-
高温胁迫不仅导致植物表型的变化,而且会引起植物体内细胞稳态失衡,生长发育受到抑制,严重影响观赏植物的品质[1]。环境温度的变化对植物光合作用的多种生理生化过程有直接的影响,包括光合碳固定和还原、蔗糖合成、光合产物的运输与分配等[2]。碳同化是光合作用中植物在同化力形成之后的第3个阶段,此阶段中,植物叶绿体基质中的Rubisco酶利用ATP和NADPH同化二氧化碳(CO2)生成淀粉、蔗糖、葡萄糖、果糖等物质。在高等植物光合作用中,果糖-1,6-二磷酸酶(Fructose-1, 6-diphosphate synthase, FBPase)在卡尔文循环和细胞质中的蔗糖生物合成途径中都起着关键作用,其中存在至少2种酶:叶绿体型FBPase和胞质型FBPase。叶绿体型FBPase主要参与还原磷酸戊糖途径,同时参与叶绿体中淀粉的合成;胞质型FBPase主要参与糖异生途径和蔗糖的合成,在蔗糖的合成中起着重要的调节作用[3-4]。有研究指出蔗糖磷酸合酶(sucrose diphosphate synthase, SPS)与淀粉积累呈现负相关关系,而与蔗糖形成呈正比关系[5-6],并与蔗糖合成酶(sucrose synthase, SS)一起成为蔗糖调节的关键酶[7],为淀粉合成提供底物及能量。淀粉是植物体中碳水化合物的主要储存形式,叶片中淀粉合成是一个动态的过程[8]。在淀粉合成通路中,腺苷二磷酸葡萄糖焦磷酸化酶(ADP-glocose pyrophosphorylase, AGP)作为限速酶,参与第一步反应,颗粒结合淀粉合酶(granule-bound starch synthase, GBSS)参与直链淀粉合成,可溶性淀粉合成酶(soluble starch synthase, SSS)和淀粉分支酶(starch branching enzyme, SBE)协作合成支链淀粉[7]。这些碳同化产物既是光合作用的产物,同时还是植物呼吸作用的底物,为植物的生长发育提供碳骨架的同时,还可增强植物的抗逆性。为进一步了解植物对高温的响应,分子生物学与转录组学等应用与解析调控植物耐热的分子基础,培育了相关观赏植物品种[1]。
景宁木兰Magnolia sinostellata是木兰科Magnoliaceae木兰属Magnolia灌木,早春开花,花色浅粉色到粉红色,具有较高的观赏价值,具有开发应用价值[9]。全球变暖带来的极端高温可能对景宁木兰的生长发育带来潜在的威胁。目前已挖掘了景宁木兰热胁迫下实时荧光定量PCR 内参基因,但还未深入研究。因此,本研究以景宁木兰幼苗为对象,采取高温胁迫处理,研究其糖代谢途径变化机制,从而探究景宁木兰对高温的响应情况,同时为木兰属植物的城市应用奠定基础。
-
景宁木兰植株均来自浙江省景宁县草鱼塘林场苗圃(海拔1 100 m)。选择1年生、生长一致的景宁幼苗栽植于装有土壤与珍珠岩按质量比为1∶1混合的容器中,常规管理。所有样品均放置于光照培养箱中[光照20 μmol·m−2·s−1,温度(25±1) ℃,相对湿度(70±10)%,光照黑暗各12 h]。当植株生长到10 cm左右高度时,选取20株健康、长势均匀的幼苗转移到温度设定为40 ℃(H)的光照培养箱中,以25 ℃为对照(ck)。处理0、3、6、9、 12、24和48 h时采集景宁木兰第2~3叶(成熟叶)为样本,迅速保存在液氮中,用于后续的测定,其中选取ck-24、ck-48、H-24、H-48用于转录组测定,每个样本3次生物学重复。
-
蔗糖、淀粉、葡萄糖和果糖质量分数的测定采用改进的苯酚-硫酸法[10],果糖1,6-二磷酸酶(FBPase)的测定方法采用CHEN等[11]的方法,蔗糖磷酸合酶(SPS)根据GROF等[12]方法测定。采用SPSS 19.0进行单因素方差分析,采用LSD法进行显著性差异分析。
-
使用Trizol试剂盒(天根,北京)提取景宁木兰叶片总RNA。质量检测合格后,经深圳华大基因科技服务有限公司使用DNaseI消化DNA、富集mRNA并反转录合成cDNA和PCR扩增,制备cDNA文库。用Agilent 2100 Bioanalyzer和ABI StepOnePlusReal-Time PCR System完成文库质量和产量检测后进行转录组测序。对原始数据(raw reads)进行质控(QC)获得滤后的数据(clean reads),再进行基因定量分析以及基于基因表达水平的各项分析(主成分、相关性、条件特异表达、差异基因筛选等),并对筛选出的样品间差异表达基因进行GO功能显著性富集分析、pathway显著性富集分析和聚类以及蛋白互作网络和转录因子等深入挖掘分析。
-
用RSEM工具[13−14]进行基因表达定量,运用FPKM法消除基因长度和测序量差异对计算基因表达的影响,计算得到的基因表达量可直接用于比较不同样品间的基因差异表达。将候选响应基因利用RefSeq non-redundant proteins(NR)数据库、Swiss-Prot数据库、Gene Ontology(GO)数据库和Kyoto Encyclopedia of Genes and Genomes(KEGG)数据库进行Blast比对,获得候选响应基因的注释信息。用WEGO软件[15]对差异基因做GO功能分类统计,利用Omicshare在线工具库中的动态KEGG富集分析程序进行候选响应基因的注释及功能预测。
-
将反转录产物cDNA稀释10倍,使用SYRB Premix Ex Tap Ⅱ (TAKARA:Code No.RR820A)实时定量PCR试剂盒,使用Light Cycler 480 Ⅱ(Roche)实时定量PCR仪进行定量分析。反应体系为SYRB Premix Ex Tap Ⅱ10 μL,cDNA模板2 μL,上下游引物各0.8 μL(10 μmol·L−1),ddH2O 6.4 μL,共20 μL体系。每个样品3次重复,扩增反应程序为:95 ℃预变性30 s,95 ℃变性5 s,60 ℃预变性30 s,共40个循环。然后95 ℃持续5 s,60 ℃持续1 min,95 ℃持续15 s作为溶解曲线分析程序,进行溶解曲线分析。最后根
${{\text{2}}^{\text{-}\Delta \Delta {{C}_{t}}}} $ 法计算目的基因的相对表达量。 -
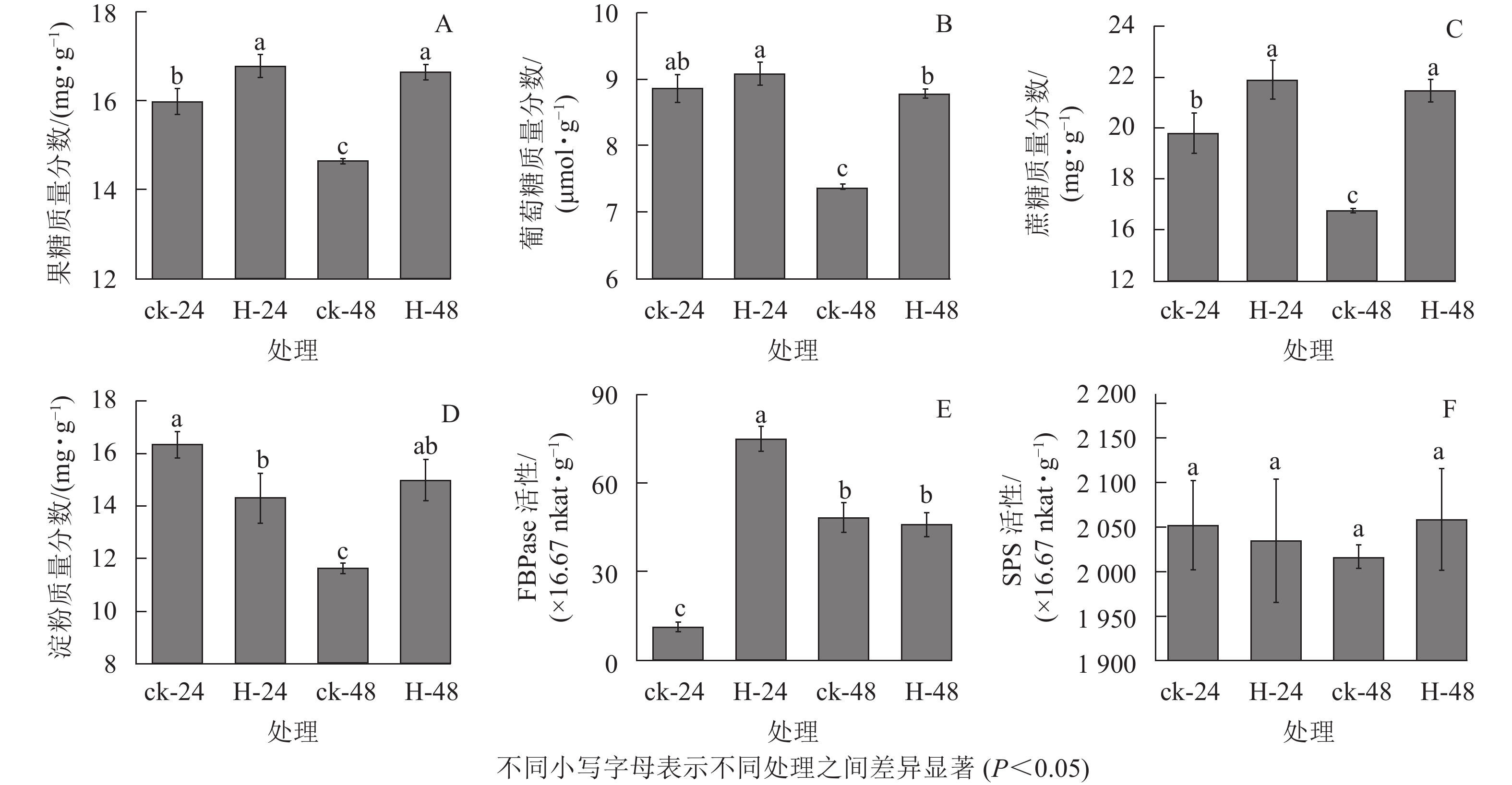
与对照相比,高温胁迫下24和48 h时景宁木兰的果糖、葡萄糖和蔗糖质量分数增加(图1A~C),除高温胁迫24 h的葡萄糖质量分数与对照无显著差异外(图1B),其他均呈显著差异(P<0.05)。与对照相比,高温胁迫24 h时的淀粉质量分数显著降低(P<0.05),而胁迫48 h时的淀粉质量分数显著升高(P<0.05)(图1D)。FBPase和SPS是蔗糖合成的关键酶,与对照相比,FBPase活性在胁迫24 h时先显著升高(P<0.05),在48 h时下降,但差异不显著(P>0.05)(图1E)。在高温胁迫下,SPS活性相比对照在24 h下降,48 h时有所上升,但并无显著差异(P>0.05)(图1F)。相关性分析结果表明(表1):蔗糖与果糖变化成极显著正相关(P<0.01),淀粉、SPS活性与FBPase活性成负相关。
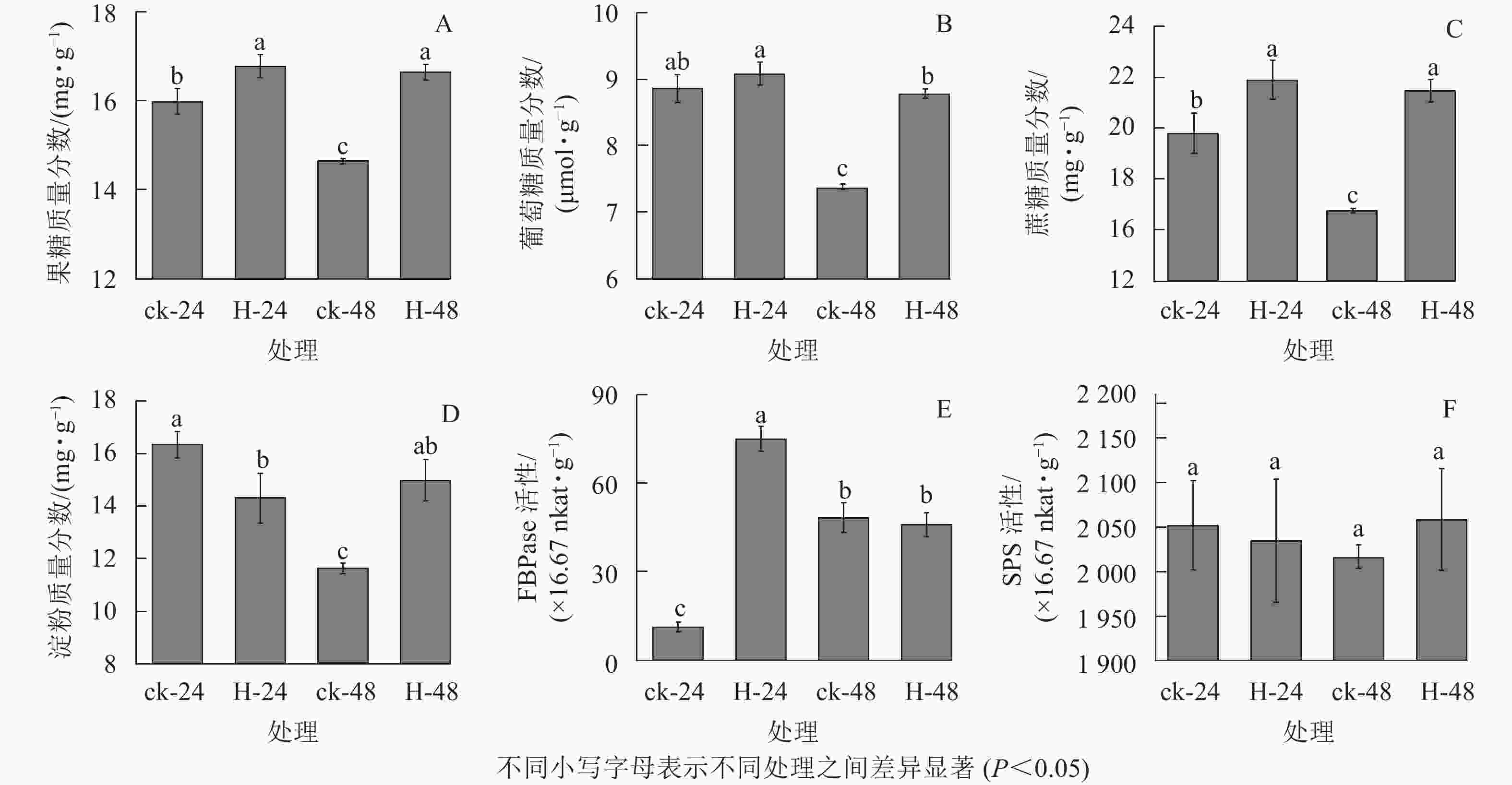
Figure 1. Effects of different heat stress treatments on the carbohydrates assimilation process of M. sinostellata
指标 果糖 葡萄糖 蔗糖 淀粉 FBPase活性 SPS活性 果糖 1 葡萄糖 0.945 1 蔗糖 0.999** 0.937 1 淀粉 0.697 0.853 0.670 1 FBPase活性 0.261 0.030 0.298 −0.493 1 SPS活性 0.707 0.738 0.680 0.891 −0.432 1 说明:**表示差异极显著(P<0.01) Table 1. Correlation coefficient between carbohydrate contents and enzyme activities of M. sinostellata under high temperature stress
-
转录组数据(表2)显示:测序碱基对总数为7 054万~ 7 313万条 。每个原始读取从一端测序,长度为50 bp。去除低质量序列后(即含有<1%的不确定碱基),各测序样本共获得70 547 818(ck-24)、72 966 866(ck-48)、73 134 592(H-24)和73 140 464(H-48) 条。每个库中clean reads的比例平均为99.53%。来自对照和高温样本的转录组都有至少7 000万个clean reads,这表明景宁木兰转录组数据质量较高,能够开展后续的生物信息学分析。数据筛选后得到的17 884个不同长度的差异unigenes[错误发生率(FDR)≤0.001, log2R≥1]。H-24与ck-24相比,4 662个基因的表达下调,6 611个基因的表达上调;H-48与ck-48相比,4 658个基因的表达下调,5 131个基因的表达上调;H-48与H-24相比,4 548个基因的表达下调,4 056个基因的表达上调(表3)。
处理 总读数 总碱基对 总比对数 高质量比对数 丢失对比数 单一比对数 非单一读数 总未比对读数 ck-24 70 547 818
(100)7 054 781 800
(100)52 601 172
(74.56)33 257 776
(47.14)19 343 396
(27.42)38 336 892
(54.34)14 264 280
(20.22)17 946 644
(25.44)ck-48 72 966 866
(100)7 296 686 600
(100)55 252 592
(75.72)35 172 380
(48.20)20 080 212
(27.52)39 734 140
(54.46)15 518 452
(21.27)17 714 272
(24.28)H-24 73 134 592
(100)73 134 592 009
(100)52 733 028
(72.10)33 645 473
(46.00)19 087 555
(26.10)38 257 910
(52.31)14 475 118
(19.79)20 401 562
(27.90)H-48 73 140 464
(100)7 314 046 400
(100)54 165 208
(74.06)34 404 377
(47.04)19 760 831
(27.02)40 219 158
(54.99)13 946 050
(19.07)18 975 254
(25.94)说明:括号内为参考基因序列的对比率(%) Table 2. Ratio of leaf sequencing results to reference gene sequences of M. sinotellata under heat stress and non-heat stress
总计 ck-24/H-24 ck-48/H-48 H-24/H-48 上调 6611 5131 4056 下调 4662 4958 4548 Table 3. Statistic of differentially expressed genes
-
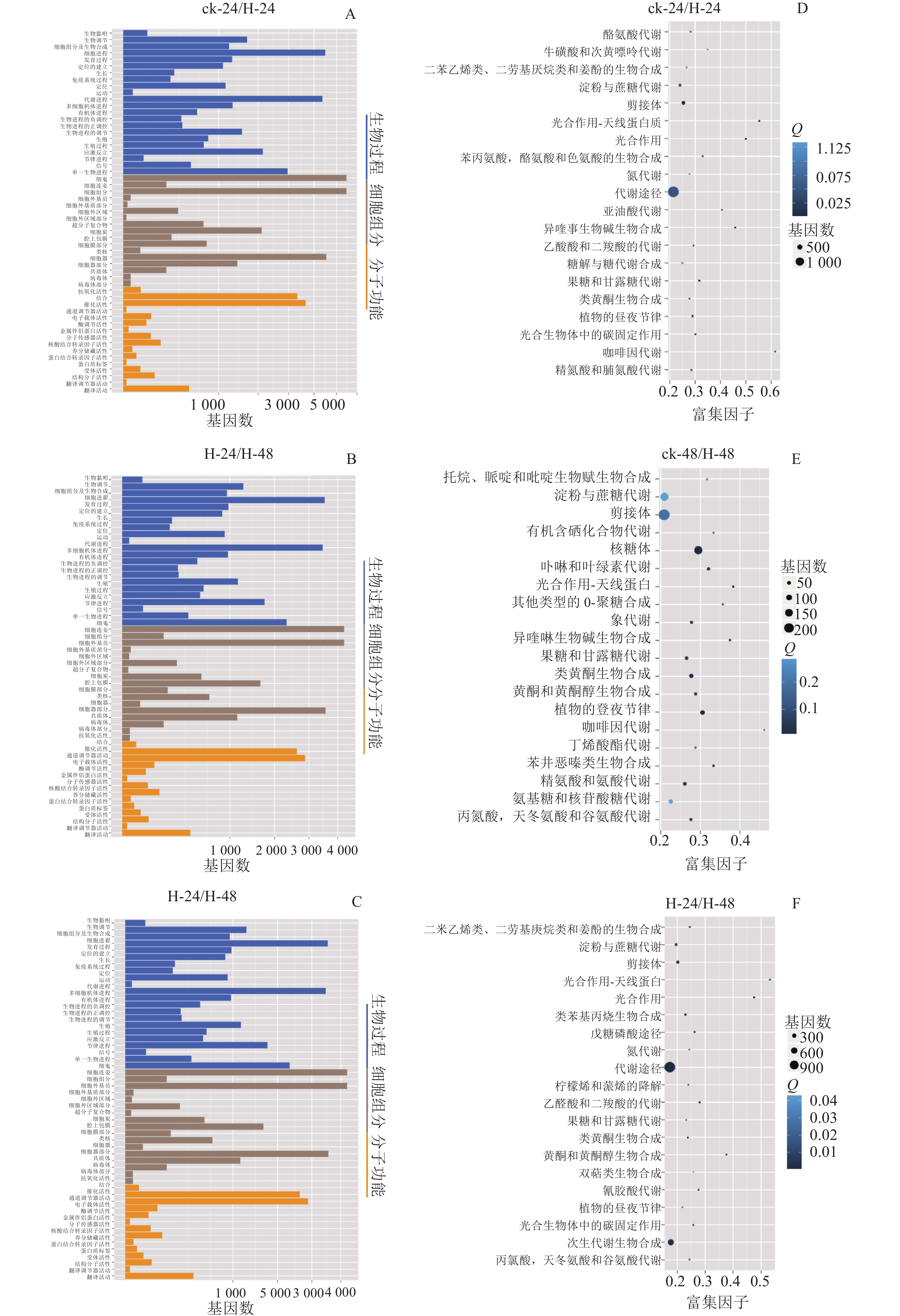
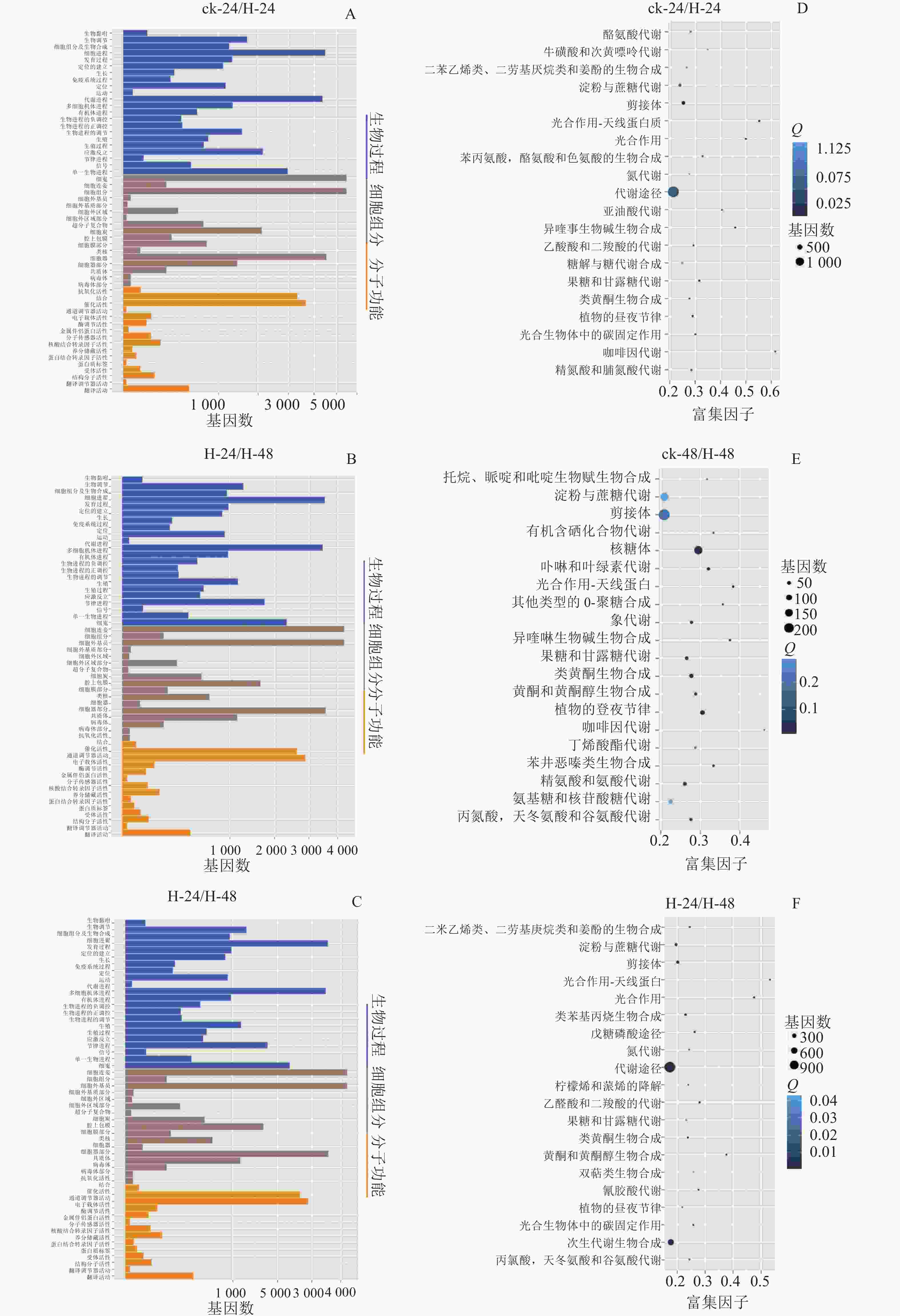
GO数据库定义了3类系统来描述基因产物的具体功能:生物过程(biological process)、细胞组分(cellar component)和分子功能(molecular function),这些基因又被具体分为56个小类,从而发挥生物功能。将组装的景宁木兰unigene与GO数据库比对分析,图2A~C显示:景宁木兰序列中有81 573条unigene可以进行功能分类。其中33.03%的基因显著富集于生物过程、34.31%的富集于细胞组分,32.66%的分子功能。生物学过程中的主要途径是细胞途径和代谢过程相关的转录调控,细胞组分中最多的是膜的构成,分子功能中最多的是结合和催化活性。
-
将组装的景宁木兰unigene与KEGG数据库比对及KEGG富集分析(图2D~E),结果发现:在景宁木兰高温胁迫的3个比较组中,ck-24与H-24、H-24与H-48代谢相关通路高度富集;ck-24与H-24、ck-48与H-48淀粉和蔗糖代谢通路富集排名第3位,H-24与H-48排名第4位。这些结果表明:淀粉和蔗糖代谢途径在景宁木兰高温胁迫响应机制中具有重要作用。
-
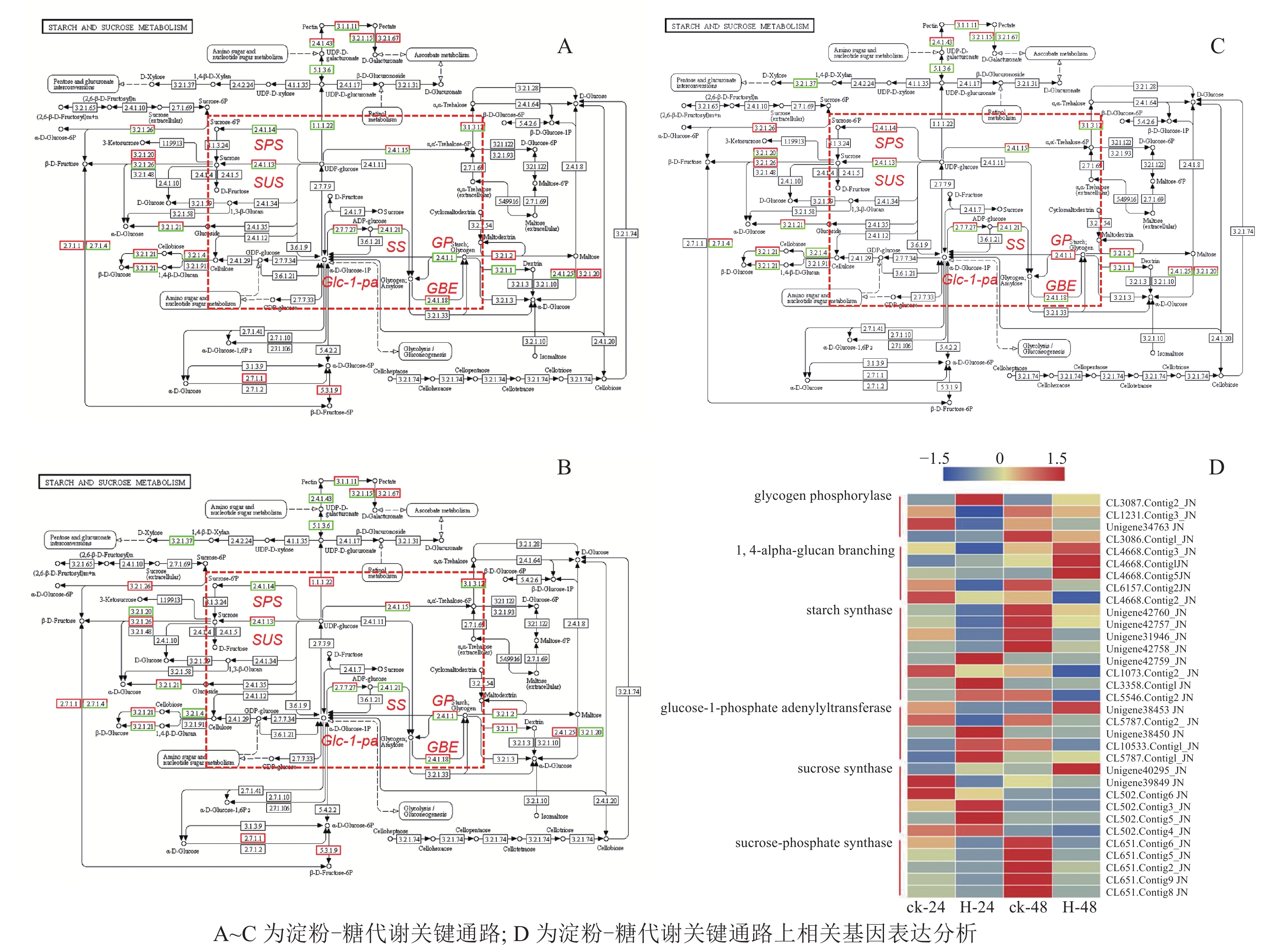
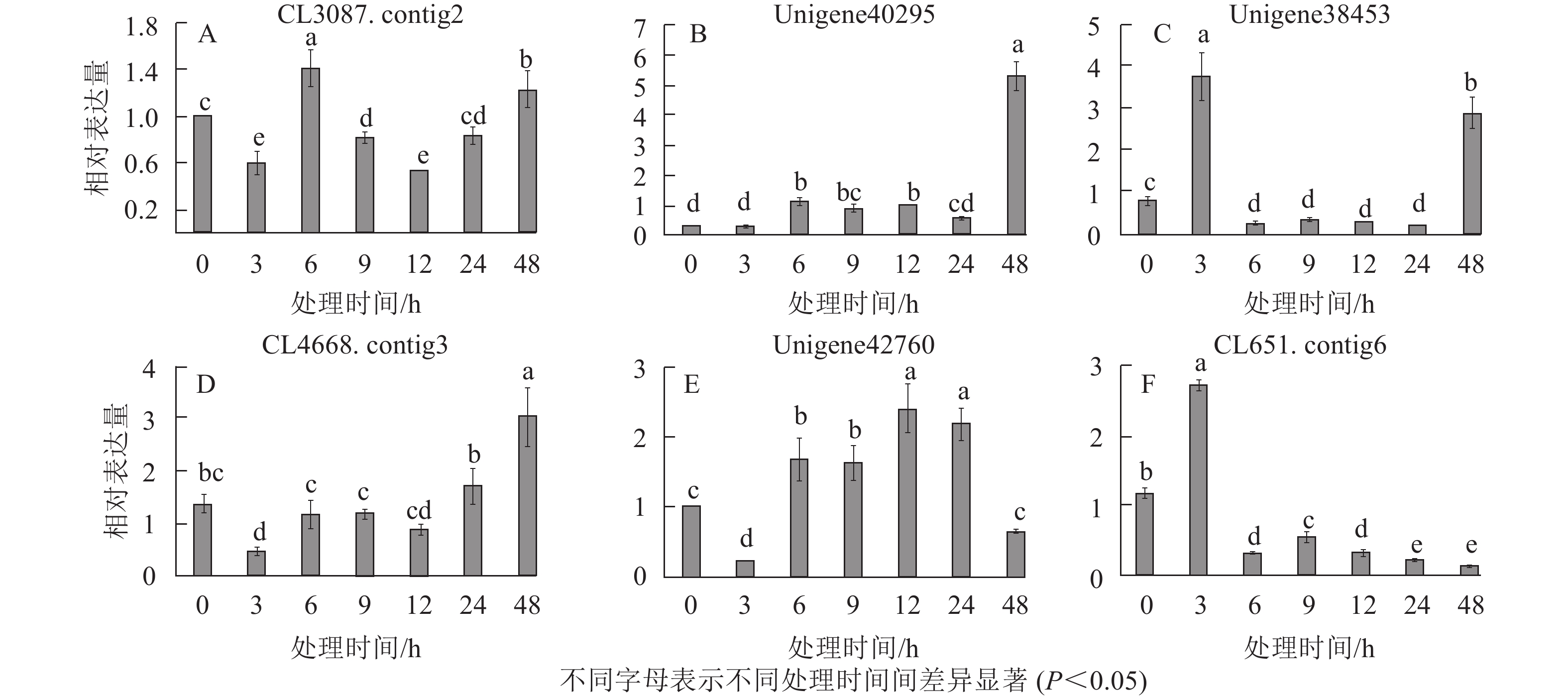
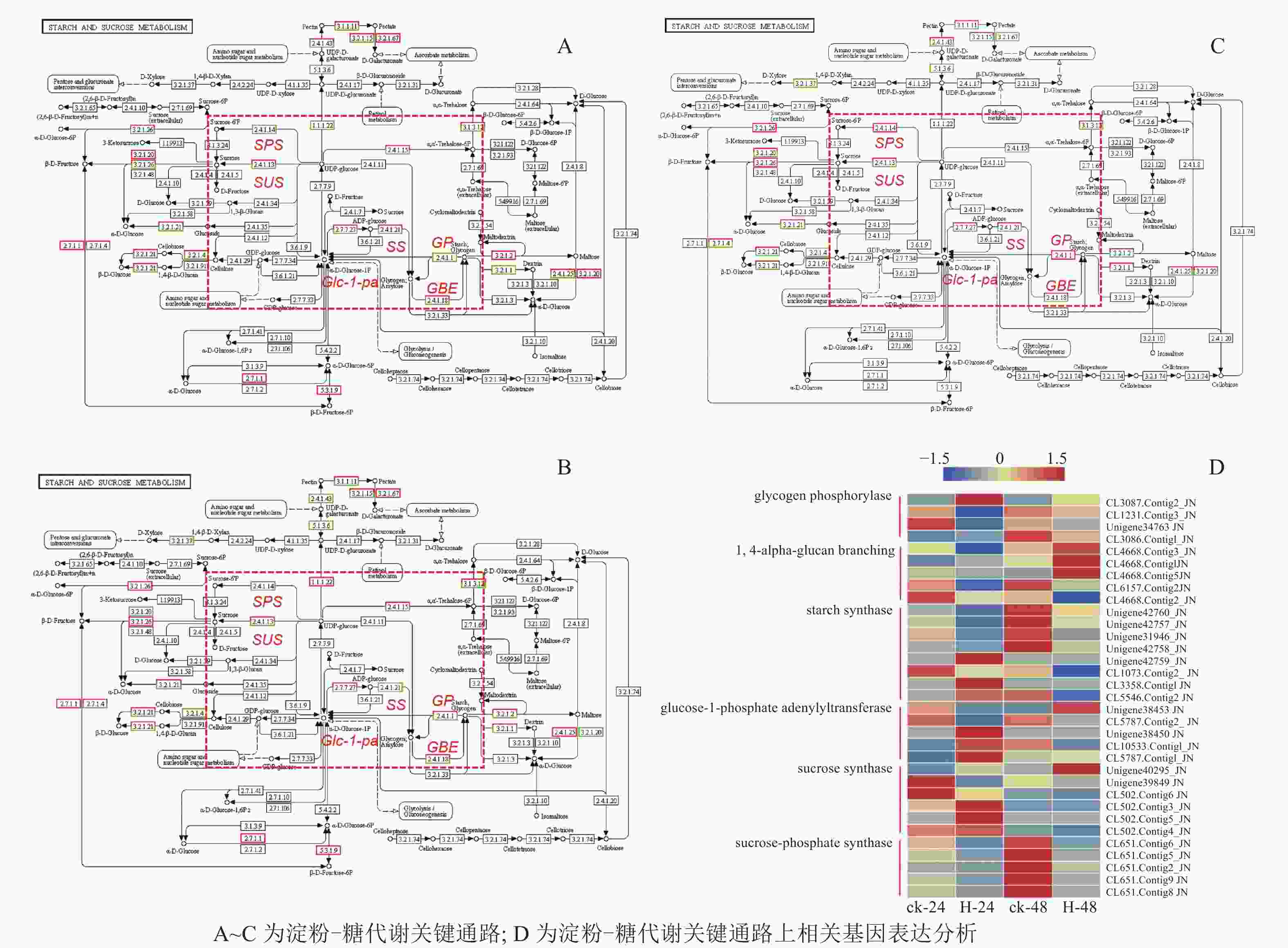
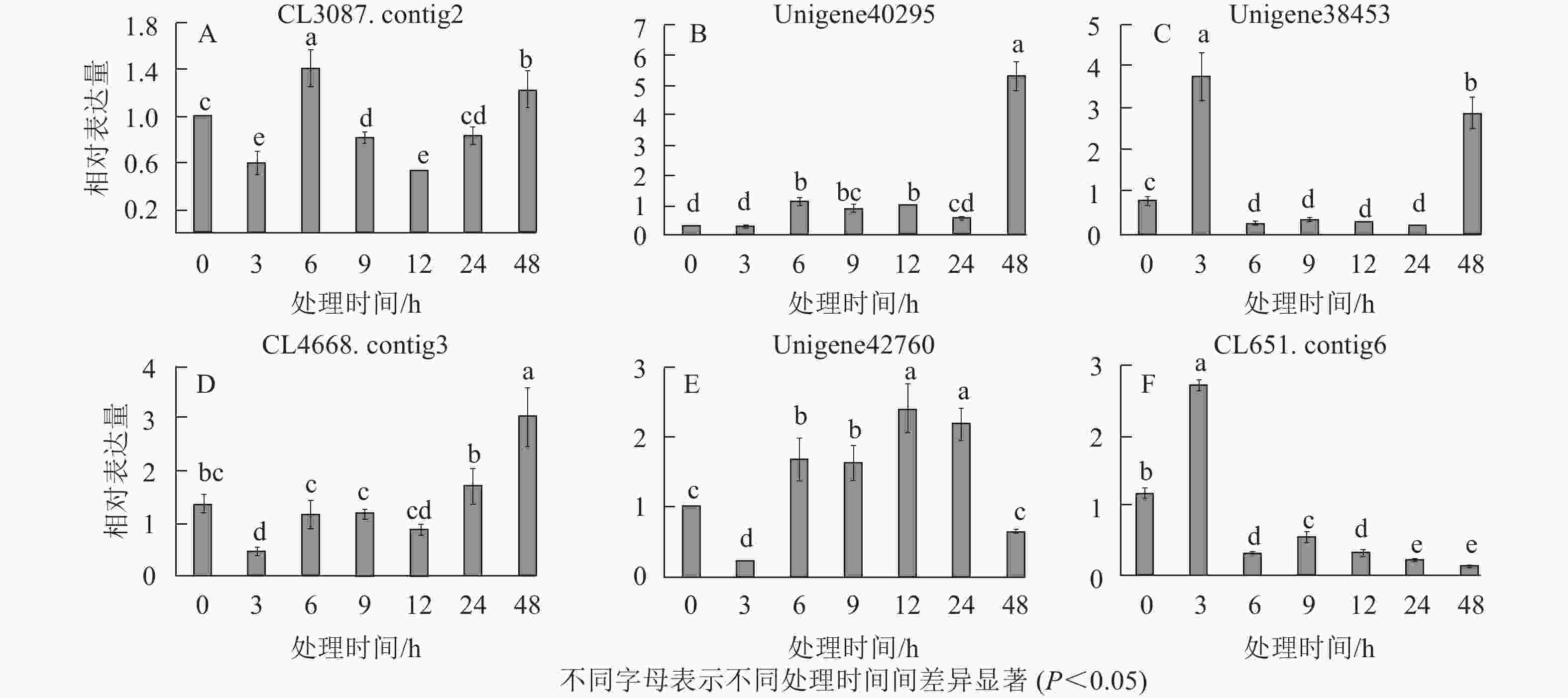
在淀粉代谢通路中共有105个差异基因。在该通路中,8个DEGs调控ADP-glucose以促进淀粉酶合成,5个DEGs调控淀粉酶以生产淀粉;淀粉也可以通过同样的途径调控ADP-glucose产生α-D-Glucose-1P,然后再形成UDP-glucose,UDP-glucose再在蔗糖合成酶基因和蔗糖6磷酸合成酶基因的作用下形成蔗糖;蔗糖代谢通路中有17个差异基因,其中有6个属于蔗糖合酶基因,5个属于蔗糖磷酸合成酶(图3A~D)。选取6个淀粉和蔗糖代谢途径高表达基因,即GP(CL3087.contig2)、SS(Unigene40295)、Glc-1-pa(Unigene38453)、GBE(CL4668.contig3)、SUS(Unigene42760)、SPS(CL651.contig6),通过qPCR定量分析,结果显示(图4):与对照相比,高温胁迫48 h处理下淀粉和糖代谢途径上的GP(CL3087.contig2)、SS(Unigene40295)、Glc-1-pa(Unigene38453)、GBE(CL4668.contig3)基因表达显著上调(P<0.05),SUS(Unigene42760)、SPS(CL651.contig6)基因表达显著下降(P<0.05)。由此说明,在高温胁迫下,淀粉合成酶基因及蔗糖6磷酸合成酶基因表达均呈现上调的趋势,以促进蔗糖产生。
-
极端高温天气势必影响植物的生长发育,最终导致部分植物处于濒危状态。本研究转录组数据显示:极端高温处理景宁木兰之后,其大部分基因可注释到生物进程,代谢功能途径中基因分布最多,这与非洲菊Gerbera jamesonii[16]、牡丹Paeonia suffruticosa[1]等相似。景宁木兰的KEGG数据库显示:在高温处理后,大部分基因高度富集于代谢通路,同时淀粉与糖代谢通路中占据重要的比例。由此推断,淀粉和蔗糖代谢对景宁木兰响应高温胁迫机制具有重要的作用。
植物叶片光合作用同化吸收的碳用于叶绿体淀粉的形成,或者运输到细胞质中合成蔗糖,蔗糖随后被运输到植物的非光合位置。不同植物叶片淀粉和蔗糖积累水平差异很大,环境因子(如温度、光照等)也影响着植物叶片所固定碳在蔗糖和淀粉之间的分配[17]。本研究中,随着高温胁迫的加深,24和48 h处理下景宁木兰叶片蔗糖和淀粉质量分数差异不显著,但是均显著高于对照。本研究中FBPase与淀粉显著正相关,与蔗糖负相关。这与马铃薯Solanum tuberosum[18]、拟南芥Arabidopsis thaliana[19]的研究结果一致。FBPase在24 h达到最高值之后,说明景宁木兰对高温具有一定的适应性,然而48 h时与对照相比差异不显著,说明高温对景宁木兰FBPase影响不大。
植物的生长发育所需要的光合产物大部分以蔗糖的形式供应和运输,其中,SPS是蔗糖合成途径的一个重要控制点,同时也是蔗糖进入各种代谢途径所必需的关键酶之一,它的活性可反映蔗糖生物合成途径的能力[20-21]。一些研究指出SPS与淀粉积累呈现负相关,而与蔗糖形成呈正相关[5-6]。本研究结果与之相似。在温州蜜柑Citurs unshiu ‘Miyagawawase’[22]、灌浆期水稻Oryza sativa ‘Koshihikari’和O. sativa ‘Sasanishiki’[23]的研究中也发现了同样的问题,可能是因为光合细胞中同化的碳水化合物在蔗糖和淀粉之间的分配受SPS的调节,但SPS不起主要作用,蔗糖和淀粉的合成还受其他因子的影响[24-25]。
淀粉和蔗糖代谢途径和多种酶密切相关,植物一般利用糖苷键将蔗糖[β-D-frucose-(2→1)-α-D-glucose]和海藻糖[α-D-glucose-(2→1)-α-D-glucose]中的己糖残基连接在一起,蔗糖和海藻糖的形成掩盖了葡萄糖和果糖的活性基团,两者反应活性低于葡萄糖[16]。本研究果糖、葡萄糖质量分数随着胁迫的加深有所下降,从而促进了淀粉质量分数的增加,虽然差异不显著,但是调节淀粉合成的SS(Unigene40295)、Glc-1-pa(Unigene38453)、GBE(CL4668.contig3)基因的表达量增加,进一步证明了高温可能促进胞质蔗糖质量分数的变化,通过ADP-glucose催化促进淀粉的合成[16]。虽然ADP-glucose是否是催化淀粉合成的关键酶还存在争议[16],但是本研究定量结果显示:SPS(CL651.contig6)基因随着高温胁迫的加深,呈现下降趋势,这进一步说明了淀粉积累与蔗糖积累之间存在相关性,但是具体机制还需要深入研究。
综上所述,景宁木兰在高温胁迫下,积累糖和淀粉以提升抵抗胁迫能力,且淀粉与蔗糖途径的关键基因表达也反映了景宁木兰响应高温胁迫的机制。具体基因的功能验证还需进一步研究。
Transcriptional analysis of starch and sucrose metabolism pathways in Magnolia sinostellata under heat stress
doi: 10.11833/j.issn.2095-0756.20220170
- Received Date: 2022-02-20
- Accepted Date: 2022-10-09
- Rev Recd Date: 2022-08-29
- Available Online: 2023-01-18
- Publish Date: 2023-01-17
-
Key words:
- heat stress /
- carbon assimilation products /
- metabolic pathway /
- key genes /
- Magnolia sinostellata
Abstract:
| Citation: | ZHENG Chenlu, WANG Qianying, LU Danying, et al. Transcriptional analysis of starch and sucrose metabolism pathways in Magnolia sinostellata under heat stress[J]. Journal of Zhejiang A&F University, 2023, 40(1): 55-63. DOI: 10.11833/j.issn.2095-0756.20220170 |










 DownLoad:
DownLoad: