-
无距虾脊兰Calanthe tsoongiana为兰科Orchidaceae虾脊兰属Calanthe地生草本,花朵娇艳,造型独特,受到人们的喜爱。近些年来,由于人为破坏,以及自身繁殖困难,导致野生无距虾脊兰种群数量锐减,现已被列为极度濒危等级[1]。
植物和真菌之间的互惠共生是自然界中普遍存在的现象[2]。兰科菌根真菌是一类与兰科植物形成共生关系并生成菌丝团的真菌,隶属于根系内生真菌,对兰科植物生长起促进作用[3]。研究表明:兰科植物的整个生命活动都需要菌根真菌的参与,菌根真菌不仅可以帮助兰花种子萌发[4−5],也在其生长发育过程中扮演着重要的角色。它可以吸收土壤中的矿质元素、有机物等,在侵染兰科植物后被宿主细胞分解并向其传输营养物质[6−7],若缺乏菌根真菌,兰科植物将无法长期存活。除了菌根真菌,兰科植物中非菌根真菌的数量也十分庞大,远远超过了菌根真菌。镰孢属Fusarium、木霉属Trichoderma和青霉属Penicillinum是兰科植物中常见的内生真菌,在适当的条件下不仅能促进兰科植物种子的萌发和生长发育,还可以有效抑制病原菌的生长[8−10]。由此可见,非菌根真菌的作用也不容忽视。兰科菌根真菌不仅广泛存在于根系内,也存在于植株周围土壤中,且距离植株越远,其相对丰度越低[11−12],但也有研究表明:菌根真菌少量或者不存在于土壤中[13−14]。蒋玉玲等[15]研究表明:通过对周围土壤中真菌类群的分析,可推断兰科植物能否长期在此生存。因此,研究根际土壤真菌类群对兰科植物保育十分重要。
目前,虾脊兰属植物的研究集中在分类鉴别、育种和繁殖等方面,具体有资源调查、遗传多样性研究、化学与药用价值研究、快速繁育以及引种驯化等等[16−17]。国内外对虾脊兰属根系内生真菌多样性研究较少,尚处于初步阶段,未得到系统且全面的数据,尤其是无距虾脊兰还未见相关报道。鉴于此,本研究以浙江省天目山野生无距虾脊兰为材料,通过分析无距虾脊兰不同时期根际土壤真菌与根系内生真菌多样性,明确其优势真菌和共有真菌类群,以期为无距虾脊兰资源有效保护提供科学参考。
-
所用材料采自浙江天目山国家级自然保护区内无距虾脊兰野生居群(海拔550 m,30°21′47″N,119°25′30″E)。该研究区属亚热带季风气候,雨水充沛,年降水量达1 870 mm,相对湿度较大,自然条件优越。
无距虾脊兰生长在海拔450~1 450 m的阔叶混交林下,生长周期可分为萌芽期、花期、果期和衰亡期[18]。选择未遭到破坏、分布集中且长势一致的植株,分别于2、5、7和11月进行根段采集。每次选取9株植株,每株取3~4个长约4~5 cm的根段,用根围土包裹装入采样袋,做好标记并放入4 ℃冰箱保存。
-
取出根系并用流水冲洗干净,无菌水冲泡,用体积分数为70%的乙醇洗涤2 min,质量分数为2.5%的次氯酸钠浸泡5 min,体积分数为70%的乙醇洗涤30 s,再经无菌水冲洗2~3次,用无菌滤纸吸去多余水分后转入液氮中紧急速冻。初步处理完成,置于−80 ℃超低温冰箱保存。
-
在超净台上抖掉根围土,保留附着在根系1 mm左右的根际土。用无菌镊子将根系转移至装有磷酸缓冲盐溶液(PBS)的离心管中,全温摇床下震荡20 min,挑出根系,将剩余悬浮液6 000 r·min−1高速离心20 min,弃上清液得到根际土。做好标签记录,将离心管放入液氮,待其彻底急速冷冻后,放入−80 ℃超低温冰箱保存。
-
使用DNA提取试剂盒提取无距虾脊兰根系与根际土壤DNA,提取后利用质量分数为1%的琼脂糖凝胶电泳检测DNA浓度,引物合成和测序由上海美吉生物医药科技有限公司进行。
PCR 仪采用ABI Gene Amp® 9700型,每个样本3个重复,20.0 μL PCR反应体系为10.0 μL 2× ChamQ SYBR Color qPCR Master Mix,0.8 μL上游引物,0.8 μL下游引物,0.4 μL 50 × ROX Reference Dye 1,2.0 μL DNA模板,ddH2O补至20.0 μL。PCR反应条件为95 ℃预变性5 min,95 ℃变性30 s,58 ℃退火30 s,35个循环;72 ℃延伸1 min,4 ℃保存。同一样本的PCR产物混合后用质量分数为2%的琼脂糖凝胶电泳检测,使用AxyPrepDNA凝胶回收试剂盒(AXYGEN公司)切胶回收PCR产物,纯化后的PCR产物使用Illumina MiSeq平台进行测序。
-
首先根据overlap关系利用FLASH (v1.2.11)软件对Illumina测序得到的原始数据进行拼接,并通过FASTP (v.0.19.6)软件对序列质量进行质控、过滤和优化。区分样本后,在97%相似度水平下使用UPARSE (v11.0)软件对优化序列进行分类操作单元(OTU)聚类分析。为了得到每个OTU对应的物种分类信息,基于97%相似水平下采用RDP classifier贝叶斯算法对OTU代表序列与UNITE (v.8.0)数据库进行分类学比对。
基于OTU聚类分析结果,通过Mothur (v1.30.2)软件进行多样性指数分析,从而得到所有样本群落中物种的丰富度、覆盖度和多样性等信息。根据分类学分析结果,通过Qiime (v1.9.1)软件生成各分类学水平丰度表,进行Beta多样性距离计算,找出各样本在某一分类学水平上的优势物种、样本中各优势物种的相对丰度以及不同样本中物种的组成相似性,并利用R语言(version 3.3.1)工具统计和作图,将差异和距离通过二维坐标图呈现出来,从总体上反映各组样本之间的差异和组内样本之间的变异度大小。基于Kruskal-Wallis秩和检验(Kruskal-Wallis H test),通过R (version 3.3.1)的stats包和python的scipy包运用DP的方法计算影响大小(effect size),从而找出多组样本的物种进行差异显著性分析。
-
无距虾脊兰萌芽期根系测序共获得31 987条优化序列。由图1可知:在门水平上担子菌门Basidiomycota和被孢霉门Mortierellomycota占比较大,属于萌芽期根系内生真菌优势门;在属水平上,被孢霉属Mortierella和红菇属Russula真菌序列占比相差不大,共同作为该时期优势内生真菌。花期根系测序共获得51 913条优化序列。其中,担子菌门为最大优势菌门;在属水平上,粗糙孔菌属Trechispora为该时期优势内生真菌。相较于萌芽期,花期真菌类群中担子菌门和子囊菌门Ascomycota相对丰度分别为萌芽期的1.33和1.97倍,被孢霉门、被孢霉属和红菇属的相对丰度呈现下降趋势,分别减少了67.82%、67.39%和75.70%。
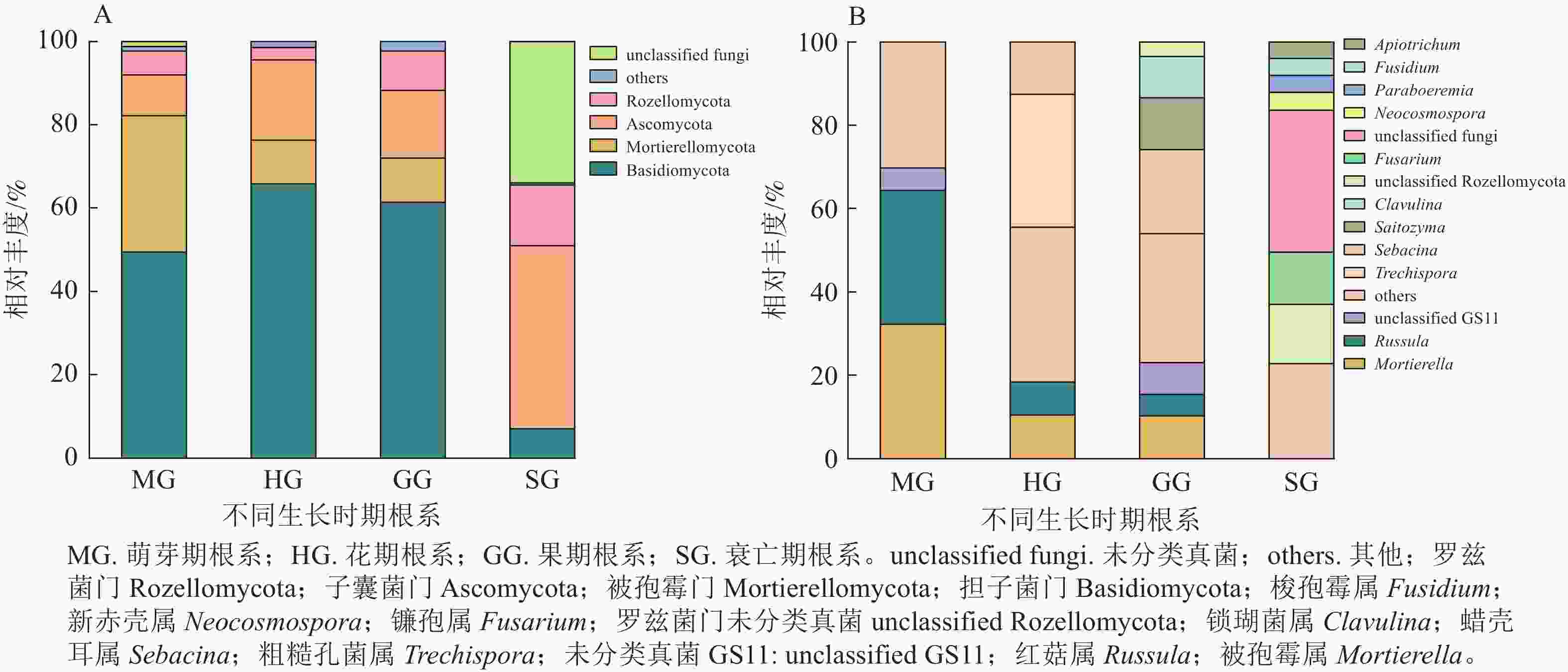
Figure 1. Community composition of root endophytic fungi in different periods of C. tsoongiana at phylum (A) and genus (B) level
果期根系测序共获得43 793条优化序列。其中担子菌门真菌在数量上占绝对优势;在属水平上,蜡壳耳属Sebacina为该时期优势内生真菌。相较于花期,担子菌门、子囊菌门、被孢霉门和红菇属相对丰度变化不大,但Saitozyma、锁瑚菌属Clavulina相对丰度有所增加,由原来占比不足3.00%增长到13.09%和10.29%,蜡壳耳属增加了67.30%。衰亡期根系测序共获得30 000条优化序列。该时期,子囊菌门属于优势菌门,镰孢属为优势内生真菌,相较于其他3个时期,衰亡期根系内生真菌多样性整体出现骤降现象。
-
在萌芽期,无距虾脊兰根际土壤测序共获得31 673条优化序列。由图2可知:萌芽期根际土壤与根系在门水平上真菌类群一致,但相对丰度稍有变化;在属水平上,红菇属相对丰度明显下降,占比仅为3.55%,其余真菌相对丰度较小,被孢霉属为该时期根际土壤优势真菌。
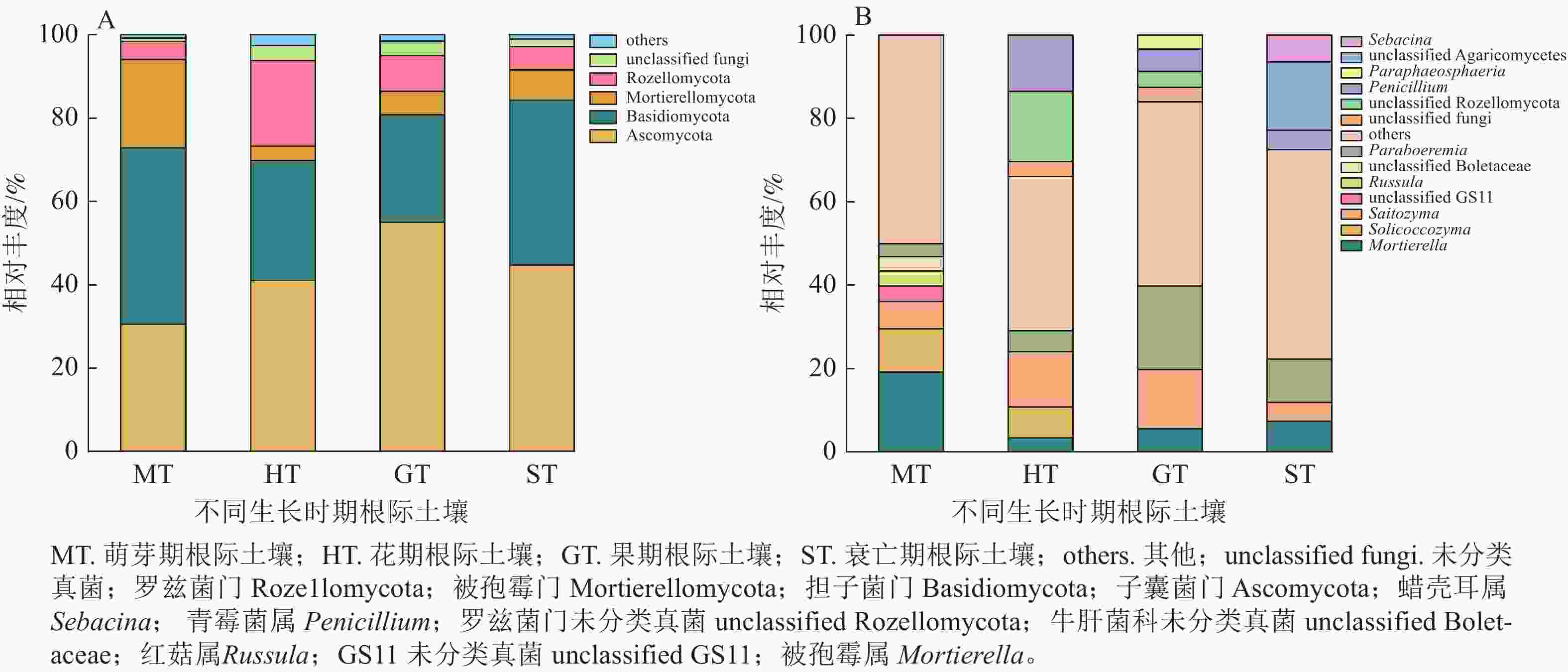
Figure 2. Community composition of rhizosphere soil fungi in different periods of C. tsoongiana at phylum (A) and genus (B) level
在花期,根际土壤测序共获得41 062条优化序列。与同时期根系内生真菌相比,担子菌门相对丰度减少,子囊菌门相对丰度增加。在属水平上,根际土壤和根系真菌类群相对丰度明显不同,其中青霉属Penicillium为该时期根际土壤优势真菌。相较于萌芽期根际土壤真菌,花期子囊菌门和罗兹菌门Rozellomycota相对丰度分别为萌芽期的1.34和4.74倍,担子菌门、被孢霉门真菌相对丰度分别减少了31.93%和67.82%。
在果期,根际土壤测序共获得47 863条优化序列。与根系内生真菌相比,门水平上子囊菌门和担子菌门变化最大,在属水平上,Paraboeremia仅存在于根际土壤真菌中,且占比较大,为该时期根际土壤优势真菌。相较于花期根际土壤真菌,果期子囊菌门、被孢霉门、Paraboeremia和Saitozyma相对丰度分别为花期的1.34、1.61、4.03和1.07倍,担子菌门和罗兹菌门相对丰度分别降低到花期的89.45%和42.21%。
在衰亡期,根际土壤测序共获得28 997条优化序列。在门水平上,担子菌门为根系内生真菌的5.61倍,根系内生真菌无被孢霉门,而在根际土壤中其真菌序列占7.29%。在属水平上,各真菌相对丰度差别较大,其中Paraboeremia为该时期根际土壤优势真菌。相较于果期根际土壤真菌,蜡壳耳属仅存在于衰亡期根际土壤中,担子菌门和被孢霉属真菌相对丰度分别为果期的1.53和1.31倍,Saitozyma相对丰度降低到果期的32.32%。
-
通过对无距虾脊兰根际土壤与根系测序分析,发现不同生长发育时期物种注释结果存在较大差别,具体差异见表1。其中萌芽期根际土壤和根系优势真菌分别为被孢霉属和红菇属,花期优势真菌分别为青霉属和粗糙孔菌属,果期优势真菌分别为Paraboeremia和蜡壳耳属,衰亡期优势真菌分别为Paraboeremia和镰孢属,不同生长发育时期优势真菌不同。
生长
时期优化序
列/条门/个 纲/个 目/个 科/个 属/个 萌芽期 63 660 11 41 91 193 359 花期 92 975 13 46 101 217 367 果期 91 656 12 48 108 224 377 衰亡期 58 997 8 33 75 162 264 Table 1. Species annotation results statistics
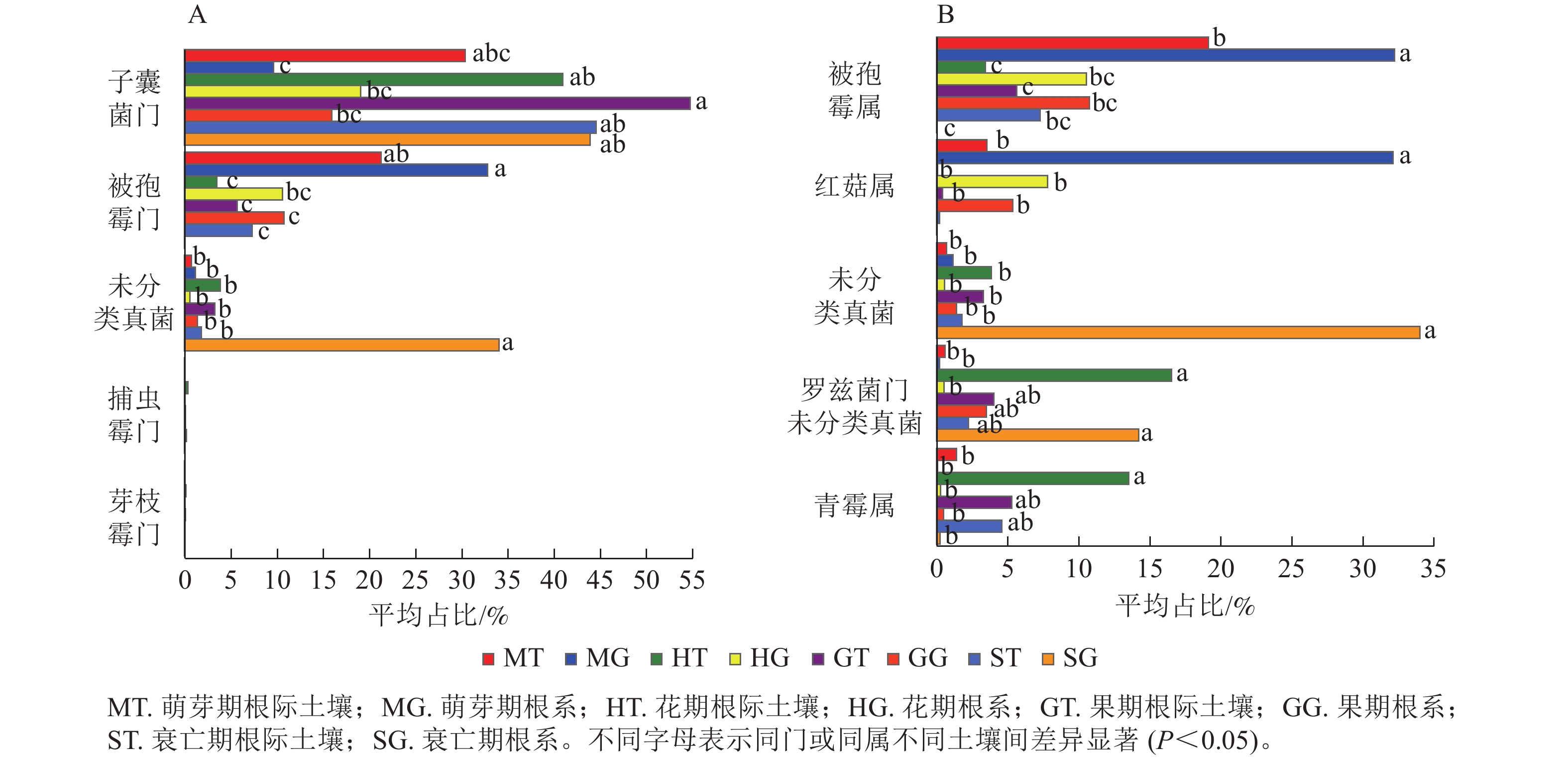
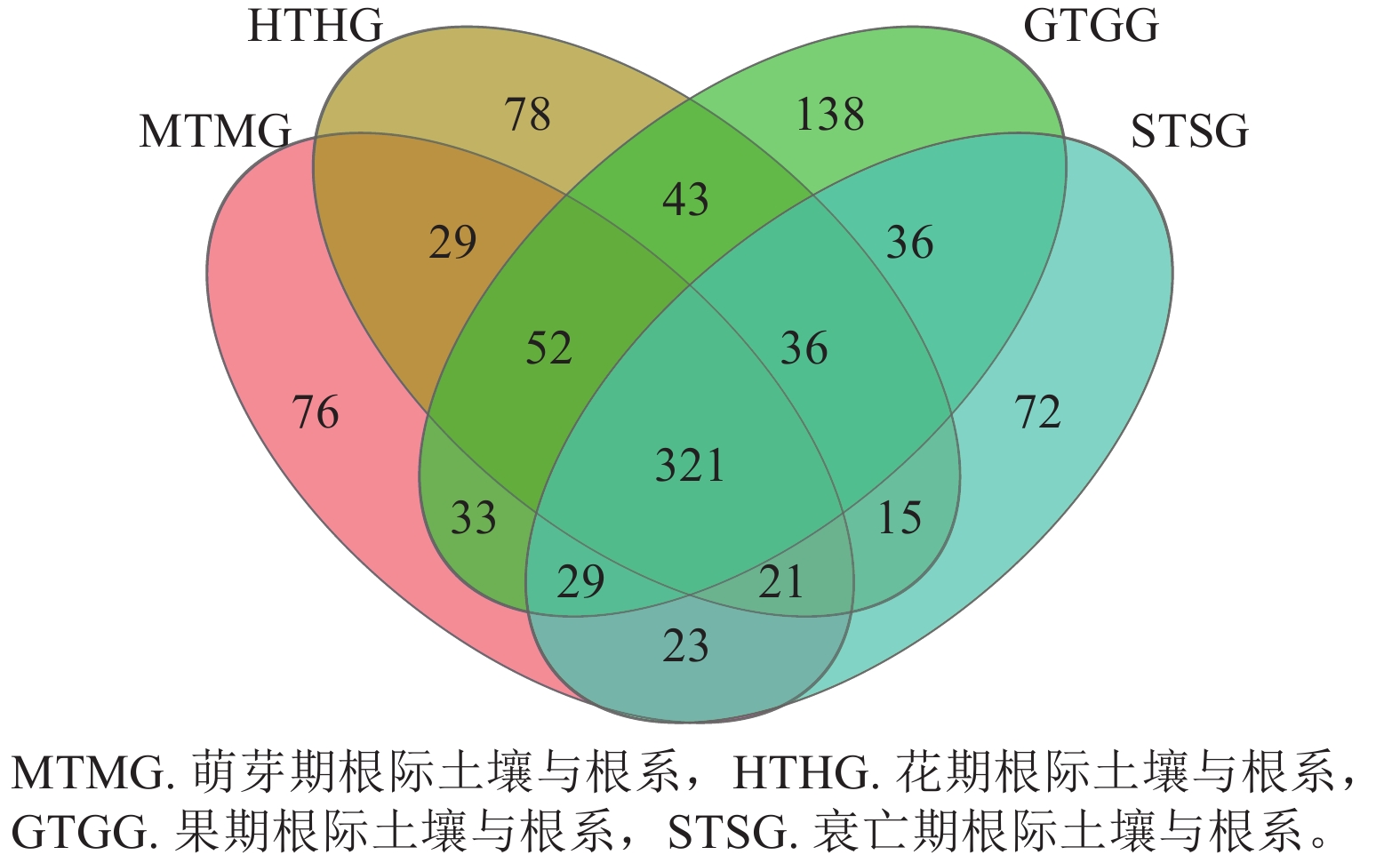
在门水平上,子囊菌门真菌在萌芽期、花期和果期的根际土壤与根系中存在显著差异;而被孢霉门真菌则在萌芽期与衰亡期的根际土壤和根系中存在显著差异(图3A,P<0.05)。在属水平上,被孢霉属、红菇属、罗兹菌门未分类属以及青霉属在萌芽期的根际土壤与根系真菌上差异较为明显。由图4可知:有虫草菌属Cordyceps等321个属为4个时期共有真菌;裸盖菇属Psilocybe等29个属为萌芽期和花期共有真菌,复膜孢酵母属Saccharomycopsis等43个属为花期和果期共有真菌,Melanopsamma等36个属为果期和衰亡期共有真菌,Stenella等23个属为衰亡期和萌芽期共有真菌。
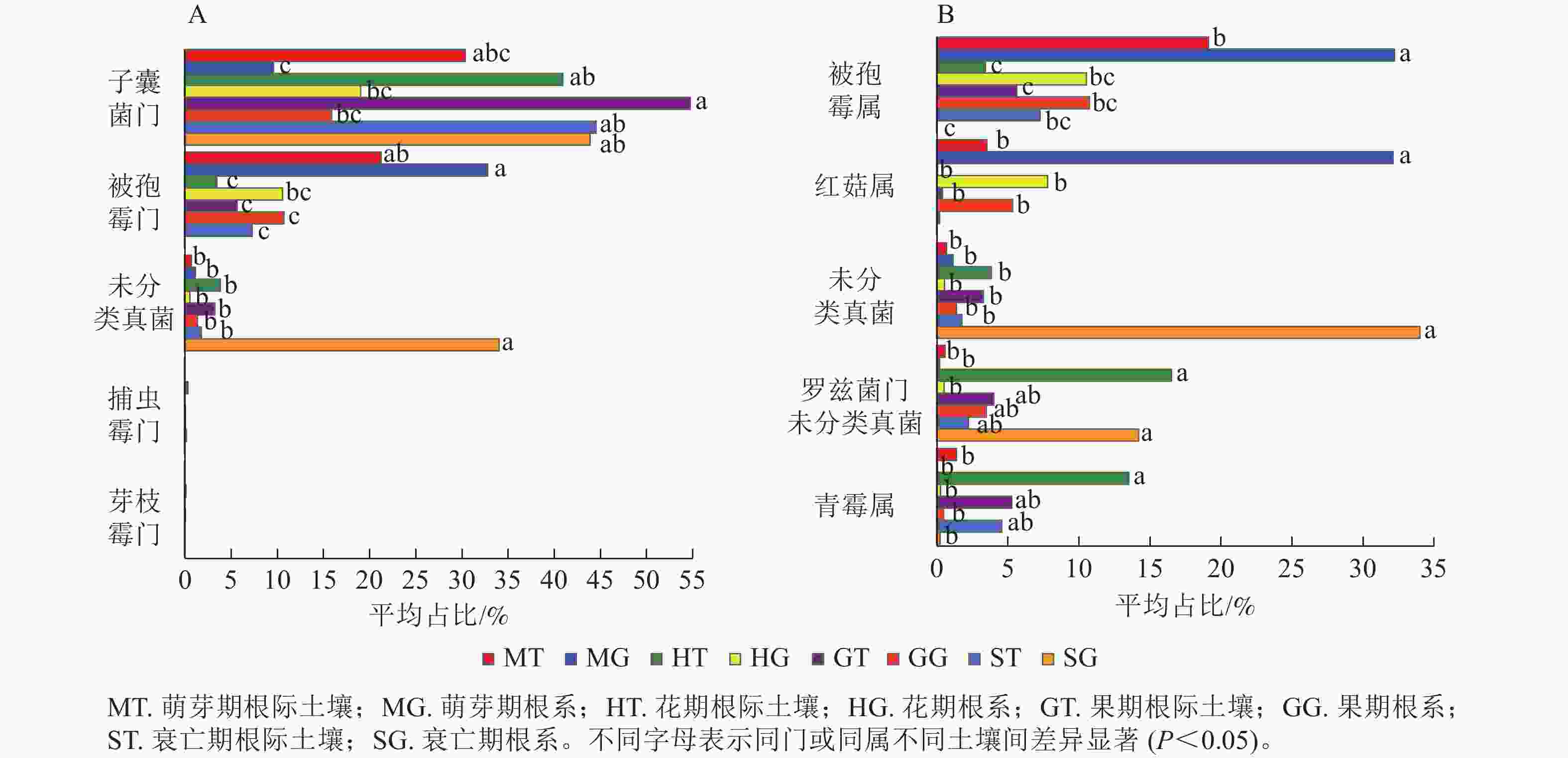
Figure 3. The endophytic fungi in rhizosphere soil and root were different at phylum (A) and genus (B) level

Figure 4. Venn diagram of endophytic fungi distribution in rhizosphere soil and root at genus level at different periods
从表2可见:果期根系内生真菌的ACE指数、Chao指数、Shannon指数和Sobs指数最高,其次是花期,然后是萌芽期,衰亡期Alpha指数最低。意味着果期根系内生真菌多样性和丰富度最高,衰亡期根系内生真菌多样性和丰富度最低。在无距虾脊兰根际土壤真菌中,花期Alpha多样性指数高于其他3个时期,果期ACE指数、Chao指数和Sobs指数高于萌芽期和衰亡期,萌芽期和衰亡期Alpha多样性指数相对一致。综合来讲,花期的根际土壤真菌多样性和丰富度最高,果期次之。
样本 Alpha多样性指数 ACE指数 Chao指数 Shannon指数 Sobs指数 Coverage指数 MG 1 983±26 c 1 379±81 ab 3.734±0.522 b 680±104 cd 0.964±0.004 a HG 4 078±3 043 bc 2 337±50 ab 3.724±1.606 b 987±423 bcd 0.968±0.009 a GG 8 681±439 ab 4 499±351 ab 4.221±0.501 b 1 342±161 abc 0.926±0.006 b SG 817±292 c 630±308 b 3.298±0.788 b 210±31 d 0.990±0.005 a MT 4 642±3 043 bc 3 057±1 539 a 5.042±0.191 a 1 243±322 abc 0.926±0.017 b HT 12 860±9 858 a 7 178±4 937 a 5.423±0.221 b 1 926±847 a 0.891±0.027 c GT 6 559±1 695 abc 4 109±826 ab 4.713±1.078 b 1 580±398 ab 0.931±0.017 b ST 5 182±642 abc 3 323±465 ab 4.713±0.684 b 1 198±229 abc 0.908±0.014 bc 说明:数据均为平均值±标准差。同列不同字母表示同一指数在不同样本间差异显著(P<0.05)。MG. 萌芽期根系;HG. 花期根系;GG. 果期根系;SG. 衰亡期根系;MT. 萌芽期根际土壤;HT. 花期根际土壤;GT. 果期根际土壤;ST. 衰亡期根际土壤。 Table 2. Alpha diversity indexes
在同一时期中,萌芽期、花期和衰亡期根际土壤真菌的Alpha多样性指数均高于根系内生真菌,说明在该时期根际土壤真菌多样性和丰富度高于根系内生真菌。果期根系内生真菌ACE指数、Chao指数高于根际土壤真菌,但是根际土壤真菌Shannon指数和Sobs指数高于根系内生真菌。
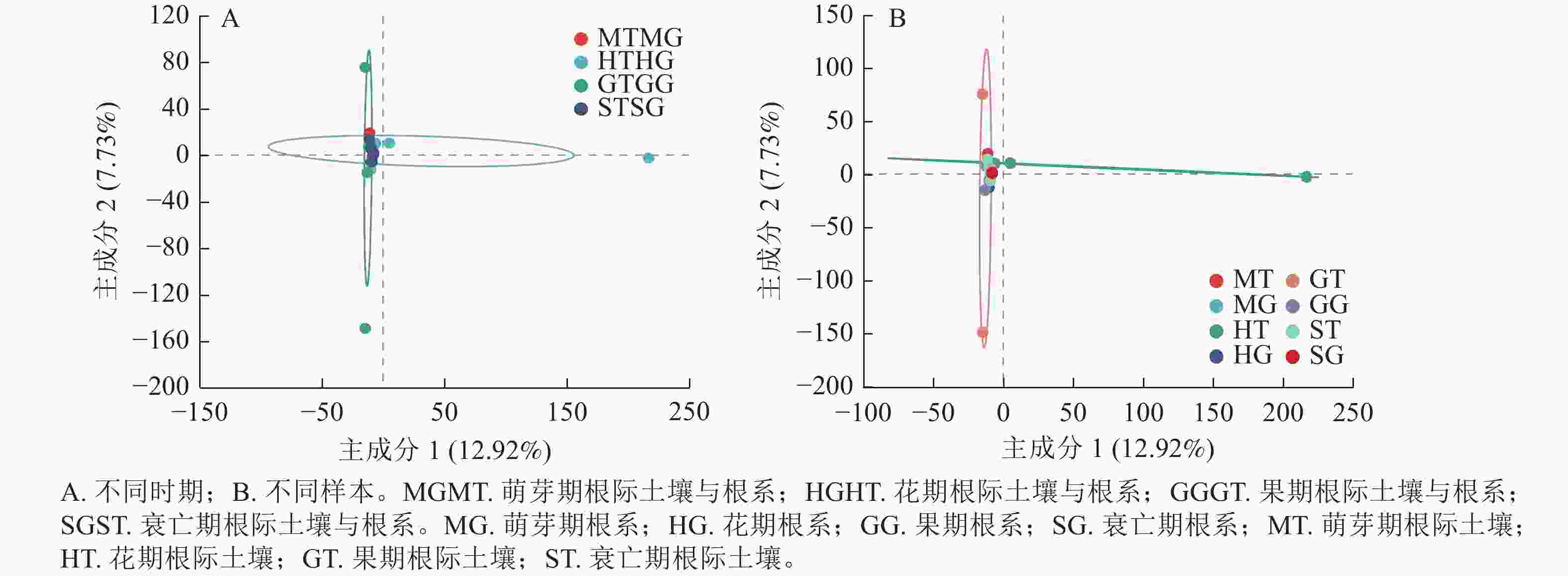
为了进一步明确无距虾脊兰不同时期根际土壤真菌和根系内生真菌群落结构的差异,本研究采用主成分分析(PCA)分析其真菌群落的相似性和差异性。PCA结果显示:无距虾脊兰在花期、果期、萌芽期、衰亡期有明显的聚类区分(图5A),主成分1解释了差异的12.92%,主成分2解释了差异的7.73%。其中在主成分1轴上花期区分较为明显,主成分2轴上果期区分较为明显,萌芽期和衰亡期距离较近,意味着衰亡期和萌芽期根际土壤真菌、根系内生真菌类群相似度高,进一步说明无距虾脊兰根际土壤真菌和根系内生真菌群落在整个生长发育时期存在阶段性差异。由图5B可知:4个时期根系内生真菌相较于根际土壤真菌,距离较近,意味着根际土壤真菌彼此间真菌类群差异较大,其中花期和果期尤为明显。另外,在每个时期中根际土壤与根系之间彼此分开较远,相较于各自内部样品之间两者真菌类群差别较为明显,其中根系样品之间内生真菌类群差别小于根际土壤。
-
本研究表明:无距虾脊兰根际土壤真菌和根系内生真菌优势菌门均为担子菌门和子囊菌门。根际土壤真菌类群与根系内生真菌类群相互重叠存在一定相似性,说明有一部分根系内生真菌来自根际土壤真菌。推测由于根系与根际土壤长期互相接触,彼此真菌类群相互影响[19],且根系对根际土壤真菌具有一定的选择性等原因造成的[20]。从另一方面来讲,彼此之间也相互独立具有一定差异性,根际土壤真菌群落的丰富度、均匀度以及多样性均高于根系内生真菌。在根际处根系经常与其他根系、土壤微生物互作,从而导致了根际土壤真菌多样性高于根内真菌。
在根系内生真菌中,真菌多样性和丰富度从萌芽期到果期依次递增,并在果期达到顶峰,衰亡期又急剧下降。而根际土壤真菌类群变化情况却与之相差较大,从萌芽期到花期逐渐增加,在花期达到顶峰后逐渐下降,衰亡期与萌芽期真菌多样性和丰富度变化相对平稳。这与吕立新等[21]的观点相吻合,夏季真菌多样性高于春季和秋季,随着季节变化,温度、土壤含水量等环境因子相继发生变化,真菌群落结构也随之发生变化。YOKOYA等[22]在研究兰花时也发现了在雨季分离出的真菌,在旱季不一定出现,而同一类真菌的相对丰度也不尽相同。花期为无距虾脊兰生长初期,此时温度适宜、光线充足,周围伴生植物数量增加,土壤和根际土壤真菌丰富度也相应增加。无距虾脊兰果期为每年的7—9月,此时温度高,周围植物数量相较于花期会有所减少,所以果期根际土壤真菌丰富度少于花期。而根系内生真菌出现上述差异情况可能是从萌芽期到果期,无距虾脊兰生长发育需要大量营养物质持续输入,且夏季温度高,需要增加自身抗逆性,因此根系内生真菌种类和丰富度显著增加并在果期达到顶峰。随着冬季的到来,周围植物逐渐进入休眠期,植被凋落物、土壤微生物的分解等都会使土壤有机质增加,从而促进微生物的生长,造成衰亡期真菌群落丰富度的降低[23]。
兰科植物在自然状态下无法自主萌发,内生真菌通过向兰科植物传输营养物质,从而促进种子萌发和原球茎的分化[24]。李孟凯等[25]用镰孢属菌液处理过的铁皮石斛Dendrobium officinale种子胚膨大,种皮有被假根冲破的迹象。内生真菌对兰科植物的促生作用在生长期也有体现。王亚妮[26]研究发现:接种了内生真菌的铁皮石斛,其鲜质量、移栽成活率相比对照均有所增加;此外,张霞[27]研究表明:接种内生真菌的铁皮石斛,其抗旱性明显增强。由此可见,内生真菌在兰科植物生长发育过程中的作用不可忽视。本研究显示:无距虾脊兰不同时期优势真菌及相对丰度不同,推测兰科植物在相应生长期会选择对自己生长益处最大的真菌共生。有研究表明:在碳的需求方面,果期需求是花期的2倍[28],而花期的优势真菌不能满足果期对生长素等方面的需求,所以不同时期优势真菌的相对丰度会随之发生变化,也进一步证实了内生真菌极易受周围环境和生长阶段影响[29]。
镰孢属和青霉属是兰科植物中常见的内生真菌,在适当的条件下不仅能促进兰科植物种子的萌发和生长发育,还可以有效抑制病原菌的生长。这2种真菌类群在花期和衰亡期属于优势真菌,由此可以推测:镰孢属和青霉属在无距虾脊兰中同样可以促进其生长,并提高其抗病性。庄鑫等[30]研究不同地点朝鲜淫羊藿Epimedium brevicornu生长时期内生真菌表明:蜡壳耳属真菌与淫羊藿中黄酮类化学成分含量成正比,证明蜡壳耳属真菌可以促进植物对磷元素的吸收,促进植物生长,同时增强植株抗灰霉病的能力。被孢霉属真菌被证实在生物分解以及土壤养分转化的过程中发挥重要作用[31]。红菇属曾被认定为菌根真菌,并与多种兰科植物存在共生关系[32]。上述几类真菌均为无距虾脊兰不同时期的优势真菌,但是这几类优势真菌是否与无距虾脊兰菌根真菌存在共生关系,是否对无距虾脊兰生长发育过程中起促进作用,还需采用石蜡切片观察是否在根细胞中形成菌丝结,并结合共生培养视植株的生长状况而定。然而,另外一些公认的菌根真菌,如胶膜菌科Tulasnellaceae在本研究中尚未见到,有研究表明可能与DNA测序区域有关[33],在后续实验进行高通量测序时可以采取不同的引物。除此之外,可能与植物种类有关,有些真菌广泛存在于多种植物体内,而有些真菌却只与某种特定植物产生共生关系[34]。
-
本研究表明:在不同生长发育时期,无距虾脊兰真菌群落多样性和丰富度存在较大差异。根际土壤真菌多样性高于根系内生真菌,花期根际土壤真菌多样性最高,果期根系内生真菌最丰富。不同时期优势真菌差别较大,萌芽期根际土壤和根系内生优势真菌分别为被孢霉属、红菇属,花期优势真菌分别为青霉属、粗糙孔菌属,果期分别为Paraboeremia、蜡壳耳属,衰亡期分别为Paraboeremia、镰孢属。
Diversity of rhizosphere soil fungi and root endophytic fungi of Calanthe tsoongiana
doi: 10.11833/j.issn.2095-0756.20230179
- Received Date: 2023-02-28
- Accepted Date: 2023-07-26
- Rev Recd Date: 2023-05-26
- Available Online: 2023-11-23
- Publish Date: 2023-11-23
-
Key words:
- Calanthe tsoongiana /
- endophytic fungi /
- diversity /
- different periods
Abstract:
| Citation: | HE Shuilian, HUANG Bei, LI Tianyuan, et al. Diversity of rhizosphere soil fungi and root endophytic fungi of Calanthe tsoongiana[J]. Journal of Zhejiang A&F University, 2023, 40(6): 1158-1166. DOI: 10.11833/j.issn.2095-0756.20230179 |










 DownLoad:
DownLoad: