP和TDCPP具有结构相似性,导致T-MIHs对两者均有吸收。而T-NIHs对3种化合物的吸附容量均较低,没有显著差异。原因是T-NIHs在制备中没有加入模板分子,水凝胶内部不存在与其相匹配的印迹孔穴,属于非特异性吸附。郭小伟等[30]研究也证明:T-MIHs网络结构中形成了三维立体孔穴和识别位点,具有良好的印迹效果。
-
以TCPP污染物为模板分子,本研究制备了1种具有温度响应与高选择识别性能于一体的T-MIHs。红外光谱分析表明T-MIHs成功制备,模板分子已被完全除去;核磁共振证实了产物的水凝胶骨架成型;SEM电镜证明制备的T-MIHs具有特异识别位点;热重分析和溶胀性能分析得到T-MIHs最低临界溶解温度为39 ℃。吸附实验表明:T-MIHs对TCPP的吸附行为符合准一级动力学模型和Langmuir吸附模型,且印迹吸附因子α为3.75,选择吸附因子β为2.75。本研究制备的T-MIHs可为识别吸附水溶液中的有机磷阻燃剂TCPP提供有益的参考。
Preparation of thermo-sensitive molecularly imprinted hydrogels and their adsorption properties for organophosphorus flame retardants
doi: 10.11833/j.issn.2095-0756.20210254
- Received Date: 2021-03-25
- Accepted Date: 2021-11-16
- Rev Recd Date: 2021-11-10
- Available Online: 2022-03-25
- Publish Date: 2022-03-25
-
Key words:
- organophosphorus flame retardant /
- thermo-sensitive hydrogel /
- molecular imprinting /
- selectivity
Abstract:
| Citation: | FANG Tao, LI Jinyun, GUO Ming, et al. Preparation of thermo-sensitive molecularly imprinted hydrogels and their adsorption properties for organophosphorus flame retardants[J]. Journal of Zhejiang A&F University, 2022, 39(2): 405-414. DOI: 10.11833/j.issn.2095-0756.20210254 |












 DownLoad:
DownLoad: