-
花型花色单一、花期短、花叶均赏品种稀缺是木兰科Magnoliaceae植物杂交育种的主要问题[1]。目前有关含笑属Michelia杂交亲和性的研究较少,而了解物种间亲和性关系是培育新品种的关键。有研究认为:在含笑属远缘杂交过程中存在不亲和现象[2−3]。王亚玲等[4]则发现含笑属内种间杂交大多表现亲和,而紫花含笑M. crassipes×金叶含笑M. foveolata则不亲和,但未对不亲和原因进行探究。金叶含笑隶属木兰科含笑属,其花淡黄白色,花被片内扣形成独特花型,叶背密被红褐色柔毛,是木兰科中珍稀的花叶共赏树种,也是木兰科优良的杂交亲本材料。紫花含笑是含笑属珍稀的紫色花色植物,含笑M. figo的花被片边缘有紫色条纹。本研究通过金叶含笑与紫花含笑和含笑的杂交育种研究,以期将三者的优良性状相结合,培育出花叶共赏新品种,提高金叶含笑利用价值,丰富含笑属种质资源,并探究不亲和的原因,以期提高含笑属杂交成功率,为含笑属远缘杂交育种提供理论依据。
-
金叶含笑和紫花含笑来自湖南省长沙市湖南省科技示范园,含笑来自中南林业科技大学校园。
-
于2021年和2022年4—5月,收集金叶含笑、含笑、紫花含笑的花粉,置于−20 ℃的冰箱保存。在授粉前进行花粉活力测定,确保各亲本的花粉活力在60%以上。培养基配方参考柴弋霞等[5]并改良,采用固体培养基。紫花含笑花粉活力测定所用培养基的配制方法:150 g·L−1 蔗糖 + 300 mg·L−1硼酸 + 300 mg·L−1氯化钙+ 8 g·L−1琼脂;含笑花粉活力测定所用培养基的配制方法:100 g·L−1 蔗糖 + 200 mg·L−1硼酸 + 300 mg·L−1氯化钙 + 8 g·L−1琼脂;作者通过预实验已筛选出金叶含笑花粉活力测定的最适培养基为50 g·L−1蔗糖 +200 mg·L−1硼酸 + 300 mg·L−1氯化钙 + 8 g·L−1琼脂。杂交组合 (♀×♂) :含笑×金叶含笑、紫花含笑×金叶含笑、金叶含笑×含笑、金叶含笑×紫花含笑。每个组合授粉30~34朵花。
-
授粉后,在当年9—12月,采集各杂交组合的果实,统计坐果率、结籽率、单个果序上的蓇葖膨大率、种子数、单个蓇葖内含种子数、形态异常种子占比和千粒重。
-
用洗涤剂将杂交种子富含油脂的外种皮搓洗掉,经质量浓度为0.3%的高锰酸钾溶液消毒30 min,再用双蒸水(ddH2O)反复清洗,随后播种,统计各杂交组合种子的出苗率。
-
参考柴弋霞等[5]的方法并改良,在母本花蕾期去除花被片及雄蕊,授予父本花粉并套袋,在授粉后2 h、4 h、6 h、8 h、10 h、12 h、1 d、2 d、4 d、6 d、8 d取下雌蕊放入FAA固定液中,待固定48 h以上后,用解剖针将雌蕊从雌蕊群柄上取下,进行体积分数为50%、30%、10%乙醇和蒸馏水逐级复水,每级复水15 min。复水后将雌蕊置于1 mol·L−1 氢氧化钠溶液中,60 ℃水浴软化3 h,随后用蒸馏水重复清洗3次,用质量分数为0.1%苯胺蓝避光染色12 h。由于金叶含笑花柱从中部至子房基部均密被银灰色短绒毛,采用常规压片时花粉管会被绒毛所覆盖,很难观察到花粉管的生长情况,因此在本研究中,将授粉12 h前的花柱切下,再将授粉12 h后各时间点的子房壁沿心皮背缝线和腹缝线方向削去直至漏出胚珠,软化后使截面呈透明。将上述处理后的材料常规压片后置于莱卡(Leica)荧光倒置显微镜下观察花粉管的生长情况。
-
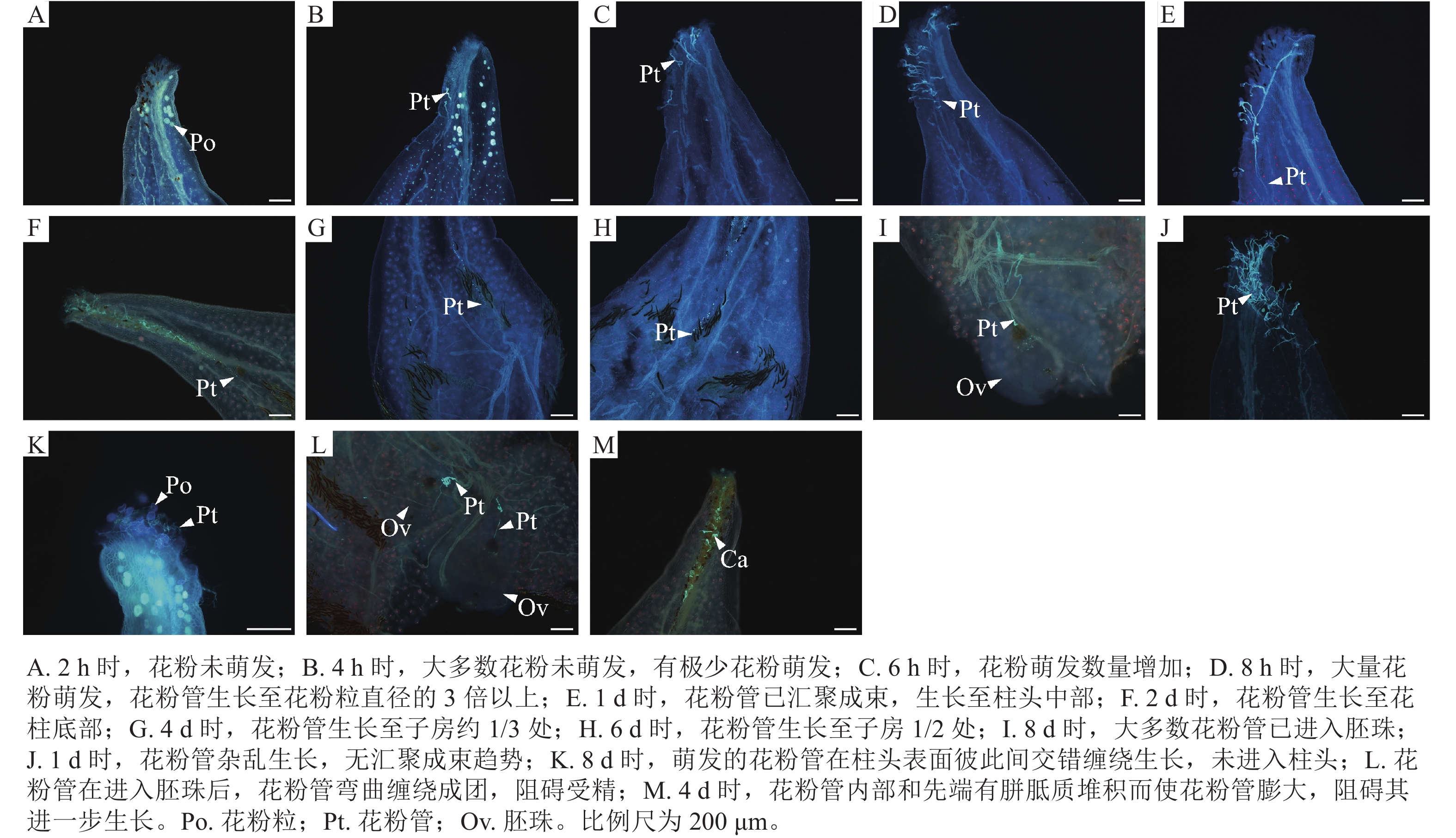
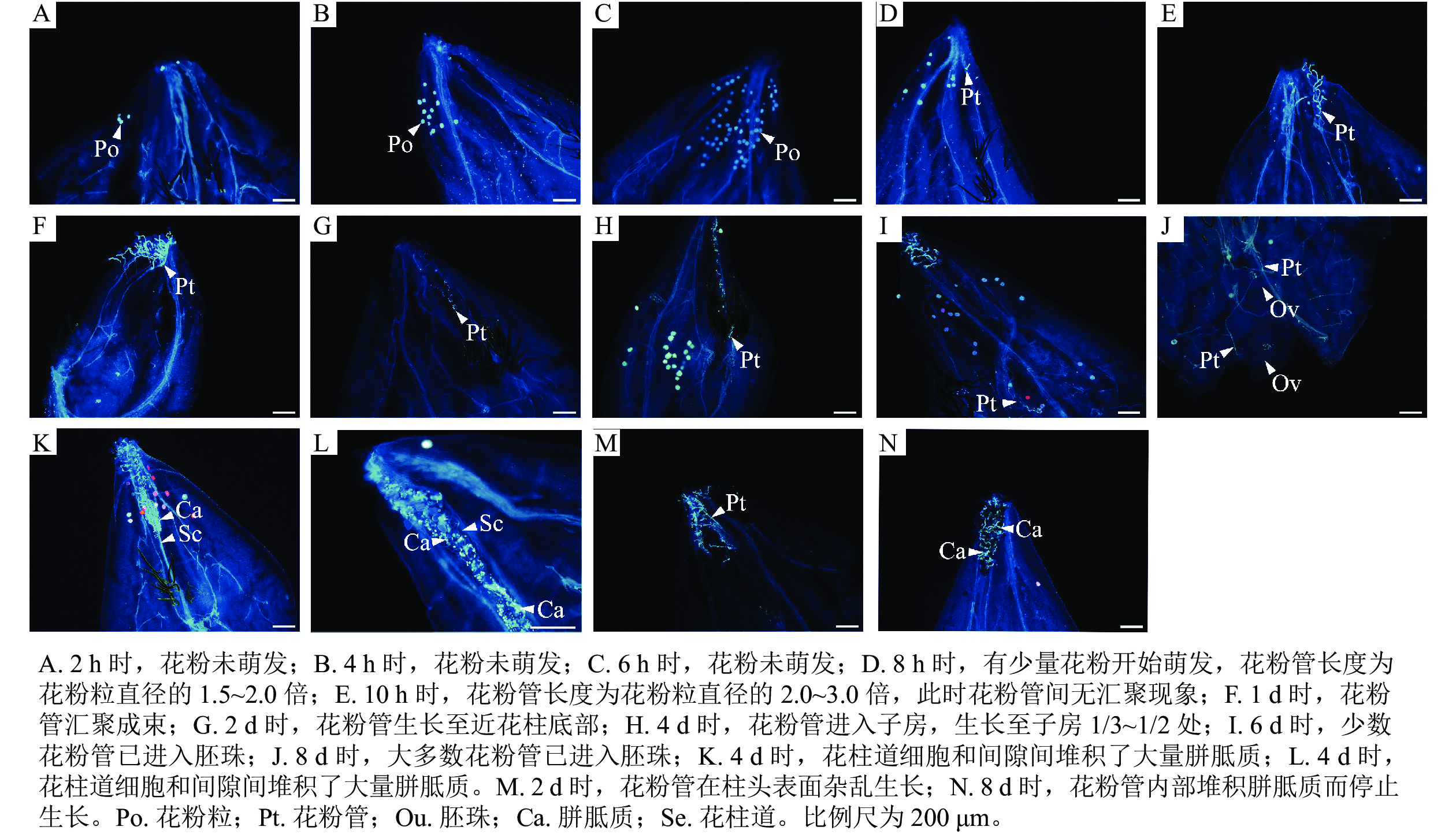
如图1所示:授粉2 h时,仅有极少量的花粉附着在含笑柱头表面且花粉未萌发(图1A);4、6 h时,附着在含笑柱头表面的花粉数量逐渐增多,但同样未萌发(图1B和C);8 h时,有少量花粉开始萌发,花粉管长度为花粉粒直径的1.5~2.0倍(图1D);10 h时,萌发的花粉数量增加,花粉管长度为花粉粒直径的2.0~3.0倍,此时花粉管无汇聚现象(图1E);1 d时,花粉大量萌发,花粉管长度生长至花粉粒直径的3.0倍以上,花粉管出现了明显的汇聚现象(图1F);2 d时,花粉管生长至近花柱底部,即将进入子房(图1G);4 d时,花粉管进入子房,生长至子房1/3~1/2处(图1H);6 d时,有少数生长较快的花粉管进入胚珠(图1I);8 d时,大多数花粉管在此时已进入胚珠(图1J)。但同时也出现了花粉管生长异常的现象:授粉4 d时,发现花柱道细胞和间隙间堆积了大量胼胝质(图1K和L),阻碍了花粉管生长,这一现象也在4、8 d时出现;2 d时,花粉管应汇聚成束进入花柱,但有些花粉管在柱头表面生长杂乱无章(图1M)。在8 d时发现,花粉管在柱头表面萌发,但因花粉管内部堆积胼胝质而停止生长(图1N)。
-
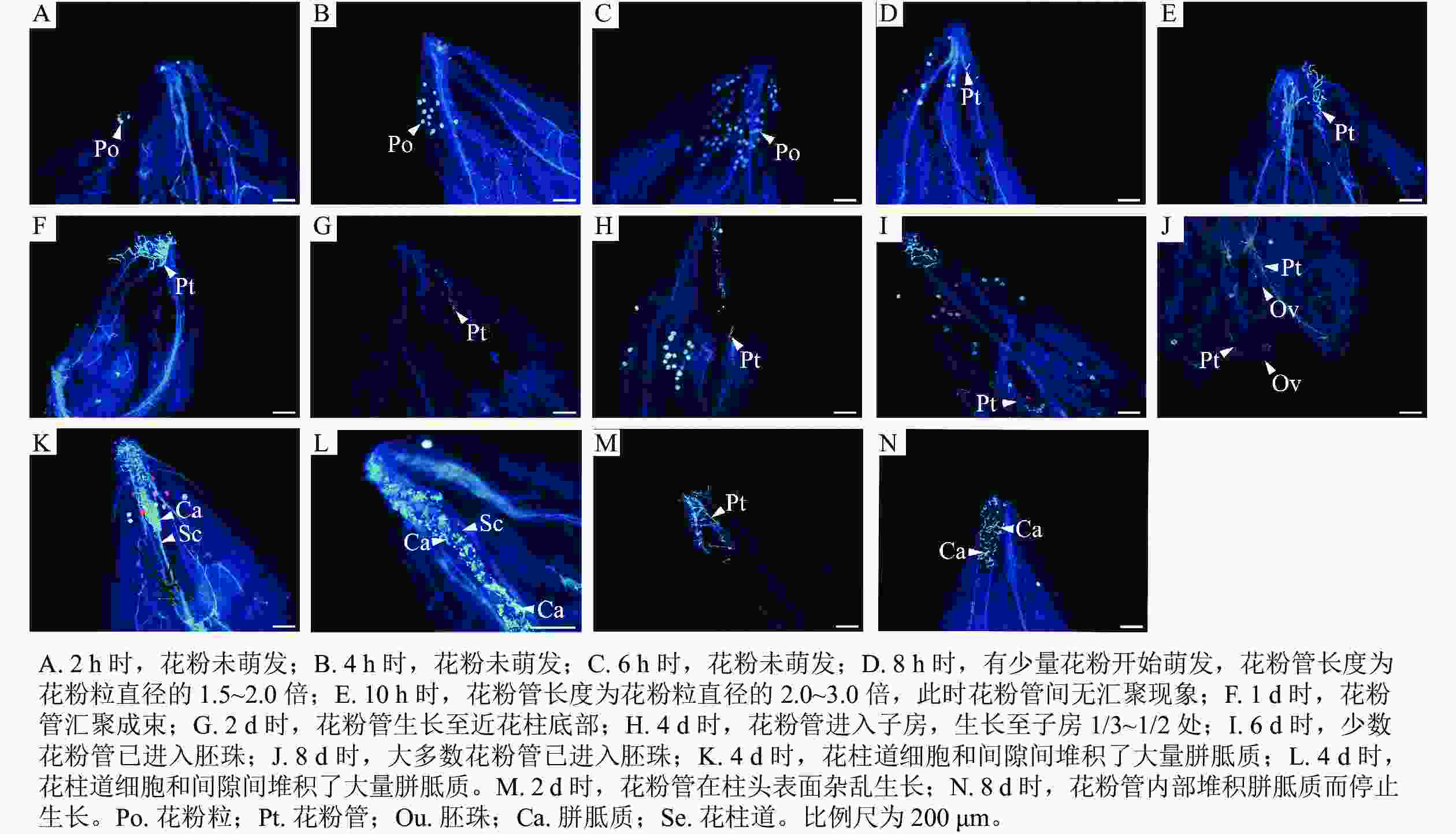
如图2所示:授粉2 h时,花粉未萌发(图2A);4 h时,柱头表面的花粉数量增多,有极少数花粉在此时开始萌发(图2B);6 h时,花粉萌发数量逐渐增多(图2C);在8 h时,大量花粉萌发,花粉管生长至花粉粒直径的3倍以上,此时花粉管存在汇聚的趋势但还没有开始汇聚(图2D);授粉后1 d,花粉管已汇聚成束,生长至柱头中部(图2E);2 d时,花粉管生长至花柱底部(图2F);4 d时,花粉管生长至子房约1/3处(图2G);6 d时,花粉管生长至子房1/2处(图2H);8 d时,大多数花粉管已进入胚珠(图2I)。该组合出现以下受精前障碍:授粉1 d时,出现花粉管生长杂乱且无汇聚现象。有的花粉萌发后,花粉管朝反方向生长(图2J);8 d时,发现有的花粉粒仍未萌发、萌发的花粉管在柱头表面彼此间交错缠绕生长,未进入柱头(图2K);花粉管在进入胚珠后,花粉管弯曲缠绕成团,阻碍受精(图2L);4 d时,花粉管内部和先端有胼胝质堆积而使花粉管膨大,阻碍其进一步生长(图2M)。
-
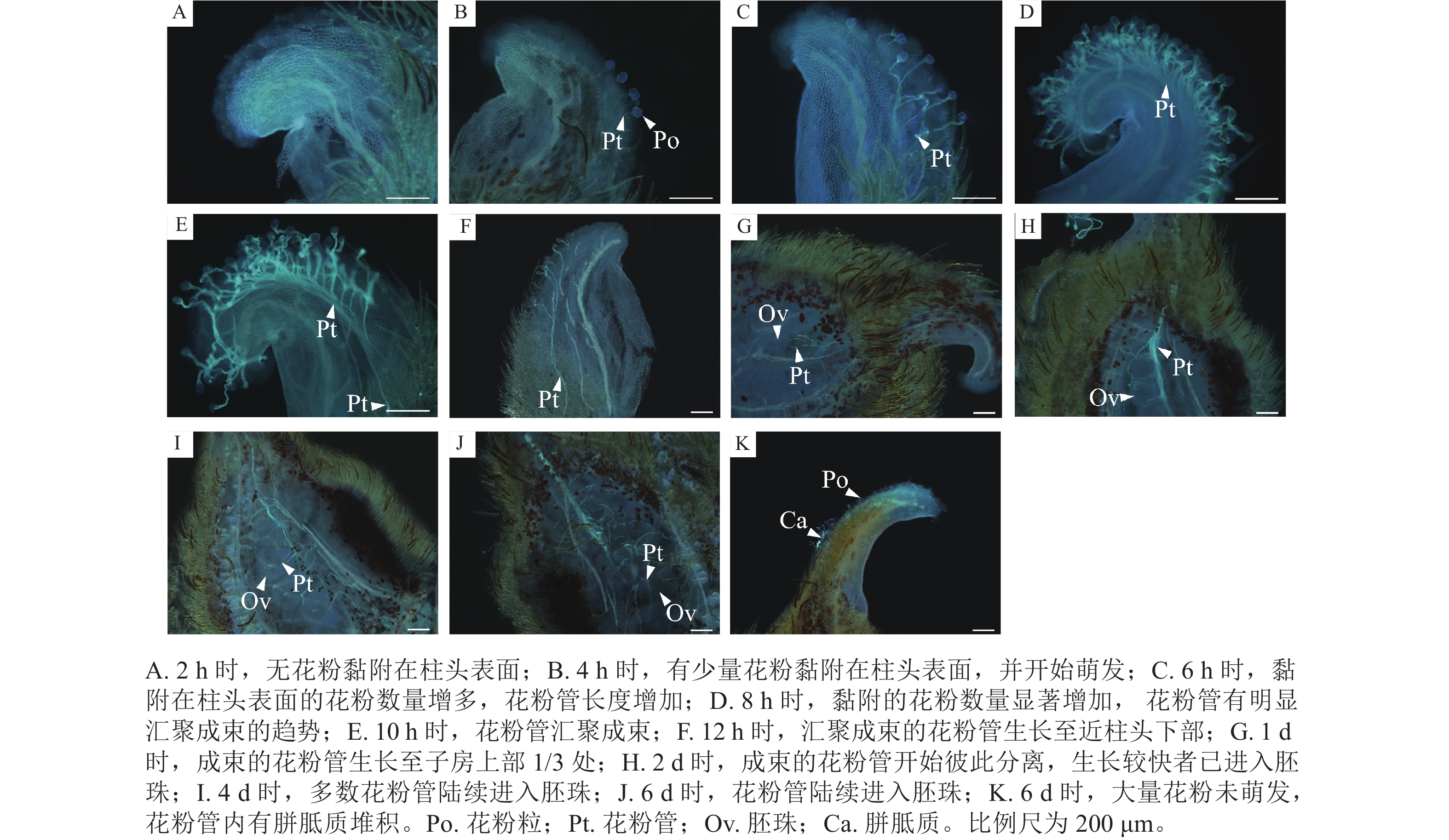
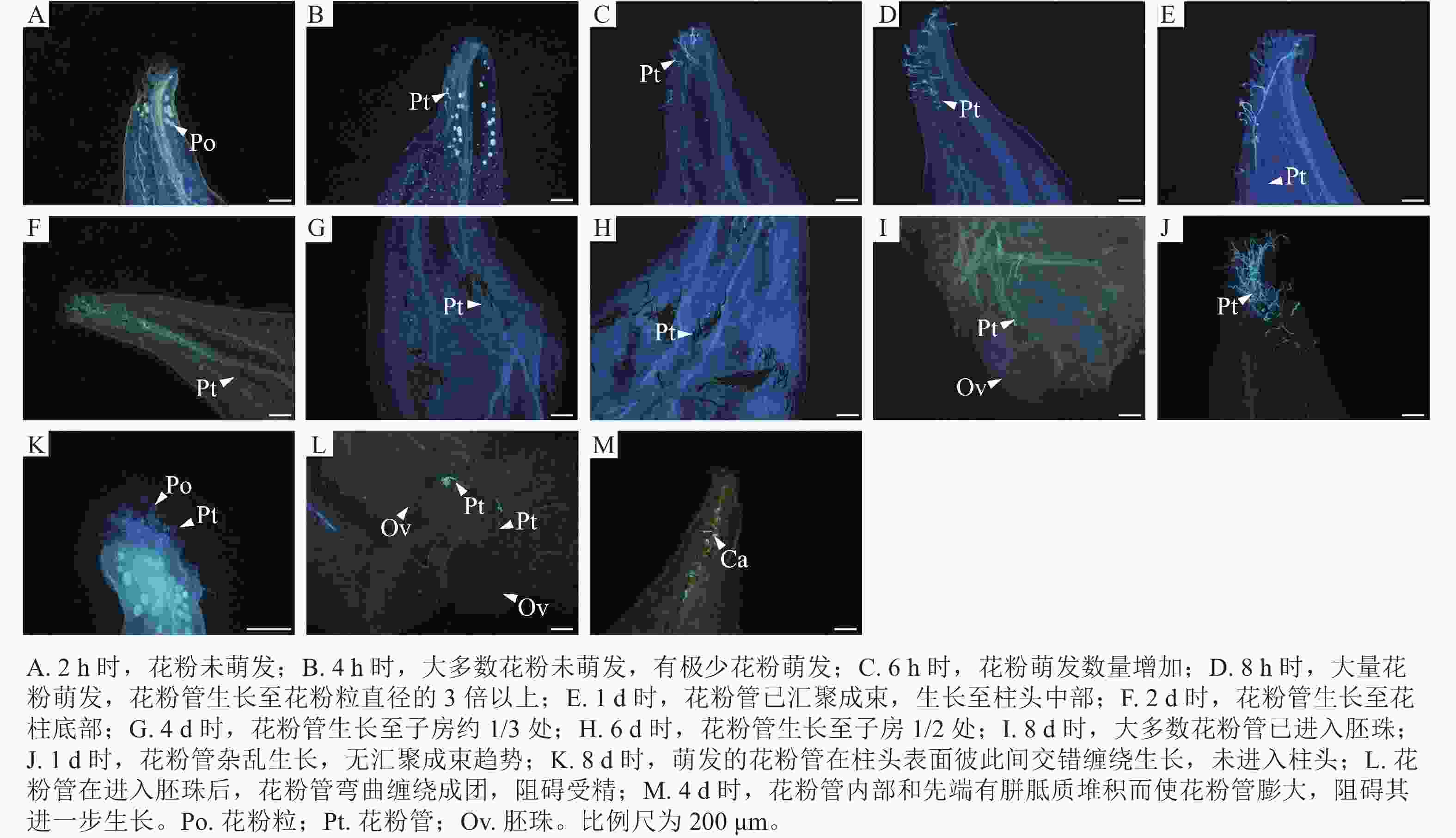
如图3所示:授粉2 h时,无花粉黏附在柱头表面(图3A);4 h时,有少量花粉黏附在柱头表面,并开始萌发,花粉管长度为花粉粒直径的2~3倍(图3B);6 h时,萌发的花粉数量增加,花粉管生长至花粉粒直径的4倍以上,生长较快的花粉管已开始汇聚成束(图3C);8 h时,萌发的花粉数量显著增加,花粉管有明显汇聚成束的趋势(图3D);10 h时,花粉管汇聚成束(图3E);12 h时,汇聚成束的花粉管生长至近柱头下部(图3F);1 d时,成束的花粉管生长至子房上部1/3处(图3G);2 d时,成束的花粉管开始彼此分离,生长较快者已进入胚珠(图3H);4 d时,多数花粉管已进入胚珠(图3I);6 d时,花粉管逐渐向下生长,进入底部的胚珠(图3J)。6 d时,极个别的柱头表面仍存在大量未萌发的花粉,少数已萌发花粉的花粉管内存在胼胝质堆积(图3K)。
-
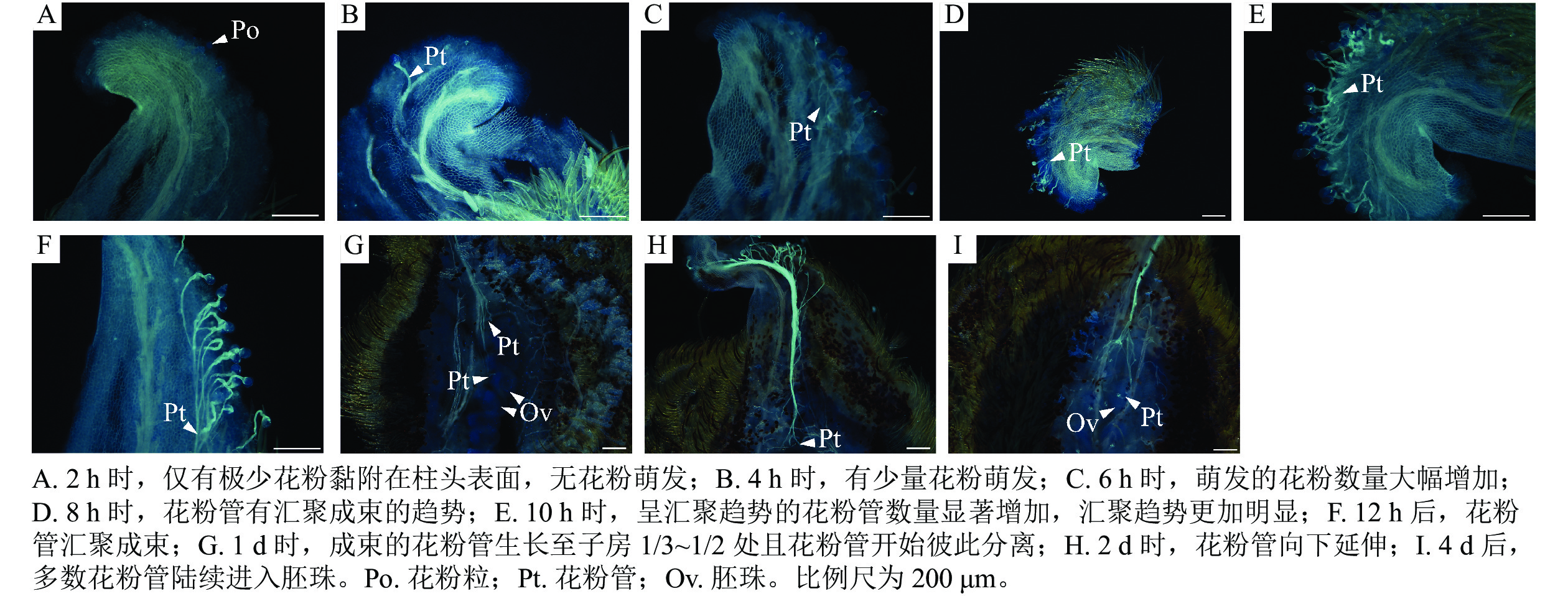
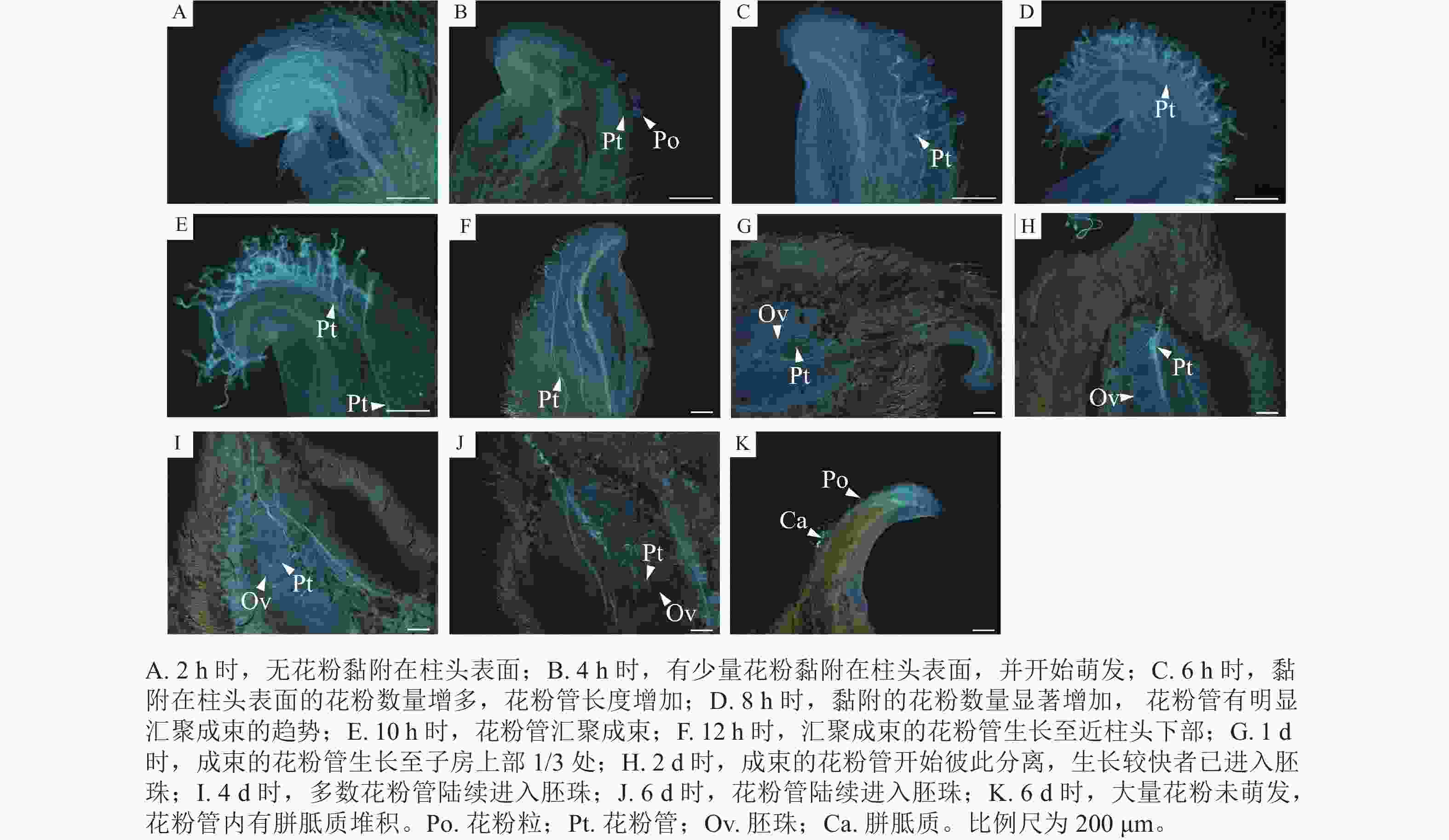
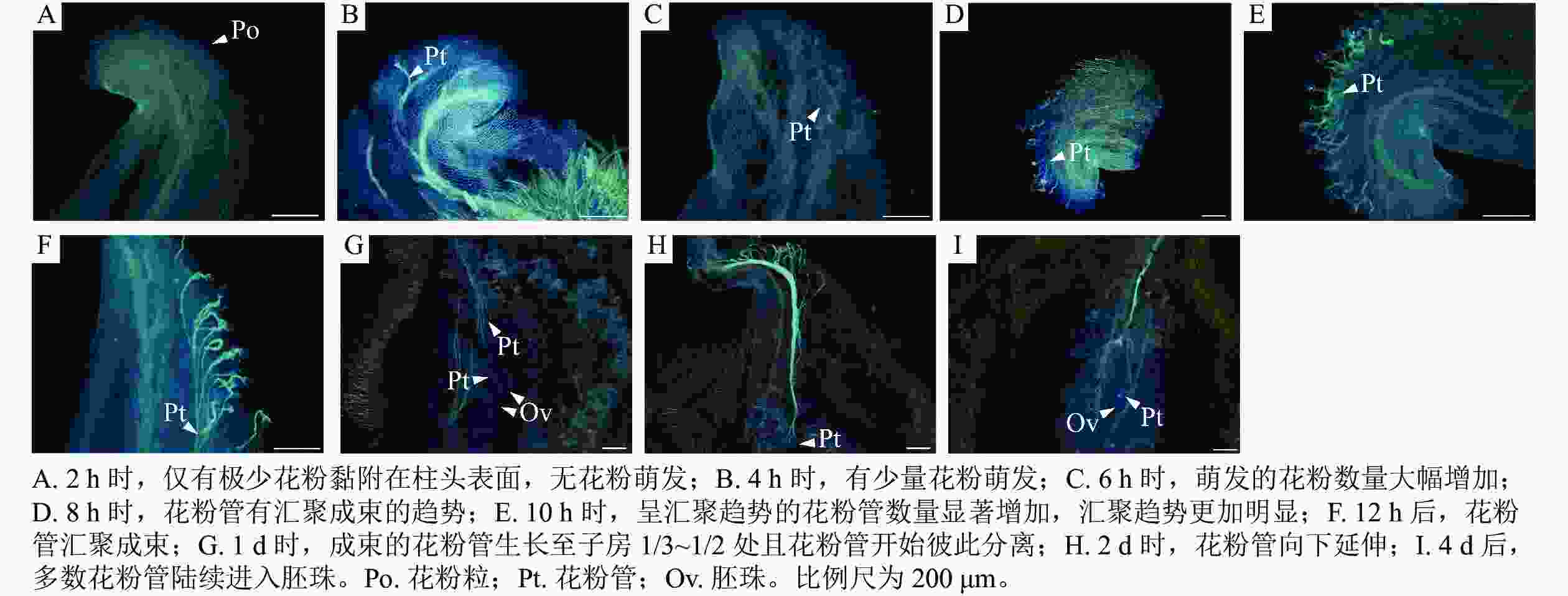
如图4所示:授粉2 h后,仅有极少花粉黏附在柱头表面,无花粉萌发(图4A);4 h时,黏附的花粉数量增加,已有少量花粉萌发,花粉管生长至花粉粒直径的2~3倍,少数生长较快的花粉管生长至花粉粒直径的4倍以上(图4B);6 h时,黏附及萌发花粉数量大幅增加,花粉管生长至花粉粒直径的4倍以上(图4C);8 h时,花粉管有汇聚成束的趋势(图4D);10 h时,呈汇聚趋势的花粉管数量显著增加,汇聚趋势更加明显(图4E);12 h时,花粉管汇聚成束(图4F);1 d时,成束的花粉管生长至子房1/3~1/2处且花粉管开始彼此分离(图4G);2 d时,花粉管继续向下延伸(图4H);4 d时,多数花粉管已进入胚珠(图4I)。
-
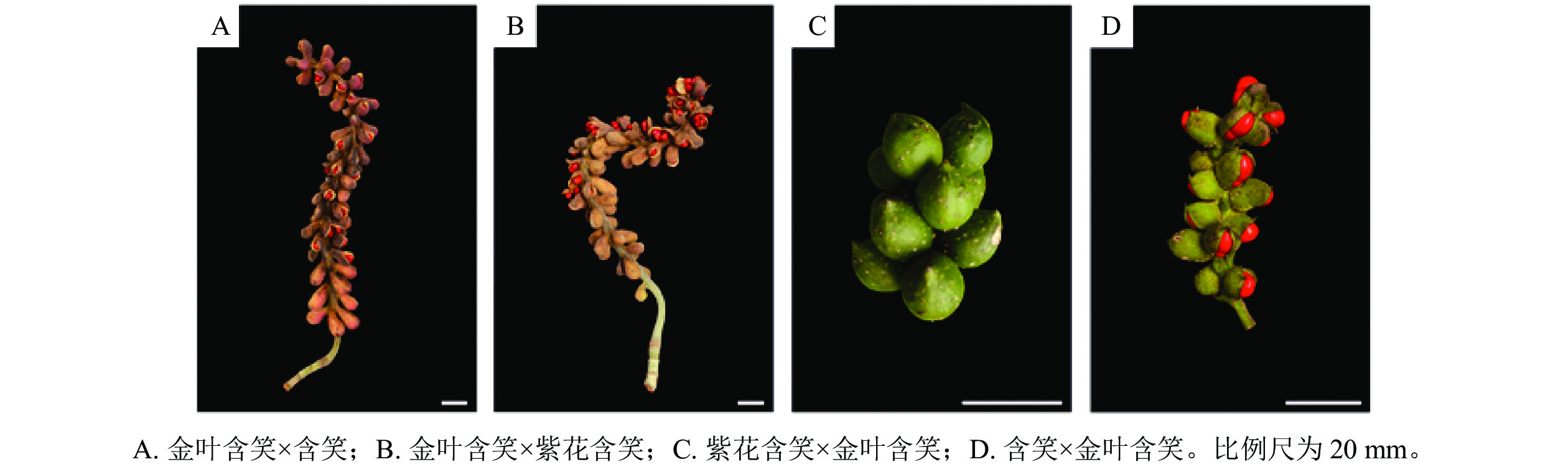
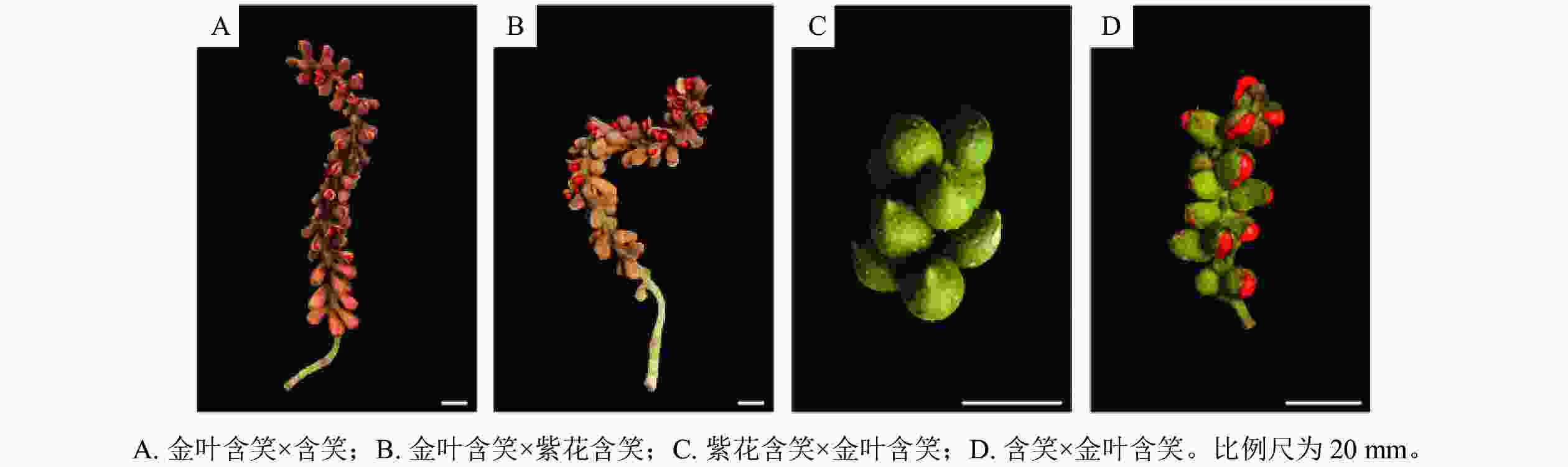
如表1和图5所示:金叶含笑与含笑正反交的坐果率均较高,分别为75.0%和78.8%。金叶含笑与紫花含笑正反交坐果率相差极大,正交的坐果率很高,达80.0%,而反交则极低,只有6.7%。金叶含笑×紫花含笑与金叶含笑×含笑的果实在坐果率、蓇葖膨大率与种子数上无显著差异,金叶含笑×含笑的种子较金叶含笑×紫花含笑略大而饱满。含笑×金叶含笑的坐果率显著高于紫花含笑×金叶含笑,蓇葖膨大率与种子数同样高于紫花含笑×金叶含笑。
杂交组合 (♀×♂) 坐果率/% 结籽率/% 单个果序蓇葖
膨大率/%种子数/粒 单个蓇葖内
含种子数/粒形态异常
种子占比/%千粒重/g 种子萌发率/% 金叶含笑×含笑 75.0 a 50.3±13.4 a 90.5±8.8 a 300.7±79.2 a 4.8±1.1 a 9.6±5.8 a 25.1 90.6 金叶含笑×紫花含笑 80.0 a 36.6±9.6 b 87.6±7.1 a 287.5±47.9 a 4.4±0.6 a 16.1±9.2 a 21.8 78.1 含笑×金叶含笑 78.8 a 27.3±9.0 bc 49.4±15.7 b 24.1±9.7 b 1.1±0.2 b 0.0±0.0 b 107.7 87.5 紫花含笑×金叶含笑 6.7 b 18.4±1.4 c 27.3±4.6 c 13.0±1.4 b 1.1±0.0 b 0.0±0.0 b 113.3 92.9 说明:不同字母表示不同杂交组合间差异显著(P<0.05)。 Table 1. Fruit and seed setting status of M. foveolata in different cross combinations
-
于2021年和2022年9—12月采集各杂交组合的种子。含笑×金叶含笑与紫花含笑×金叶含笑的种子经低温沙藏处理后于翌年1月播种,种子在3月初至4月初开始陆续萌发。金叶含笑×含笑与金叶含笑×紫花含笑的种子属于薄皮种子,很容易因失水、氧化而失去活性,因此采用即采即播的方式,种子在翌年2月中旬陆续萌发。由表1所示:各杂交组合的种子出苗情况良好,萌发率较高,均在75.0%以上,其中最高的为紫花含笑×金叶含笑杂交组合的种子,萌发率高达92.9%。
-
杂交障碍分为受精前障碍与受精后障碍。受精前障碍主要表现为:花粉在柱头上不能萌发;即使花粉在柱头表面萌发,但花粉管不能伸入柱头;花粉管可以穿过柱头在花柱中生长但生长受阻,不能进入子房到达胚囊;花粉管虽能达到胚囊,但不能完成双受精[6]。受精后障碍主要表现为受精后的合子不分裂或原胚发育异常或早期发育停止;胚乳发育异常或胚乳与胚发育不协调,最终导致胚体发育异常而降解;可获得种子但种子不能发芽或发芽后成株前夭亡或不能正常开花和结果[7]。
本研究中含笑×金叶含笑杂交的坐果率较高,达78.8%。平均每个果序上有49.4%的蓇葖膨大,当果序上有极少数蓇葖膨大时依然可以实现坐果。然而结籽率却较低,仅为27.3%,平均单个蓇葖内含种子数为1.1个,含笑的每心皮内含2枚胚珠,有近一半的胚珠没有成功受精或受精后胚发育异常。综合分析,在荧光显微观察中发现的花柱道细胞和间隙间堆积了大量胼胝质,阻碍了花粉管的生长;花粉管在柱头表面杂乱生长,导致花粉管无法汇聚成束进入花柱道,最终停止生长;花粉管内部和先端有胼胝质堆积,形成胼胝质塞,使花粉管生长受阻等障碍与结实情况,认为上述杂交障碍并不影响坐果,而是影响结籽。王亚玲[8]发现花粉管两型性在木兰科植物中广泛存在,绝大多数花粉是短花粉管型,极少数是长花粉管型,而只有长花粉管能完成受精。香港木兰M. championii的花粉大量萌发,在花柱道汇聚成束后,随着花粉管生长,越靠近胚珠的花粉管neng数量越少,仅有少数花粉管能到达胚珠。这种现象与本研究中含笑和紫花含笑的胚珠附近未见明显的花粉管分离现象相似。因此推测:可能是由于花粉的生理特性加上胼胝质阻碍花粉管进入胚珠完成受精,进而影响结籽率。也有研究发现木兰科植物存在大孢子发生和雌配子体发育异常的现象[9−11],这会对木兰科植物结实产生影响。
金叶含笑×紫花含笑坐果率与结籽率显著高于紫花含笑×金叶含笑,可见金叶含笑作母本时表现亲和,而作父本时亲和性较差。目前已有许多学者发现杂交亲和性具有单向性[12−14]。在王亚玲[8]研究中,紫花含笑×金叶含笑的坐果率为0,判定紫花含笑和金叶含笑杂交不亲和。本研究证实该杂交组合坐果率虽然很低,但仍可以结实。产生差异的原因可能是由于王亚玲仅对7朵花进行杂交试验,样本量过少。虽然在紫花含笑×金叶含笑中出现花粉在柱头表面未萌发,花粉管在柱头表面盘旋生长,未进入柱头,花粉管内部、先端堆积胼胝质而变得膨大,生长受阻,花粉管在胚珠内弯曲缠绕成团,阻碍受精等不亲和现象,但经观察发现绝大多数金叶含笑的花粉管已到达紫花含笑胚珠部位。杨占辉等[15]在鸢尾Iris spp.杂交试验中发现鸢尾花粉在柱头表面缠绕扭曲,但对花粉管伸长影响不大,最终都可见花粉管进入胚珠。何兴波等[16]发现‘风味玫瑰’Prunus domestica × P. armeniaca ‘Fengweimeigui’自交的花粉管在胚珠附近时,花粉管先端出现团状胼胝质,阻碍花粉管进入胚珠。张赛阳等[17]发现杂交石竹Dianthus hybridus的花粉管即使可以从珠孔进入胚囊,但在珠孔附近卷曲盘绕生长,并堆积胼胝质,阻碍双受精。在紫花含笑×金叶含笑杂交1个月后,紫花含笑的雌蕊群上仅有极少数雌蕊膨大,但未落果。在2个月时大多数雌蕊的颜色由绿色转为黄色,随后脱落。当下层雌蕊脱落时会连带上层膨大的雌蕊一起脱落,果实脱落率高达93.34%。王亚玲[8]发现没有完成双受精的香港木兰雌蕊会在授粉后10 d左右开始凋落。也有学者认为:在授粉后子房生长停滞并大量落果,是胚胎发育不良引起的[18−19]。吴美娇等[20]发现百合Lilium spp.花粉管在受精前无明显障碍,但在受精和胚胎发育过程中出现不同程度的胚败育现象。综合分析,推测紫花含笑×金叶含笑杂交不亲和主要是由受精后障碍引起的,因此,需要对受精情况和胚胎发育进一步研究。
在金叶含笑×含笑与金叶含笑×紫花含笑中均存在形态异常的种子,大多数为空瘪的种子。在2个组合中形态异常种子占比分别为9.6%和16.1%,而反交则均为饱满的种子,王亚玲[8]发现:在香港木兰授粉后部分合子出现败育,如果胚乳核继续发育,最后可以形成无胚种子。同时也存在受精胚乳核败育,胚乳没有形成营养丰富的胚乳核细胞,而是形成海绵状物质。目前已有许多方法用于克服受精前障碍,如蒙导法、重复授粉法等。贺丹等[21]研究发现:延迟授粉法可以减缓甚至克服母本柱头或花柱对异源花粉萌发及花粉管生长的抑制,克服受精前障碍,提高育种效率。对于杂交胚早期败育可采用胚抢救的方法[22−23]。因此,在今后研究中可对紫花含笑×金叶含笑的杂交胚采取胚抢救的方法克服不亲和障碍。
-
金叶含笑与含笑正反交均亲和,金叶含笑花粉管与含笑花柱道内的胼胝质堆积,但不影响坐果率,只对结籽率有一定影响。金叶含笑与紫花含笑表现为单向杂交亲和,金叶含笑×紫花含笑亲和性较强,紫花含笑×金叶含笑亲和性较差是由受精后障碍所引起的。
Compatibility between Michelia foveolata and two Michelia species
doi: 10.11833/j.issn.2095-0756.20230021
- Received Date: 2023-02-01
- Accepted Date: 2023-06-06
- Rev Recd Date: 2023-06-06
- Available Online: 2023-09-26
- Publish Date: 2023-09-26
-
Key words:
- Michelia foveolata /
- cross-compatibility /
- pollen tube /
- fruit setting rate /
- seed setting rate
Abstract:
| Citation: | ZHANG Zheng, SHAO Fengxia, JIN Xiaoling, et al. Compatibility between Michelia foveolata and two Michelia species[J]. Journal of Zhejiang A&F University, 2023, 40(5): 961-969. DOI: 10.11833/j.issn.2095-0756.20230021 |









 DownLoad:
DownLoad: