-
铁皮石斛Dendrobium catenatum是兰科Orchidaceae石斛属Dendrobium植物,其所含的石斛多糖成分具有抗氧化和抑制癌细胞等药用活性,使其具有重要的经济价值[1-6]。铁皮石斛在野外多附生于高大乔木或山石上,适宜其生长环境条件通常为海拔800~1 600 m,树冠透光率约60%,大气相对湿度60%~75%,日间温度20~25 ℃,夜间温度10~13 ℃,日温差为10~15 ℃,年均降水量为1 100~1 500 mm。目前,铁皮石斛已实现规模化人工种植,常采用温室控温控湿栽培于树皮土壤复合基质等方式[2, 7-9]。据2018年统计,中国人工栽培铁皮石斛的面积已经超过6 667 hm2,产量超1万t[10]。不同的生长环境对铁皮石斛的有效成分会产生影响,一般野外环境更利于铁皮石斛积累有效的次生代谢物[2, 7-11]。因此,近年来越来越多的栽培基地采用模拟野外生长环境,将铁皮石斛附生于立木表面进行栽培[7-8]。众多研究发现:植物内生菌对于宿主植物的营养代谢、抗逆适生和抵御病虫害等方面都发挥重要作用[12-16]。兰科植物通常与菌根真菌共生,铁皮石斛的多种内生真菌具有促进组培植株生长的作用,如镰刀菌属Fusarium内生真菌可产生赤霉素促进铁皮石斛生物量和氮磷养分的积累等[6, 17-20]。此外,分离自铁皮石斛的内生细菌被发现可诱使铁皮石斛种子萌发,具备固氮、解磷等功能[10, 20-21]。挖掘铁皮石斛内生菌资源,深度解析菌群结构与宿主植株的内在关联,对于植株种质选育、栽培方式改进、产量及品质提升等都具有积极意义。本研究探讨了铁皮石斛2种人工栽培方式(温室土壤基质培养和露天立木培养)对铁皮石斛内生细菌菌群结构的影响,并对铁皮石斛根、茎、叶各部多糖质量分数与内生细菌种类分布之间的潜在联系进行了分析,以期为开发具有促进铁皮石斛适生和营养等功能的内生细菌资源提供研究基础。
-
铁皮石斛(品系C9)植株取自浙江省杭州市天目山种植基地。该品系具有较强的抗霜冻能力,以防露天立木培养的植株在10 ℃下受到霜害。于6月花期,采集2种栽培方式下的3年生健康植株,分别为大棚内的盆栽植株(土培,相对湿度50%~75%,温度15~25 ℃)和栖于树干部(马尾松Pinus massoniana)近野生植株(树培,霜期130~156 d,年降水量1 390~1 870 mm)。选取生长状态较一致的植株,分别采集其根、茎、叶、花苞、果实等部分。所采集的铁皮石斛材料,及时用自来水冲洗进行表面处理,用于后续的总DNA提取和多糖测定。
-
分别从土培和树培植株的根、茎、叶、花苞或果实部分(当年新生部分,表面无病斑,每份100 mg)共选取24份样品。将每份样品浸没于5 mL体积分数为70%的乙醇,振荡消毒30 s后,用无菌水振荡清洗3次,每次30 s左右。晾干后,液氮碾磨,随后用Takara细菌基因组提取试剂盒(MiniBEST Bacterial Genomic DNA Extraction Kit Ver. 5.0)提取总DNA。操作步骤按试剂盒手册进行,为使内生菌破壁完全,其中裂解细胞阶段延长作用时间至2.0 h。另准备各部分样品表面消毒后置于R2A培养基上检验表面除菌效果,28 ℃培养2~3 d,无菌落生长则为消毒操作合格。
-
细菌16S V3~V4可变区(450 bp)为测序目标片段,由杭州沃森生物科技有限公司利用Illumina Miseq平台进行高通量测序。用QⅡME 2.0软件去除低质量序列,剩余序列按照相似度97%以上进行分类操作单元(operational taxonomic unit, OTU)聚类,以丰度最大的序列为代表序列。利用Greengenes数据库(版本13.8)和美国国立生物技术信息中心(National Center for Biotechnology Information, NCBI)比对进行序列注释,并除去注释为植株叶绿体和线粒体细胞器16S OTUs信息。统计各样品OTU数量及每个OTU对应的有效序列数,计算相对丰度(占每个样品总序列数的比例),并进行多样性分析。
-
将新鲜的土培和树培铁皮石斛根、茎、叶(每份样品鲜质量100.0 g)在80 ℃烘干2~3 d,粉碎后过筛(60目)收集。将每份样品的粉末分别称取3份(每份为0.3 g),放置于250 mL圆底烧瓶200 mL水中,100 ℃水浴加热2.5 h。将烧瓶内样品进行抽滤处理,滤出液体倒入250 mL容量瓶,待冷却至室温用水进行定容。取5.0 mL滤液与25.0 mL无水乙醇混合后进行4 ℃冷藏1.0 h。样品离心1.0 h(转速6 000 r·min-1),弃上清液后加20.0 mL体积分数为80%的乙醇,离心20 min(转速6 000 r·min-1),重复2次。弃上清液,加100 ℃热水溶解沉淀,转移至50 mL容量瓶中待冷却至室温后,进行定容。量取样品溶液0.5 mL,置于10.0 mL具塞试管中,加入蒸馏水0.5 mL以及体积分数为5 %苯酚溶液1.0 mL,摇匀,再加入浓硫酸5.0 mL摇匀,沸水中加热20 min后冰浴5 min。以蒸馏水为空白对照组,分别取200 μL处理液,测定波长488 nm处各组的光密度。配置不同质量分数多糖溶液,测量光密度,绘制标准曲线。重复3次,计算根、茎、叶各部分多糖质量分数。
-
利用数据统计软件DPS对2种栽培方式下根、茎、叶、花、果各部分的OTUs、多样性指数和多糖质量分数进行方差分析。利用R软件制作韦恩图、热图等。同时,将筛选出来根、茎、叶各部的OTU相对丰度与相应各部多糖质量分数进行逐步回归分析,筛选出与多糖质量分数关联度最高的OTU信息。
-
2种栽培方式下,铁皮石斛各组织部位菌群测序结果分析显示,OTU数量、Shannon-Wiener和Simpson多样性指数,多呈现差异显著(OTU:栽培方式F1, 16=18.405, P=0.000 6;组织部位F3, 16=12.638, P=0.000 2。Shannon-Wiener指数:栽培方式F1, 16=2.504, P=0.133 1;组织部位F3, 16=6.017, P=0.000 6。Simpson指数:栽培方式F1, 16=84.739, P=0.000 1;组织部位F3, 16=34.673, P=0.000 1),说明栽培方式对铁皮石斛内生细菌群落结构产生重要影响,且内生细菌在植株上的分布呈组织特异性。如表 1所示:树培的植株除根部外,其他叶和茎部分OTU数量少于土培环境中的植株;不同组织部分中,叶部、花部的OTU菌群分布数量较少,根、茎、果的部分数量较多。多样性指数方面也呈相似规律。
表 1 2种栽培方式下铁皮石斛内生细菌多样性
Table 1. Diversity of bacterial symbionts of Dendrobium catenatum under two cultivation methods
栽培
方式部位 分类操单元
(OTU)Shannon-Wiener
指数Simpson指数 树培 根 630.67 ± 226.48 a 1.52 ± 0.59 a 0.94 ± 0.09 a 果 447.33 ± 57.84 b 1.58 ± 0.21b 0.92 ± 0.03 b 茎 122.67 ± 26.76 c 0.37 ± 0.20d 0.07 ± 0.04 c 叶 28.33 ± 6.81 c 0.03 ± 0.01 d 0.00 ± 0.00 c 土培 根 112.67 ± 24.19 c 0.34 ± 0.05d 0.06 ± 0.01 c 花 86.67 ± 19.55 c 0.24 ± 0.08d 0.04 ± 0.01 c 茎 310.67 ± 107.58 c 1.62 ± 0.91 c 0.37 ± 0.24 b 叶 73.00 ± 84.92 c 0.27 ± 0.34 d 0.07 ± 0.10 c 说明:数值为3个样品的平均值+标准差;同列不同字母表示差异显著(LSD统计方法,P<0.05) -
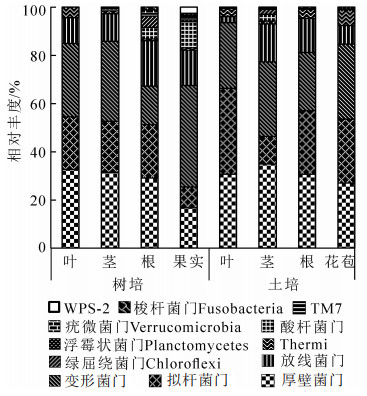
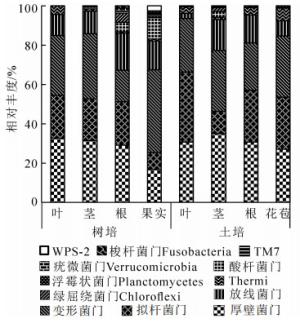
由图 1可见:厚壁菌门Firmicutes(16.7%~34.9%)、拟杆菌门Bacteroidetes(8.7%~35.6%)、变形菌门Proteobacteria(15.9%~42.0%)、放线菌门Actinobacteria(2.6%~19.0%)在各组织部位相对丰度较高。厚壁菌门在土培的茎部占比最高(34.9%),与树培茎部(31.3%)相近。拟杆菌门在土培叶部最高(35.6%),为树培叶部(21.7%)的1.6倍。放线菌门在树培根部最高(19.0%),是土培根部(14.1%)的1.3倍。酸杆菌门Acidobacteria在树培果实部位占比最高,达10.9%,但在叶部和花苞部未检出。
-
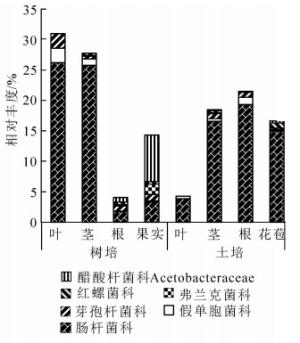
氮元素是植物生长所必需的大量元素,自然条件下常呈局部紧缺状态。红螺菌科Rhodospirillaceae中的固氮螺菌属Azospirillum,弗兰克菌科Frankiaceae中的弗兰克菌属Frankia,肠杆菌科Enterobacteriaceae中的肠杆菌属Enterobacter,假单胞菌科Pseudomonadaceae的假单胞菌属Pseudomonas和芽孢杆菌科Bacillaceae的芽孢杆菌属Bacillus中的部分种类有内生固氮功能。这些科属在铁皮石斛各组织中都有分布,除了红螺菌科和弗兰克菌科不存在于叶部。从2种栽培下铁皮石斛内生细菌功能上看,树培状态下具有这些固氮潜能的OTUs占比明显高于温室土培植株(图 2)。
-
从图 3可见:栽培环境对组织内生细菌优势属(相对丰度大于1.0%)影响较大。栖水菌属Enhydrobacter在树培果中占优势,比例高达35.2%;甲醇杆菌属Methylobacterium在土培叶中为优势菌属,比例达35.8%;薄层菌属Hymenobacter仅在土培叶中分布,占9.9%。拟杆菌属Bacteroides、双歧杆菌属Bifidobacterium、韦荣球菌属Veillonella在各组织部位中多有分布,在树培根部、果实和土培叶部相对丰度较低;颤螺旋菌属Oscillospira在树培茎部分布较少;巨单胞菌属Megamonas在树培茎部相对较多,栖热菌属Thermus则在土培花苞内最多;不动杆菌属Acinetobacter则存在于树培和土培的茎部。
-
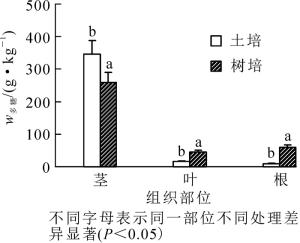
如图 4所示:铁皮石斛茎中多糖质量分数显著高于叶部和根部。方差分析结果显示:2种栽培方式下铁皮石斛不同部位多糖质量分数呈极显著差异(根:F1, 4=11.15, P=0.029;茎:F1, 4=9.44, P=0.037;叶:F1, 4=39.31, P=0.003),说明栽培方式影响多糖在植株内的积累。通过韦恩图发现:不同栽培方式对根、茎、叶菌群结构有明显影响,其中根部和叶部影响大,共有的OTUs占24%~26%,而茎部共有部分占49%,这些OTUs的分布差异可能与多糖质量分数间存在一定关联。将2种栽培方式下根、茎和叶优势内生细菌属相对丰度(x自变量,x1~x53分别表示不同属)和各部多糖质量分数(y因变量)进行相关性分析,得逐步回归方程y=0.058+29.688x17-4.146x13-0.424x52-0.085x2(相关系数R2=0.999 8,P < 0.05)。方程中通过逐步回归筛选出的4个属的相对丰度与多糖质量分数最为密切(系数绝对值越大代表其相关性越高),其中:不动杆菌属(x17)影响最大,其次为假单胞菌属(x13)、薄层菌属(x52)和韦荣球菌属(x2)。
-
在门水平上,不同栽培环境下铁皮石斛的优势内生细菌门类多集中在厚壁菌门、拟杆菌门、变形菌门和放线菌门,且在不同栽培环境和不同组织中占比有所不同,显示内生细菌分布受外界环境和宿主植物组织特异性的影响较大。其中,果实部位与其他部位差异较明显,变形菌门和酸杆菌门的种类占优势,这很可能跟开花和果实发育过程中植株表面环境菌群进入果实内部有关,或与不同组织营养成分种类和含量不同相关。铁皮石斛内生细菌在属水平上显示,拟杆菌属、双歧杆菌属和韦荣球菌属相对丰度占优势,这些属中通常包含了常见的植物源乳酸菌,广泛存在于各类植物中。其中,不同栽培方式下的韦荣球菌属、栖热菌属、颤球菌属和甲醇杆菌属差异较大,这很可能与露天树培环境复杂,风吹、雨溅、虫噬等使得环境中部分菌属进入植株内部。氮元素往往是野外自然条件下植株的生长限制因子,从高通量测序结果看,铁皮石斛具有多种固氮潜能的菌种包括根瘤菌Rhizobiales和弗兰克菌等[20, 22-23]。尤其在树培环境中固氮菌占比显著高于温室土培植株,这很可能与环境中氮养分含量多寡有关,树培环境中固氮菌具有促进铁皮石斛植株生长的可能。
植物内生细菌对宿主植物积累光合产物等糖类物质的有益作用在不同植物上多有报道。例如,甘蔗Saccharum officinarum茎干部的内生细菌具有促植株生长及糖类产物积累的作用[24],内生荧光假单胞菌利于草本药用植物苍术Atractylodes lancea植株内光合产物和多糖的提高[25]。有些内生细菌本身可在植物体内合成大量多糖,如分离自禾本科Gramineae芒草Miscanthus sinensis茎秆部的内生菌Mucilaginibacter endophyticus可产生多种外分泌多糖[26]。本研究的2种栽培环境中,铁皮石斛根、茎、叶各部OTU重叠部分仅为24.2%~49.0%,显示出内生菌群的组织分布差异性。从逐步回归分析结果看:仅4个属的内生细菌与多糖质量分数呈相关性。尽管不同栽培方式下铁皮石斛多糖质量分数有所差别,但与多糖质量分数关联最高的不动杆菌属在2种栽培方式下茎部相对丰度都达1.0%,明显高于根部和叶部。从序列比对结果看:该OTU与约氏不动杆菌Acinetobacter johnsonii一致,后者是一种革兰氏阴性反硝化聚磷耐冷菌,其是否与铁皮石斛C9品系耐冷特性及多糖合成相关有待进一步验证[27]。与多糖质量分数相关联仅次于不动杆菌属的是假单胞菌属,但与多糖质量分数呈负相关。该属细菌往往具有固氮、高分子多聚物降解等多种生理功能,铁皮石斛内发现的这株假单胞菌很可能参与糖类分解代谢,不利于多糖积累。这与苍术中发现的促生假单胞菌的作用不同,说明同属菌种在不同宿主植物中作用的多样性。
综上所述,不同栽培条件对铁皮石斛内生细菌菌群结构有显著影响,功能上固氮和耐冷等与宿主营养和抗逆相关的属种变化明显,说明内生细菌群对铁皮石斛适应环境很可能发挥重要作用。同时,发现相对丰度与多糖质量分数相关的4个OTUs,为进一步分析铁皮石斛内生细菌功能与多糖积累之间的关联提供启示。
Effects of two cultivation environments on diversity of bacterial symbionts in Dendrobium catenatum
-
摘要:
目的 揭示不同栽培环境对铁皮石斛Dendrobium catenatum内生细菌群落结构的影响,解析与石斛多糖含量存在潜在关联的功能菌种。 方法 针对温室人工基质培养和仿野生立木附生培养2种栽培体系下的铁皮石斛C9品系根、茎、叶等各部组织内生细菌,采用Illumina测序平台的Miseq进行高通量测序分析其菌落结构和多样性,同时利用逐步回归法分析内生细菌分布与组织多糖质量分数的关联性。 结果 铁皮石斛在2种栽培环境不同组织中的菌群结构差异显著(P < 0.05),附生立木仿野生环境比温室环境下物种丰富度更高。同时,根部所含分类操作单元(OTUs)数量最多(112~630个),叶部则最少(28~73个)。其中,厚壁菌门Firmicutes(16.7%~34.9%)、拟杆菌门Bacteroidetes(8.7%~35.6%)和变形菌门Proteobacteria(15.9%~42.0%)的OTUs占优势。从菌种生理功能上看,固氮相关的菌种如弗兰克菌科Frankiaceae在树培环境下相对丰度更高,推测可能受树培寡氮环境的影响使固氮菌富集。逐步回归分析结果显示:不动杆菌Acinetobacter在根、茎、叶不同组织中的相对丰度与多糖质量分数变化关联度最大,其次为假单胞菌Pseudomonas。 结论 铁皮石斛内生细菌资源相当丰富,而栽培方式影响菌群分布,且与石斛多糖质量分数具有关联性。 Abstract:Objective Aimed to discover the effect of different cultivation measures on endophytic bacterial communities and polysaccharides accumulation in Dendrobium catenatum, this research is conducted to compare D. catenatum in two environments of soil cultivation in green house and truck-attaching cultivation in open field. Method With the Miseq high-throughput sequencing on Illumina platform conducted, efforts are made to discover the community structures, diversity, and potential functions of bacterial symbionts in different tissues such as roots, stems and leaves of D. catenatum. Besides, the correlation of polysaccharide content with bacterial community was also analyzed employing the stepwise method. Result (1) There is significant difference (P < 0.05) in bacterial communities in different tissues with different cultivation environments; (2) The bacterial richness was higher in truck-attaching cultivation than that in greenhouse, and the operational taxonomic units (OTUs) in root tissues are the largest in number (112-630 OTUs) while those in leaf tissues (28-73 OTUs) are the smallest in number. Comparatively, the OTUs in phyla of Firmicutes (16.7%-34.9%), Bacteriodetes (8.7%-35.6%) and Proteobacteria (15.9%-42.0%) dominate in tissues; (3) Physiologically, OTUs of nitrogen-fixation related bacteria such as Frankiaceae are larger in number in truck-attaching cultivation environment, reflective of the influence of nitrogen-lacking environment. The stepwise procedure results have shown that the relative abundance of Acinetobacter has the highest correlation with the polysaccharide contents in different tissues of root, stem and leaf, followed by Pseudomonas. Conclusion D. catenatum are rich in endophytic bacteria, cultivation measures exert effect on the bacterial community and some bacteria are correlated with the polysaccharides contents. These results shall shed light on further exploration of endophytic bacterial resources in D. catenatum. -
表 1 2种栽培方式下铁皮石斛内生细菌多样性
Table 1. Diversity of bacterial symbionts of Dendrobium catenatum under two cultivation methods
栽培
方式部位 分类操单元
(OTU)Shannon-Wiener
指数Simpson指数 树培 根 630.67 ± 226.48 a 1.52 ± 0.59 a 0.94 ± 0.09 a 果 447.33 ± 57.84 b 1.58 ± 0.21b 0.92 ± 0.03 b 茎 122.67 ± 26.76 c 0.37 ± 0.20d 0.07 ± 0.04 c 叶 28.33 ± 6.81 c 0.03 ± 0.01 d 0.00 ± 0.00 c 土培 根 112.67 ± 24.19 c 0.34 ± 0.05d 0.06 ± 0.01 c 花 86.67 ± 19.55 c 0.24 ± 0.08d 0.04 ± 0.01 c 茎 310.67 ± 107.58 c 1.62 ± 0.91 c 0.37 ± 0.24 b 叶 73.00 ± 84.92 c 0.27 ± 0.34 d 0.07 ± 0.10 c 说明:数值为3个样品的平均值+标准差;同列不同字母表示差异显著(LSD统计方法,P<0.05) -
[1] 陈晓梅, 王春兰, 杨峻山, 等.铁皮石斛化学成分及其分析的研究进展[J].中国药学杂志, 2013, 48(19):1634-1640. CHEN Xiaomei, WANG Chunlan, YANG Junshan, et al. Research progress on chemical composition and chemical analysis of Dendrobium officinale[J]. Chin Pharm J, 2013, 48(19):1634-1640. [2] 王玲, 唐德强, 王佳佳, 等.铁皮石斛原球茎与野生铁皮石斛多糖的抗菌及体外抗氧化活性比较[J].西北农林科技大学学报(自然科学版), 2016, 44(6):167-172, 180. WANG Ling, TANG Deqiang, WANG Jiajia, et al. Comparison of antibacterial and in vitro antioxidant activities of polysaccharides from Dendrobium candidum protocorms and wild Dendrobium[J]. J Northwest A&F Univ Nat Sci Ed, 2016, 44(6):167-172, 180. [3] XIA Linjing, LIU Xiaofei, GUO Huiyuan, et al. Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale (Tiepishihu) in vitro[J]. J Funct Foods, 2012, 4(1):294-301. [4] XING Shangping, ZHANG Xiaofeng, KE Hannu, et al. Physicochemical properties of polysaccharides from Dendrobium officinale by fractional precipitation and their preliminary antioxidant and anti-HepG2 cells activities in vitro[J]. Chem Cent J, 2018, 12:100. doi:10.1186/s13065-018-0471-9. [5] NG T B, LIU Jingyi, WONG J H, et al. Review of research on Dendrobium, a prized folk medicine[J]. Appl Microbiol Biotechnol, 2012, 93(5):1795-1803. [6] YAN Liang, WANG Xiao, LIU Hui, et al. The genome of Dendrobium officinale illuminates the biology of the important traditional Chinese Orchid herb[J]. Mol Plant, 2015, 8(6):922-934. [7] 陈晓梅, 田丽霞, 单婷婷, 等.铁皮石斛种质资源和遗传育种研究进展[J].药学学报, 2018, 53(9):1493-1503. CHEN Xiaomei, TIAN Lixia, SHAN Tingting, et al. Advances in germplasm resources and genetics and breeding of Dendrobium officinale[J]. Acta Pharm Sin, 2018, 53(9):1493-1503. [8] 王淑媛, 吴令上, 董洪秀, 等.铁皮石斛种质和附生立木对其内生真菌菌群的影响[J].中国中药杂志, 2018, 43(8):1588-1595. WANG Shuyuan, WU Lingshang, DONG Hongxiu, et al. Effects of germplasm and host tree trunk on endophytic fungal communities in epiphytic Dendrobium catenatum[J]. China J Chin Mater Med, 2018, 43(8):1588-1595. [9] 李俊, 陈秀秀, 王英瑛, 等.不同品种、产地和栽培基质铁皮石斛的指纹图谱研究[J].中药新药与临床药理, 2019, 30(2):206-209. LI Jun, CHEN Xiuxiu, WANG Yingying, et al. Fingerprint of Dendrobium officinale from different varieties, origins and cultural substrates[J]. Tradit Chin Drug Res Clin Pharm, 2019, 30(2):206-209. [10] 陈晓梅, 田丽霞, 单婷婷, 等.铁皮石斛种质资源和遗传育种研究进展[J].药学学报, 2018, 53(9):1493-1503. CHEN Xiaomei, TIAN Lixia, SHAN Tingting, et al. Advances in germplasm resources and genetics and breeding of Dendrobium officinale[J]. Acta Pharm Sin, 2018, 53(9):1493-1503. [11] DING Jinting, TU Hangyao, ZANG Zelin, et al. Precise control and prediction of the greenhouse growth environment of Dendrobium candidum[J]. Comput Electron Agric, 2018, 151:453-459. [12] van der HEIJDEN M G, de BRUIN S, LUCKERHOFF L, et al. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment[J]. ISME J, 2015, 10(2):389-399. [13] 林标声, 宋昭昭, 张丽丽, 等.巨菌草不同生长时期根、茎、叶内生细菌组成的多样性及差异[J].福建农林大学学报(自然科学版), 2018, 47(3):352-360. LIN Biaosheng, SONG Zhaozhao, ZHANG Lili, et al. Composition diversity and differences of endophytic bacteria in root, stem and leaf at different growth stages of Pennisetum sp.[J]. J Fujian Agric For Univ Nat Sci Ed, 2018, 47(3):352-360. [14] 陈丽莹, 张玉满, 陈晓英, 等.茶树叶际选择性富集的内生细菌的鉴定[J].微生物学报, 2018, 58(10):1776-1785. CHEN Liying, ZHANG Yuman, CHEN Xiaoying, et al. Identification of endophytic bacteria selectively enriched in Camellia sinensis leaf[J]. Acta Microbiol Sin, 2018, 58(10):1776-1785. [15] 刘学周, 赵智灵, 李绍宾, 等.西洋参内生菌群落结构与多样性[J].微生物学报, 2015, 55(3):330-340. LIU Xuezhou, ZHAO Zhiling, LI Shaobin, et al. The community structure and diversity of the endophytes in American ginseng[J]. Acta Microbiol Sin, 2015, 55(3):330-340. [16] 李盼盼, 袁晓龙, 李金海, 等.湖北烟草内生真菌生物多样性和种群结构分析[J].微生物学报, 2018, 58(10):1853-1863. LI Panpan, YUAN Xiaolong, LI Jinhai, et al. Biodiversity and community structure of endophytic fungi isolated from Nicotiana tabacum L.[J]. Acta Microbiol Sin, 2018, 58(10):1853-1863. [17] 胡克兴, 侯晓强, 郭顺星.铁皮石斛内生真菌分布[J].微生物学通报, 2010, 37(1):37-42. HU Kexing, HOU Xiaoqiang, GUO Shunxing. Distribution of endophytic fungi in Dendrobium officinale[J]. Microbiol China, 2010, 37(1):37-42. [18] ZHU Bo, WU Lingshang, WAN Haitong, et al. Fungal elicitors stimulate biomass and active ingredients accumulation in Dendrobium catenatum plantlets[J]. Biologia, 2018, 73(10):917-926. [19] SHI Sanjun, LI Yuanyuan, MING Yue, et al. Biological activity and chemical composition of the endophytic fungus Fusarium sp. TP-G1 obtained from the root of Dendrobium officinale Kimura et Migo[J]. Rec Nat Prod, 2018, 12(6):549-556. [20] 杨波, 陈晏, 李霞, 等.植物内生菌促进宿主氮吸收与代谢研究进展[J].生态学报, 2013, 33(9):2656-2664. YANG Bo, CHEN Yan, LI Xia, et al. Research progress on endophyte-promoted plant nitrogen assimilation and metabolism[J]. Acta Ecol Sin, 2013, 33(9):2656-2664. [21] 陈泽斌, 李冰, 高熹, 等.铁皮石斛不同部位内生细菌群落高通量分析[J].河南农业科学, 2017, 46(11):98-102, 112. CHEN Zebin, LI Bing, GAO Xi, et al. High throughput analysis of the endophytic bacterial community in different parts of Dendrobium candidum[J]. J Henan Agric Sci, 2017, 46(11):98-102, 112. [22] KUYKENDALL L D, YOUNG J M, MARTINEZ-ROMERO E, et al. Genus rhizobium, a highly divergent genus in a revised family, the Rhizobiaceae[C]//GARRITY G. Bergey's Manual of Systematic Bacteriology(2nd Vol). 2nd Ed. New York: Springer-Verlag, 2005: 324-340. [23] SCHWINTZER C R, TJEPKEMA J D. Factors affecting the acetylene to I5N2 conversion ratio in root nodules of Myrica gale L.[J]. Plant Physiol, 1994, 106(3):1041-1047. [24] DOS-SANTOS C M, de SOUZA D G, BALSANELLI E, et al. A culture-independent approach to enrich endophytic bacterial cells from sugarcane stems for community characterization[J]. Microb Ecol, 2017, 74(2):453-465. [25] ZHOU Jiayu, SUN Kai, CHEN Fei, et al. Endophytic Pseudomonas induces metabolic flux changes that enhance medicinal sesquiterpenoid accumulation in Atractylodes lancea[J]. Plant Physiol Biochem, 2018, 130:473-481. [26] ZHANG Zhendong, SUN Feng, CHEN Yan, et al. Mucilaginibacter endophyticus sp. nov., an endophytic polysaccharide-producing bacterium isolated from a stem of Miscanthus sinensis[J]. Antonie van Leeuwenhoek, 2019, 112:1087-1094. [27] 辛小雪, 王雪香, 李明宇, 等.铁皮石斛不同花期及花朵不同部位活性组分分析[J].浙江农林大学学报, 2019, 36(1):200-205. XIN Xiaoxue, WANG Xuexiang, LI Mingyu, et al. Active components of flowers in different flowering stages and floral structures of Dendrobium officinale[J]. J Zhejiang A&F Univ, 2019, 36(1):200-205. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2020.02.012






 下载:
下载: