-
在全球气候变暖的大背景下,由温室效应不断加剧而导致的高温天气日趋严重,已成为全世界广为关注的环境问题之一。高温不仅会危害人体健康,也会对农林业生产造成严重影响[1-2]。在高温胁迫下,植物种子萌发受抑[3]、光合能力降低[4-5]、活性氧(reactive oxygen species, ROS)大量产生并造成次生氧化胁迫[6]。这不仅会影响植物生长发育,甚至会引发细胞程序性死亡[6-7]。光合作用是植物最主要的物质与能量代谢过程,也是对环境因子变化最为敏感的生理过程[8-9]。高温胁迫可破坏光系统Ⅱ(PSⅡ)结构,抑制电子供体侧水的光解,并降低气孔导度、反应中心光化学效率、二氧化碳同化能力和水分利用效率等[4, 10-12]。在这些影响中,往往几种共同作用以降低植物的光合性能。樟树Cinnamomum camphora主要生长在热带和亚热带地区,是优良的园林绿化树种,被广泛作为庭荫树、行道树、防护林以及风景林使用,具有巨大的经济与生态价值。目前,对樟树研究主要集中于组织培养和无性繁殖等育苗技术[13-14],以及低温[15]、干旱[16-17]和盐碱胁迫[18-19]等对生理特性的影响以评估其向北方和盐碱滩涂引种的适应性。随着高温天气的加剧,其生长过程中不可避免的会经历高温胁迫,以其主要生长区域之一浙江省为例,2013-2017年间夏季高温可达40 ℃,甚至某些区域会高达42 ℃[20]。然而高温对樟树生长与光合作用具有何种影响,尚未见相关研究报道。因此,本研究从叶绿素荧光诱导动力学、气体交换速率和水分利用效率等方面研究高温胁迫对樟树光合性能的影响,以期揭示高温环境对樟树的危害。
-
在3月选取生长一致的3年生樟树幼苗(高度约90 cm)移栽至花盆(高30 cm,直径30 cm)中,并置于室外缓苗备用。在5月选取生长状况一致的幼苗进行高温胁迫处理。将樟树幼苗放入光照培养箱中(光16 h/暗8 h),分别进行25,35和45 ℃(光照时的温度)温度处理,黑暗时温度分别为20,25和35 ℃。35和45 ℃高温处理前分别经25和35 ℃适应2 d后进行,温度升高时升速为3 ℃·h-1。以25 ℃处理作为对照。每个温度处理幼苗4株,视为重复1次·株-1。高温胁迫2 d后,测定幼苗的光合性能。
-
对樟树叶片暗适应15 min后,采用Yaxin-1161叶绿素快速荧光仪测定其叶绿素荧光诱导动力学曲线[21],并根据CHRISTEN等[22]的方法计算叶绿素荧光动力学参数:单位反应中心吸收的光能总量(ABS/RC),单位反应中心捕获的光能总量(TRO/RC),单位反应中心内电子传递的量子产额(ETO/RC),单位反应中心热耗散的能量(DIO/RC),光合性能指数(PIABS)和单位吸收面积上天线色素能量吸收的驱动力(DFABS)。
-
樟树叶片的气体交换速率采用LI-6400便携式光合仪进行测量,光强设定为2 000,1 500,1 200,1 000,800,600,400,300,200,100,50,20,10和0 μmol·m-2·s-1,二氧化碳摩尔分数为400 μmol·mol-1,叶室温度为30 ℃。对光合速率(Pn),气孔导度(Gs),胞间二氧化碳摩尔分数(Ci)和蒸腾速率(Tr)的光响应变化进行分析,并参考YE等[23]直角双曲线修正模型计算光补偿点、光饱和点和最大光合速率。
-
水分利用效率是指植物或叶片每蒸腾一定量的水分所同化的二氧化碳的量,即Pn与Tr的比值,以mmol·mol-1(二氧化碳/水)表示。在25和35 ℃时,樟树处于最大光合速率时的光强接近于1 200 μmol·m-2·s-1,45 ℃时接近1 000 μmol·m-2·s-1,分别采用此时的光合速率和蒸腾速率计算相应的水分利用效率。
-
实验数据采用SPSS 13.0进行分析,Origin 8.0进行作图。
-
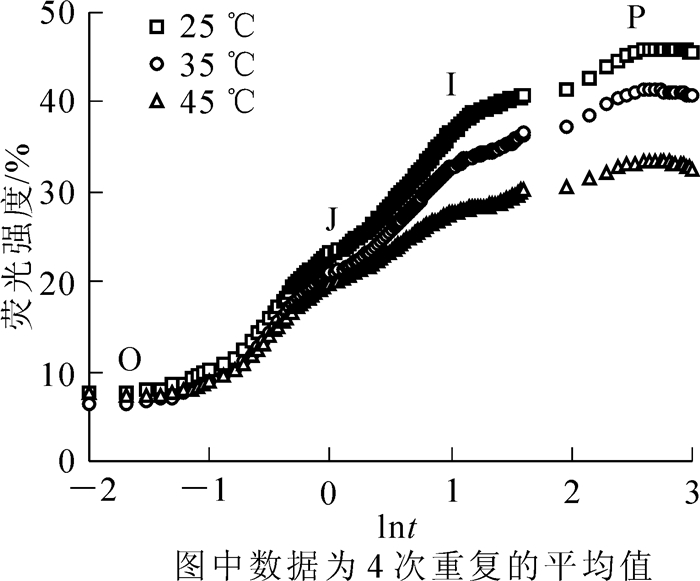
在35和45 ℃高温胁迫下,樟树叶绿素荧光诱导动力学曲线O到P点的荧光强度均明显降低,其中在45 ℃高温胁迫后降至最低(图 1)。

图 1 高温对樟树叶绿素荧光诱导动力学曲线的影响
Figure 1. Effects of high temperature on chlorophyll fluorescence transients in Cinnamomum camphora
樟树经35和45 ℃高温胁迫处理后,其ABS/RC分别降低了4.9%和21.7%,其中在45 ℃高温胁迫时差异达到极显著(P<0.01)水平。与ABS/RC相似,35和45 ℃高温胁迫均明显降低TRO/RC,ETO/RC,PIABS和DFABS,并且在45 ℃高温胁迫时均达到极显著(P<0.01)水平。对于DIO/RC而言,35和45 ℃高温胁迫均使其明显增加,与对照相比分别增加了13.5%(P<0.05)和78.4%(P<0.01)(表 1)。
表 1 高温胁迫对樟树叶绿素荧光诱导动力学参数的影响
Table 1. Effects of high temperature stress on chlorophyll fluorescence transient parameters in Cinnamomum camphora
参数名称 不同处理温度下的参数值 25 (对照) 35 45℃ ABS/RC 2.63 ± 0.26 2.50 ± 0.16 2.06 ± 0.08** TRO/RC 2.10 ± 0.16 1.88 ± 0.10* 1.73 ± 0.06** ETO/RC 1.21 ± 0.09 1.12 ± 0.08* 0.74 ± 0.10** DIO/rc 0.37 ± 0.08 0.42 ± 0.04* 0.66 ± 0.09** PIARS 3.49 ± 0.34 3.39 ± 0.54 1.39 ± 0.47** DFARS 0.52 ± 0.04 0.53 ± 0.07 0.38 ± 0.10** 说明: *表示与对照相比,差异在P < 0.05水平上显著;**表示与对照相比,差异在P < 0.01水平上极显著。表中数据为4次重复的平均值±标准差 -
随着光照强度增加,樟树光合速率呈现先增加后趋于稳定的明显的光响应曲线特征。在35和45 ℃高温胁迫后,樟树的光响应曲线明显降低,其中在45 ℃时降至最低,这表明高温明显抑制了樟树光合速率(图 2A),其中最大光合速率分别降低了42.3%(P<0.05)和67.6%(P<0.05)。同时,光补偿点分别增加了20.3%(P<0.01)和1.6倍(P<0.01),而光饱和点则分别降低了6.7%和11.9%(P<0.05)(表 2)。

图 2 高温胁迫对樟树光合作用的影响
Figure 2. Effects of high temperature stress on photosynthesis in Cinnamomum camphora
表 2 高温胁迫对樟树光响应参数的影响
Table 2. Effects of high temperature stress on light response parameters in Cinnamomum camphora
参数名称 不同处理温度下的参数值/(μmol·m-2·s-1) 25 (对照) 35 45℃ 光补偿点 17.72 ± 2.65 21.33 ± 3.08** 46.09 ± 4.85** 光饱和点 1 211.02 ± 68.44 1 129.72 ± 54.81 1 066.6 7 ± 69.15* 最大光合速率 14.18 ± 3.92 8.21 ± 2.24* 4.59 ± 0.63** 说明:*表示与对照相比,差异在P<0.05水平上显著;**表示与对照相比,差异在P<0.01水平上极显著。表中数据为4次重复的平均值±标准差 气孔导度对光强的响应与光合速率相似,亦随光强增加而呈现先增加后趋于稳定的变化趋势,同时随高温胁迫增强而逐渐降低(图 2B)。对于胞间二氧化碳摩尔分数而言,其随光强增加而逐渐降低。在高温胁迫后,胞间二氧化碳摩尔分数升高,其中在光强≥1 000 μmol·m-2·s-1后45 ℃时最高(图 2C)。
-
随着光照强度增加,樟树在25,35和45 ℃的蒸腾速率均呈现逐渐增加趋势。与25 ℃相比,樟树在35和45 ℃高温胁迫后,其蒸腾速率明显降低,其中在45 ℃高温胁迫时降至最低(图 3A)。采用樟树处于最大光合速率时的光合速率和蒸腾速率计算水分利用效率,其在25 ℃时为5.0 μmol·mmol-1,在35和45 ℃高温胁迫后分别降低了16.0%和44.6%,并且差异均达到显著(P<0.05)和极显著(P<0.01)水平(图 3B)。
-
叶绿素荧光是研究植物光合作用有效的非损伤探针,其诱导动力学曲线可反映PSⅡ反应中心原初光化学反应。在O—P点的荧光动态变化过程中,O点表示PSⅡ反应中心可最大限度的接受光量子;O—J表示PSⅡ反应中心受光激发后将电子传给PSⅡ原初醌受体(QA)并还原为QA-;J—I—P表示质体醌(PQ)库的还原状态[24]。在高温胁迫下,樟树O—P点的荧光强度明显降低,这与高温胁迫对苹果Malus domestica[25]和黄瓜Cucumis sativus[26]叶片叶绿素荧光诱导动力学曲线的影响相一致。在此过程中,樟树O—J点荧光强度降低,表明其QA-积累导致了PSⅡ受体侧电子传递受阻[21, 27];J—P降低则表明PSⅡ供体侧电子传递受阻,并引发P680+积累[28-29]。
在光反应过程中,光能的吸收、分配与利用可通过叶绿素荧光诱导动力学参数进行反应[30]。在高温胁迫下,辣椒Capsicum annum叶片反应中心单位面积上吸收、捕获和传递的光能均明显降低[31]。在本研究中,樟树亦有相似结果,ABS/RC,TRO/RC和ETO/RC在高温胁迫下均明显低于对照(表 1)。在高温胁迫时,樟树DIO/RC增加,这表明其吸收的光能通过叶黄素循环进行热耗散增加[33]。PIABS由光能吸收、激发能捕获和激发能到电能的传导3方面构成[32],在高温胁迫下樟树PIABS降低表明光反应过程受到明显抑制。
在高温胁迫后,黄瓜[34]、芍药Paeonia lactiflora[35]和油菜Brassica napus[36]的光合速率、气孔导度和蒸腾速率以及芍药水分利用效率均明显降低,同时黄瓜[34]和西洋杜鹃Rhododendron hybridum[37]胞间二氧化碳摩尔分数明显升高,本研究结果与之相似。樟树在高温胁迫后,气孔导度降低限制了水分蒸发从而导致蒸腾速率降低,同时光合速率降低量明显高于蒸腾速率降低量,进而导致水分利用效率明显降低。植物光合速率降低的原因可分为气孔限制与非气孔限制[38],如果胁迫降低气孔导度而叶肉细胞仍可活跃的进行光合作用,其胞间二氧化碳摩尔分数显著降低,这就是典型的气孔限制;反之则为非气孔限制。在高温胁迫下,虽然樟树气孔导度明显降低,但是胞间二氧化碳摩尔分数却明显升高,这表明高温导致樟树光合速率降低不是由气孔限制原因造成的。
由此可见,高温胁迫可通过降低樟树PSⅡ的光能吸收、量子产量和电子传递,并促进吸收的光能进行热耗散,从而降低PSⅡ效率,进而导致同化力(三磷酸腺苷ATP和还原型烟酰胺腺嘌呤二核苷酸磷酸NADPH)减少以影响二氧化碳同化并降低光合速率。
Photosynthetic abilities in Cinnamomum camphora with high temperature stress
-
摘要: 光合作用是植物对环境变化最为敏感的生理过程。通过研究高温胁迫对樟树Cinnamomum camphora叶绿素荧光诱导动力学、气体交换速率和水分利用效率的影响,以期从光合作用的角度揭示高温对樟树的危害机制。结果表明:35和45℃高温胁迫后,樟树叶绿素荧光诱导动力学曲线O到P点的荧光强度均随胁迫增强而明显降低,同时诱导动力学参数单位反应中心吸收的光能总量、单位反应中心捕获的光能总量、单位反应中心内电子传递的量子产额、光合性能指数和单位吸收面积上天线色素能量吸收的驱动力均明显降低,其中在45℃时降至最低,与对照(25℃)相比分别降低了21.7%(P < 0.01),17.6%(P < 0.01),38.8%(P < 0.01),60.2%(P < 0.01)和26.9%(P < 0.01)。35和45℃高温胁迫后,单位反应中心热耗散的能量明显升高,与对照相比分别增加了13.5%(P < 0.05)和78.4%(P < 0.01);此外,樟树光合速率、气孔导度、蒸腾速率和水分利用效率亦均明显降低,其中最大光合速率分别降低了16.0%(P < 0.05)和44.6%(P < 0.01)。由此可见,高温胁迫可通过降低樟树的光能吸收、量子产量和电子传递,并促进吸收光能进行热耗散,降低光系统Ⅱ效率,进而减少同化力以降低光合速率。Abstract: Photosynthesis is the most sensitive physiological process for environmental variation. To uncover any detriment of high temperature from photosynthesis on Cinnamomum camphora, the effects of high temperature stress on chlorophyll fluorescence transients, gas exchange rate, and water use efficiency in the plants treated with high temperature at 25℃ (the control), 35℃ and 45℃, respectively, were studied, and one-way analyses of variance was performed to analyze the effects of high temperature. Results showed that fluorescence intensity from O to P point in chlorophyll fluorescence transients in C. camphora declined as temperature increased. Fluorescence transient parameters significantly decreased by 21.7% (P < 0.01) in absorption flux per reaction center, 17.6% (P < 0.01) in trapped energy flux per reaction center, 38.8% (P < 0.01) in electron transport flux per reaction center, 60.2% (P < 0.01) in performance index based on absorption, and 26.9% (P < 0.01) in driving force based on absorption at 45℃. Compared to the control, dissipated energy flux per reaction center significantly increased by 13.5% (P < 0.05) and 78.4% (P < 0.01), respectively, under high temperature at 35 and 45℃. In addition, the photosynthetic rate, stomatal conductance, transpiration rate, and water use efficiency declined at 35 and 45℃. The maximum photosynthetic rate significantly reduced by 16.0% (P < 0.05) and 44.6% (P < 0.01), respectively, at 35 and 45℃. Therefore, high temperature stress reduced C. camphora photosystem Ⅱ efficiency by reducing light energy absorption, quantum yield, and electron transportation as well as by increasing the absorbed light energy dissipated as heat, which resulted in an assimilatory power reduction and photosynthetic rate decline.
-
表 1 高温胁迫对樟树叶绿素荧光诱导动力学参数的影响
Table 1. Effects of high temperature stress on chlorophyll fluorescence transient parameters in Cinnamomum camphora
参数名称 不同处理温度下的参数值 25 (对照) 35 45℃ ABS/RC 2.63 ± 0.26 2.50 ± 0.16 2.06 ± 0.08** TRO/RC 2.10 ± 0.16 1.88 ± 0.10* 1.73 ± 0.06** ETO/RC 1.21 ± 0.09 1.12 ± 0.08* 0.74 ± 0.10** DIO/rc 0.37 ± 0.08 0.42 ± 0.04* 0.66 ± 0.09** PIARS 3.49 ± 0.34 3.39 ± 0.54 1.39 ± 0.47** DFARS 0.52 ± 0.04 0.53 ± 0.07 0.38 ± 0.10** 说明: *表示与对照相比,差异在P < 0.05水平上显著;**表示与对照相比,差异在P < 0.01水平上极显著。表中数据为4次重复的平均值±标准差 表 2 高温胁迫对樟树光响应参数的影响
Table 2. Effects of high temperature stress on light response parameters in Cinnamomum camphora
参数名称 不同处理温度下的参数值/(μmol·m-2·s-1) 25 (对照) 35 45℃ 光补偿点 17.72 ± 2.65 21.33 ± 3.08** 46.09 ± 4.85** 光饱和点 1 211.02 ± 68.44 1 129.72 ± 54.81 1 066.6 7 ± 69.15* 最大光合速率 14.18 ± 3.92 8.21 ± 2.24* 4.59 ± 0.63** 说明:*表示与对照相比,差异在P<0.05水平上显著;**表示与对照相比,差异在P<0.01水平上极显著。表中数据为4次重复的平均值±标准差 -
[1] LOBELL D B, SCHLENKER W, COSTA-ROBERTS J. Climate trends and global crop production since 1980[J]. Science, 2011, 333(6042):616-620. [2] GIRARDIN M P, HOGG E H, BERNIER P Y, et al. Negative impacts of high temperatures on growth of black spruce forests intensify with the anticipated climate warming[J]. Global Change Biol, 2016, 22(2):627-643. [3] FERNÁNDEZ I C D, LUQUE E G, MERCADO F G, et al. Influence of temperature and salinity on the germination of Limonium tabernense Erben from Tabernas Desert (Almería, SE Spain)[J]. Flora, 2016, 218(1):68-74. [4] MATHUR S, AGRAWAL D, JAJOO A. Photosynthesis:response to high temperature stress[J]. J Photochem Photobiol B Biol, 2014, 137(8):116-126. [5] ZUO Zhaojiang, WANG Bin, YING Binbin, et al. Monoterpene emissions contribute to thermotolerance in Cinnamomum camphora[J]. Trees, 2017, 31(6):1759-1771. [6] MITTLER R, FINKA A, GOLOUBINOFF P. How do plants feel the heat[J]. Trends Biochem Sci, 2012, 37(3):118-125. [7] PETROV V, HILLE J, MUELLER-ROEBER B, et al. ROS-mediated a biotic stress-induced programmed cell death in plants[J]. Front Plant Sci, 2015, 6(1):1-16. [8] LAWLOR D W. Preface:musings about the effects of environment on photosynthesis[J]. Ann Bot, 2009, 103(4):543-549. [9] KUMAR T A, CHARAN T B. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat[J]. Plant Physiol, 1998, 117(3):851-858. [10] ASHRAF M, HARRIS P J C. Photosynthesis under stressful environments:an overview[J]. Photosynthetica, 2013, 51(2):163-190. [11] ENAMI I, KITAMURA M, TOMO T, et al. Is the primary cause of thermal in activation of oxygen evolution in spinach PSⅡmembranes release of the extrinsic 33 kDa protein or of Mn?[J]. Bioch Biophys Acta Bioenerg, 1994, 1186(1/2):52-58. [12] CRAFS-BRANDNER S J, LAW R D. Effects of heat stress on the inhibition and recovery of ribulose-1, 5-bisphosphate carboxylase/oxygenase activation state[J]. Planta, 2000, 212(1):67-73. [13] 李富福, 吴幼媚, 易平, 等.香樟无性系扦插林和实生人工幼林生长性状比较研究[J].西部林业科学, 2010, 39(4):15-19. LI Fufu, WU Youmei, YI Ping, et al. Comparative study on growth performance of Cinnamomum camphora plantations of clone cuttings and seedlings[J]. J West China For Sci, 2010, 39(4):15-19. [14] DU Li, LI Yongpeng, YAO Yao, et al. An efficient protocol for plantlet regeneration via direct organogenesis by using nodal segments from embryo-cultured seedlings of Cinnamomum camphora L.[J]. PLoS One, 2015, 10(5):e0127215. doi:10.1371/journal.Pone. 0127215. [15] 张旻桓, 张汉卿, 刘二冬.樟树北移耐寒性与形态特征的相关性研究[J].北方园艺, 2011(13):94-97. ZHANG Minheng, ZHANG Hanqing, LIU Erdong. Camphortree and the morphological characteristics of cold north correlation[J]. North Hortic, 2011(13):94-97. [16] 胡义, 胡庭兴, 胡红玲, 等.干旱胁迫对香樟幼树生长及光合特性的影响[J].应用与环境生物学报, 2014, 20(4):675-682. HU Yi, HU Tingxing, HU Hongling, et al. Effects of drought stress on growth and photosynthetic characteristics of Cinnamomum camphora saplings[J]. Chin J Appl Environ Biol, 2014, 20(4):675-682. [17] 韩博, 李志勇, 郭浩, 等.干旱胁迫下5种幼苗光合特性的研究[J].林业科学研究, 2014, 27(1):92-98. HAN Bo, LI Zhiyong, GUO Hao, et al. Studies on seedling photosynthetic characteristics of five tree species under drought stress[J]. For Res, 2014, 27(1):92-98. [18] 梅海军, 王宁, 李自阳, 等. NaCl和Na2SO4胁迫对香樟幼苗生理特性的影响[J].西北林学院学报, 2011, 26(6):30-34. MEI Haijun, WANG Ning, LI Ziyang, et al. Effects of NaCl and Na2SO4 saline stress on ecophysiological characteristics of Cinnamomum camphora seedlings[J]. J Northwest For Univ, 2011, 26(6):30-34. [19] 张丽华, 王晓立, 王梦秋, 等.苏打盐碱胁迫对香樟幼苗光合特性的影响[J].北方园艺, 2012(23):91-93. ZHANG Lihua, WANG Xiaoli, WANG Mengqiu, et al. Effect on camphor seedling photosynthetic characteristic of soda saline-alkali stress[J]. North Hortic, 2012(23):91-93. [20] 张小泉, 王文, 傅帅. 2013年浙江省夏季异常高温天气特征及其成因分析[J].气象与环境学报, 2017, 33(1):80-86. ZHANG Xiaoquan, WANG Wen, FU Shuai. Characteristics and causes of an extremely high-temperature event in the summer of 2013 in Zhejiang Province[J]. J Meteorol Environ, 2017, 33(1):80-86. [21] STRASSERF R J, SRIVASTAVA A, GOVINDJEE. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria[J]. Photochem Photobiol, 1995, 61(1):32-42. [22] CHRISTEN D, SCHÖNMANNA S, JERMINI M, et al. Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress[J]. Environ Exp Bot, 2007, 60(3):504-514. [23] YE Z P, YU Q, KANG H J. Evaluation of photosynthetic electron flow using simultaneous measurements of gas exchange and chlorophyll fluorescence under photorespiratory conditions[J]. Photosynthetica, 2012, 50(3):472-476. [24] STRASSER R J, GOVINDJEE. On the O-J-I-P fluorescence transients in leaves and D1 mutants of Chlamydomonas reinhardtii[G]//MURATA N. Research in Photosynthesis. Dordrecht: Kluwer Academic Publishers, 1992: 29-32. [25] CHEN L S, CHENG L. Photosystem 2 is more tolerant to high temperature in apple (Malus domestica Borkh.) leaves than in fruit peel[J]. Photosynthetica, 2009, 47(1):112-120. [26] 金立桥, 车兴凯, 张子山, 等.高温、强光下黄瓜叶片PSⅡ供体侧和受体侧的伤害程度与快速荧光参数Wk变化的关系[J].植物生理学报, 2015, 51(6):969-976. JIN Liqiao, CHE Xingkai, ZHANG Zishan, et al. The Relationship between the changes in Wk and different damage degree of PSⅡdonor side and acceptor side under high temperature with high light in cucumber[J]. Plant Physiol J, 2015, 51(6):969-976. [27] HALDIMANN P, STRASSER R J. Effects of anaerobiosis as probed by the polyphasic chlorophyll a fluorescence rise kinetic in pea (Pisum sativum L.)[J]. Photosynth Res, 1999, 62(1):67-83. [28] PAN Xiangliang, DENG Chunnuan, ZHANG Daoyong, et al. Toxic effects of amoxicillin on the photosystem Ⅱ of Synechocystis sp. characterized by a variety of in vivo chlorophyll fluorescence tests[J]. Aquat Toxicol, 2008, 89(4):207-213. [29] GOVINDJE E. Sixty-three years since Kautsky:chlorophyll a fluorescence[J]. Austr J Plant Physiol, 1995, 22(2):131-160. [30] GAO Peijun, ZUO Zhaojiang, WU Xingbo, et al. Effects of cycloheximide on photosynthetic abilities, reflectance spectra and fluorescence emission spectra in Phyllostachys edulis[J]. Trees, 2016, 30(3):719-732. [31] 刘倩倩, 马寿宾, 冯希环, 等.嫁接对高温和低温胁迫下辣椒幼苗快速叶绿素荧光诱导动力学特性的影响[J].园艺学报, 2016, 43(5):885-896. LIU Qianqian, MA Shoubin, FENG Xihuan, et al. Effects of grafting on the fast chlorophyll fluorescence induction dynamics of pepper seedlings under temperature stress[J]. Acta Hortic Sin, 2016, 43(5):885-896. [32] STRAUSS A J, KRÜGER G H J, STRASSER R J, et al. Ranking of dark chilling tolerance in soybean genotypes probed by the chlorophyll a fluorescence transient O-J-I-P[J]. Environ Exp Bot, 2006, 56(2):147-157. [33] IVANOV A G, HURRY V, SANE P V, et al. Reaction center quenching of excess light energy and photoprotection of photosystem Ⅱ[J]. J Plant Biol, 2008, 51(2):85-96. [34] 孙胜楠, 王强, 孙晨晨, 等.黄瓜幼苗光合作用对高温胁迫的响应与适应[J].应用生态学报, 2017, 28(5):1603-1610. SUN Shengnan, WANG Qiang, SUN Chenchen, et al. Response and adaptation of photosynthesis of cucumber seedlings to high temperature stress[J]. Chin J Appl Ecol, 2017, 28(5):1603-1610. [35] 郝召君, 周春华, 刘定, 等.高温胁迫对芍药光合作用、叶绿素荧光特性及超微结构的影响[J].分子植物育种, 2017, 15(6):2359-2367. HAO Zhaojun, ZHOU Chunhua, LIU Ding, et al. Effects of high temperature stress on photosynthesis, chlorophyll fluorescence and ultrastructure of herbaceous peony (Paeonia lactiflora Pall.)[J]. Mol Plant Breed, 2017, 15(6): 2359-2367. [36] 黄纯倩, 朱晓义, 张亮, 等.干旱和高温对油菜叶片光合作用和叶绿素荧光特性的影响[J].中国油料作物学报, 2017, 39(3):342-350. HUANG Chunqian, ZHU Xiaoyi, ZHANG Liang, et al. Effects of drought and high temperature on photosynthesis and chlorophyll fluorescence characteristics of rapeseed leaves[J]. Chin J Oil Crop Sci, 2017, 39(3):342-350. [37] 郑宇, 何天友, 陈凌艳, 等.高温胁迫对西洋杜鹃光合作用和叶绿素荧光动力学参数的影响[J].福建农林大学学报(自然科学版), 2012, 41(6):608-615. ZHENG Yu, HE Tianyou, CHEN Lingyan, et al. Effects of high temperature stress on photosynthesis and chlorophyll fluorescence of Rhododendron hybridum[J]. J Fujian Agriv For Univ Nat Sci Ed, 2012, 41(6):608-615. [38] DRAKE J E, POWER S A, DUURSMA R A, et al. Stomatal and non-stomatal limitations of photosynthesis for four tree species under drought:a comparison of model formulations[J]. Agric For Meteorol, 2017, 247:454-466. doi:10.1016/j.agrformet.2017.08.026. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2019.01.007






 下载:
下载: