-
干旱作为植物重要的非生物胁迫之一,是全球面临的重大环境问题,严重影响植物的生长、发育和生存[1]。植物会通过形态、生理生化、光合作用及分子水平等层次的响应抵御干旱胁迫[2−9]。植物受干旱胁迫伤害时,脂膜过氧化导致丙二醛(MDA)积累,使蛋白质和核酸变性,导致膜流动性降低,膜透性增强,因此MDA的多少可衡量植物细胞受伤害的程度[10];活性氧保护酶过氧化物酶(POD)、超氧化物歧化酶(SOD)和过氧化氢酶(CAT)协同作用可有效清除植物体内过多的自由基,从而维持植物体内活性氧代谢系统的平衡[11−12];植物还会积累可溶性糖等渗透调节物质以提高细胞保水能力,从而维持细胞的正常生理过程来响应干旱胁迫[13−15];干旱胁迫还能破坏植物水分代谢,导致叶绿素分解,影响光能电子传递和转换、光合磷酸化及暗反应等过程,使植物光合速率下降,严重时还可导致叶绿体光合机构的破坏,对植物体造成不可逆影响。叶绿素荧光参数对干旱胁迫的响应非常灵敏,可作为监测光合作用过程中光能的吸收、传递、耗散和分配的指标,判断植物干旱胁迫的程度;董斌等[16]利用叶绿素荧光检测油茶Camellia oleifera的干旱抗性,选育出抗干旱的油茶品种。
植物根系利用细胞水势低于土壤水势的原理进行吸水,聚乙二醇6000(PEG 6000)可降低溶液水势,根系不易从周围吸收水分,从而造成干旱胁迫[17]。已有学者[18−19]利用不同质量分数PEG 6000模拟不同程度的干旱胁迫对小麦Triticum aestvum和玉米Zea mays的影响。铁皮石斛Dendrobium candidum是兰科Orchidaceae多年生草本植物,兼具很高的药用和观赏价值,分布于中国安徽、浙江、福建等地[20−21]。目前,已将铁皮石斛野生资源成功进行驯化和人工移栽,实现了铁皮石斛的大面积规模化产业种植和近野生栽培,不仅提高了铁皮石斛的经济价值,还推动中国中药材产业的可持续发展[22]。在铁皮石斛产业化种植和近野生栽培过程中,水分是一个重要的限制性生态因子,因缺水引起的干旱严重影响铁皮石斛的产量和品质,因此了解干旱胁迫下铁皮石斛生理生化和叶绿素荧光参数的变化特征,对研究铁皮石斛耐干旱机制及铁皮石斛的近野生栽培意义重大。近年来,已有关于干旱对铁皮石斛的生长和生理特性变化的报道。阮凌暄等[23]研究发现:随着PEG 6000模拟干旱胁迫时间的延长和胁迫强度的增加,3年生盆栽铁皮石斛叶片活性氧积累呈上升趋势,保护酶POD和SOD活性先增加后降低,第8天时达到最大值,叶片叶绿素最大荧光(Fm)、最大光化学效率(Fv/Fm)和光化学猝灭系数(qp)逐渐降低,光反应能力降低。吕朝燕等[24]研究指出:3年生盆栽铁皮石斛叶片净光合速率和气孔导度随干旱胁迫时间的延长呈先升高后降低的趋势,而胞间二氧化碳 (CO2)摩尔分数和蒸腾速率则相反,呈先降低再升高的趋势,认为铁皮石斛为干旱避免型植物。为进一步探讨铁皮石斛抵抗干旱胁迫的生理特性,本研究拟通过不同质量分数PEG 6000模拟干旱胁迫,对铁皮石斛幼苗MDA、可溶性糖和可溶性蛋白、POD、CAT等生理生化指标和叶绿素质量分数、叶绿素荧光参数等进行分析,探讨铁皮石斛幼苗抗旱的生理和叶绿素荧光特性,以期为铁皮石斛的品种选育及产业化栽培和近野生栽培技术的建立提供参考。
-
试验材料铁皮石斛‘晶品1号’D. candidum ‘Jingpin No. 1’幼苗于光照1500~3000 lx和(25±2) ℃的植物组织培养室培养60 d,每天光照和黑暗时间分别为14 h和10 h。
-
在MS液体培养基中添加不同质量分数PEG 6000(5%、10%、20%、30%)模拟干旱胁迫,以不含PEG 6000 的MS液体培养基为对照(ck)[25]。选择长势良好,生长较一致的‘晶品1号’幼苗,接种于不同质量分数PEG 6000的培养液中进行培养,光照1500~3000 lx,每天光照和黑暗时间分别为 14 h和10 h,培养温度(25±2) ℃,每个处理60株,处理后每隔2 d定时取5株植株叶片和茎段观察细胞学结构(分别记为D2、D4、D6、D8、D10和D12);取5株混合样测定铁皮石斛幼苗的MDA、POD、CAT、可溶性糖、可溶性蛋白和叶绿素等各项生理生化指标,选择5株植株测定叶片的叶绿素各荧光参数。每个处理3个生物学重复。
-
采用琼脂糖包埋法对铁皮石斛‘晶品1号’茎段和叶片进行固定。具体做法为:试验当天将新鲜茎段和叶片样品置于磷酸盐(PBS)缓冲溶液(pH 7.4)中,配制质量分数为5%琼脂糖凝胶,待凝胶温度为50~60 ℃时,将新鲜茎段和叶片置于凝胶中,于室温冷却至凝胶凝固,完成样品的固定;将固定好的茎段和叶片置于震荡切片机上进行横切,切片厚度为50~100 μm,切好的茎段和叶片横切片置于载玻片上,滴加1滴PBS缓冲液(pH 7.4),盖上盖玻片,于莱卡荧光显微镜(Leica DM-4000)下观察茎段和叶片的细胞结构变化,并拍照记录[26]。
-
采用硫代巴比妥酸法(TBA法)测定丙二醛(MDA)质量摩尔浓度[27−28];采用愈创木酚法测定POD酶活性;紫外分光光度法的CAT活性测定[11];蒽酮法测定茎段的可溶性糖质量分数[29];考马斯亮蓝比色法测定可溶性蛋白质量分数[30]。
-
用紫外分光度法测定叶片的叶绿素质量分数[31]。分别取不同处理‘晶品1号’幼苗叶片,去叶脉叶缘,剪碎叶片,将0.1 g新鲜叶片放入10 mL离心管,加入8 mL体积分数为95% 乙醇提取液,避光浸提 24 h后,测定波长 663、645和470 nm处吸光度,并计算叶绿素质量分数。
-
采用便携式调制叶绿素荧光仪(PAM-2500),于9:00—11:00,选择从上往下数的第3片叶子,先用暗适应夹夹住叶片暗适应20 min,然后开始测定不同质量分数PEG 6000处理的铁皮石斛‘晶品1号’幼苗叶片的电子传递速率(ETR)、qP、光合效率(α)、非化学猝灭系数(qNP)和Fv/Fm等叶绿素荧光参数。每个处理测定5株,每株测定3次,取平均值。
-
通过Excel 2018 整理所有原始数据,Graphpad 8.0作图,SPSS 20 进行方差分析,并采用单因素方差分析法和新复极差法对数据进行差异性分析和显著性比较,误差线用标准差表示。显著性水平为0.05。
-
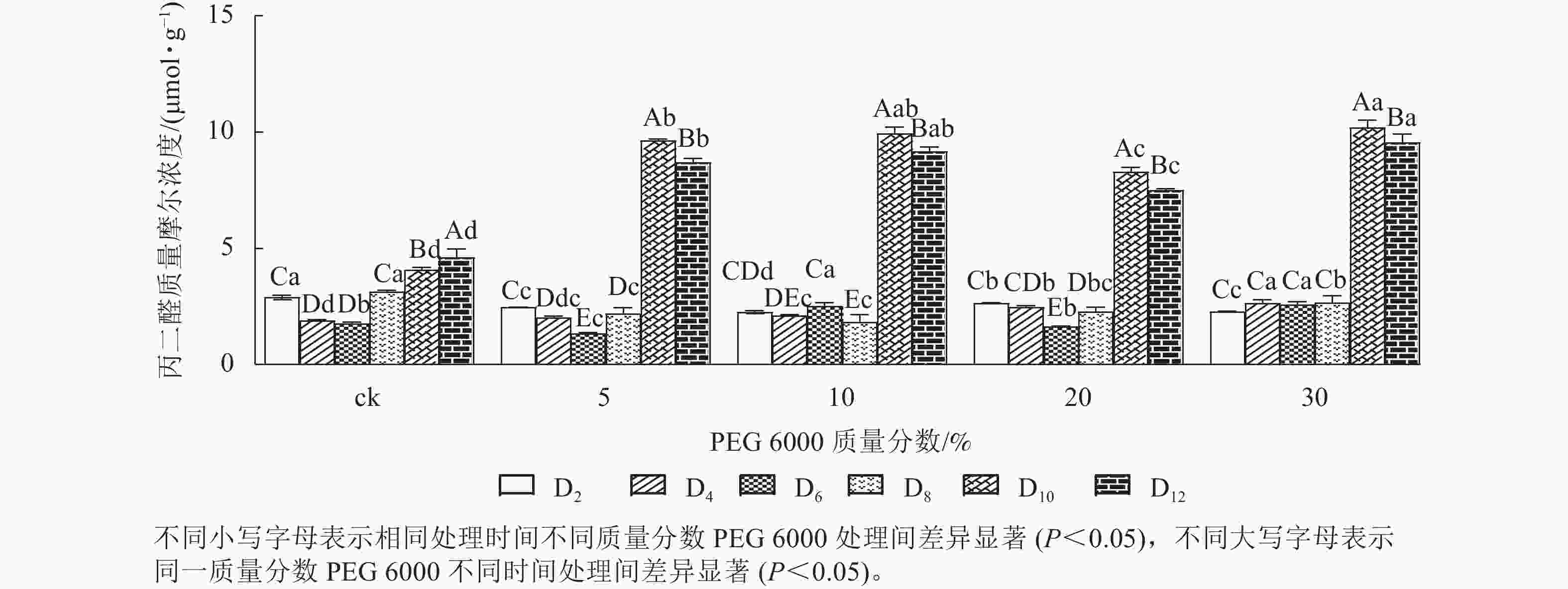
PEG 6000模拟干旱胁迫处理不同时间对铁皮石斛幼苗丙二醛质量摩尔浓度的影响差异显著(P<0.05)。不同质量分数PEG 6000模拟干旱胁迫处理2~8 d时的铁皮石斛幼苗茎段丙二醛比较稳定,说明短时间(≤8 d)的不同质量分数PEG 6000模拟干旱胁迫处理对铁皮石斛细胞膜的影响较小,而到第8天时丙二醛急剧上升,到第10天时达最大值,比同期ck组分别增加2.36 (5% PEG 6000)、2.43 (10% PEG 6000)、2.03 (20% PEG 6000)和2.50倍 (30% PEG 6000)(图1),说明不同质量分数PEG 6000模拟干旱胁迫对铁皮石斛幼苗细胞膜造成不同程度的伤害,PEG 6000质量分数越高(30%)对铁皮石斛细胞膜的伤害越大,抵御干旱的能力下降。
-
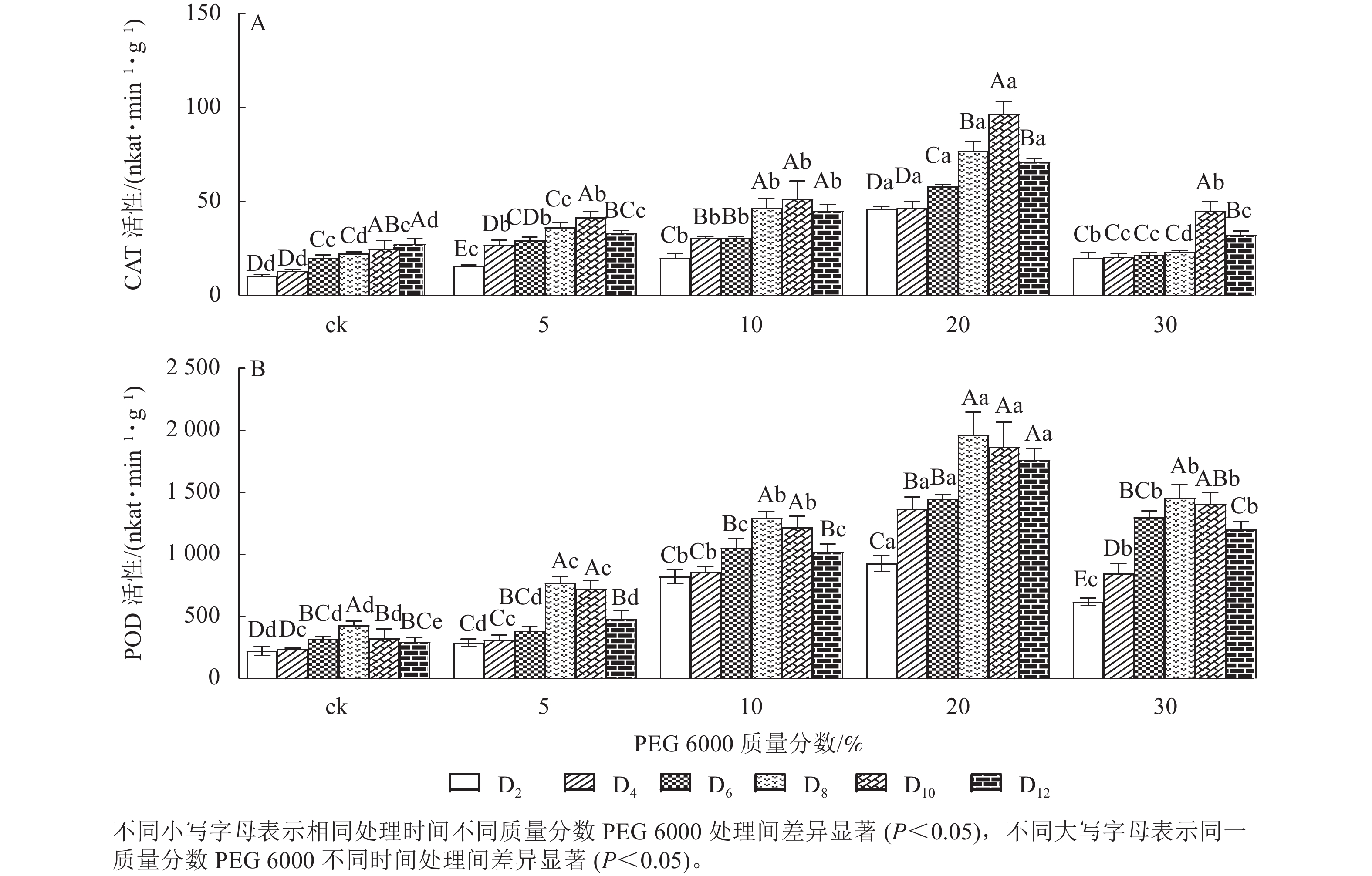
PEG 6000模拟干旱胁迫处理不同时间对铁皮石斛幼苗CAT和POD活性的影响差异显著(P<0.05)。由图2A可以看出:铁皮石斛幼苗CAT活性随着不同质量分数PEG 6000处理时间的延长呈先上升后下降的趋势,且在处理10 d时CAT活性达到最高值,12 d时CAT活性下降(图2A);铁皮石斛幼苗CAT活性随着PEG 6000质量分数的增加呈先上升后下降的趋势,PEG 6000质量分数为20%时CAT活性明显高于其他处理,且在处理第10天时,CAT活性是ck组的3.87倍;PEG 6000质量分数为30%时CAT活性降低,可能由于高质量分数PEG 6000可使细胞脱水,严重影响细胞正常生活,降低CAT活性。
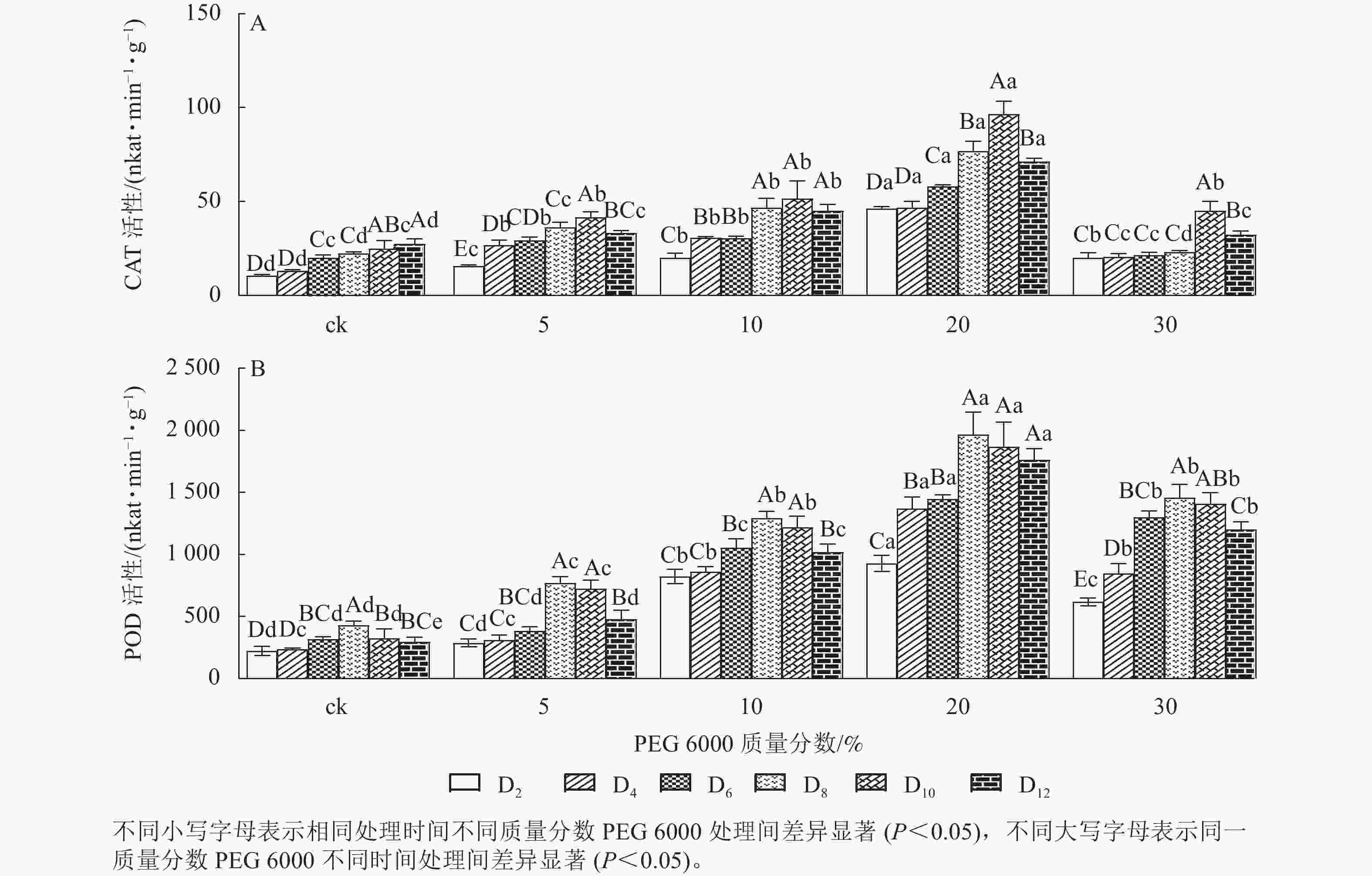
Figure 2. Changes of CAT and POD enzyme activities in D. candidum seedlings after PEG 6000 simulated drought stress
由图2A和图2B可知:PEG 6000模拟干旱胁迫后,铁皮石斛幼苗POD活性的变化趋势与CAT活性基本一致。没有PEG 6000处理的铁皮石斛幼苗的POD活性几乎保持不变,随着PEG 6000处理时间延长,铁皮石斛POD活性均呈现先上升后下降的趋势,在处理第8天时达到最高值(图2B),而后开始缓慢下降;PEG 6000模拟干旱处理后铁皮石斛幼苗POD活性均高于ck组,且随着PEG 6000质量分数的增加,POD活性呈先上升后下降的趋势,当PEG 6000质量分数为20%时达到峰值,为119×16.67 nkat·min−1·g−1 (图2B)。
-
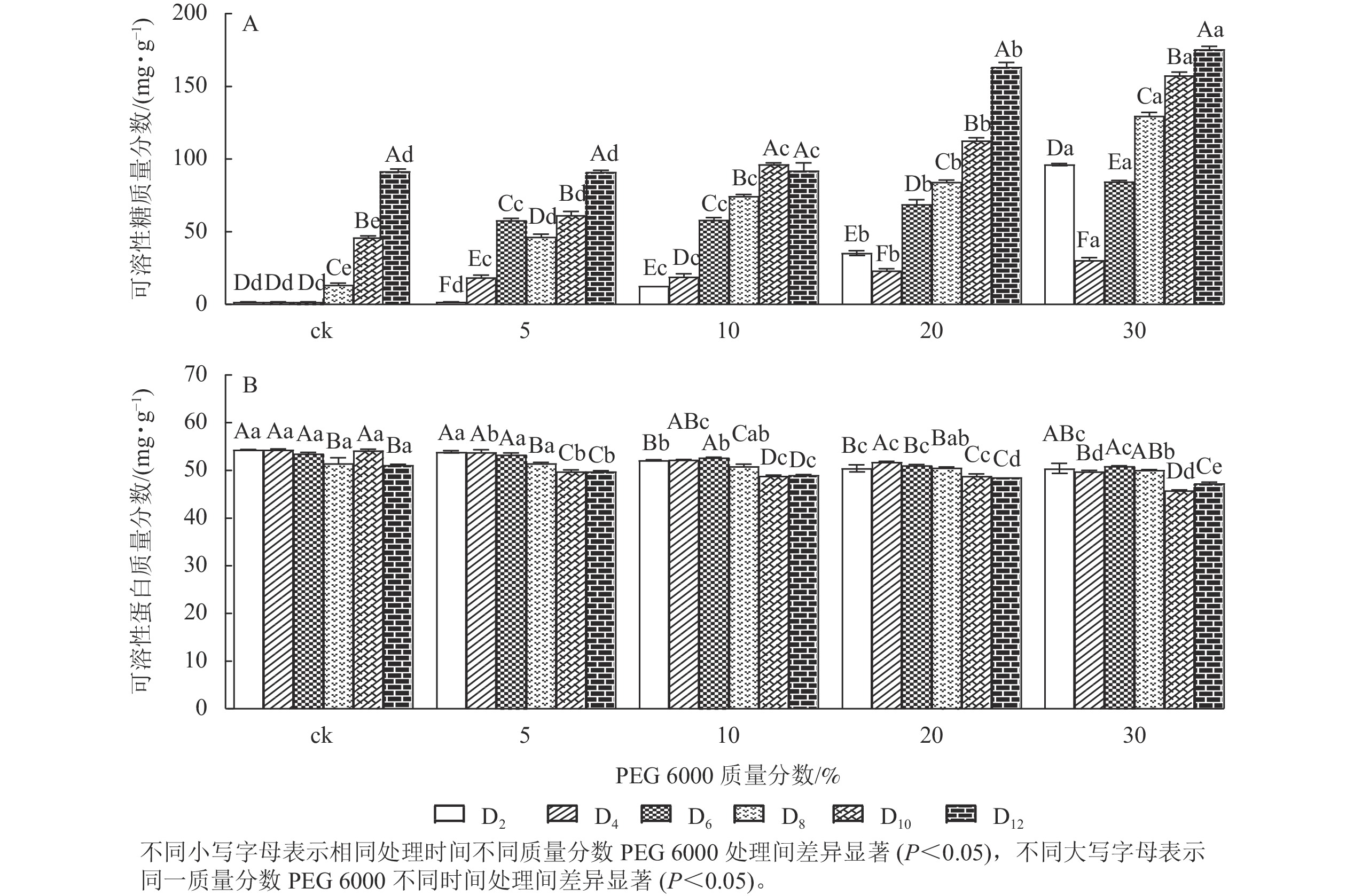
PEG 6000模拟干旱胁迫处理不同时间对铁皮石斛幼苗可溶性糖和可溶性蛋白质量分数的影响差异显著(P<0.05)。可溶性糖不仅能够为植物提供碳源物质,还能维持一定的渗透压。从图3A可看出:铁皮石斛幼苗的可溶性糖质量分数随着PEG 6000质量分数的增加和处理时间的延长均呈上升趋势,到第12天时达最高,且随着PEG 6000质量分数的增加,可溶性糖质量分数呈上升趋势,PEG 6000为30%时,可溶性糖上升显著,说明铁皮石斛植株受到不同质量分数PEG 6000模拟干旱胁迫时,通过增加可溶性糖来维持植物体内细胞的渗透压。
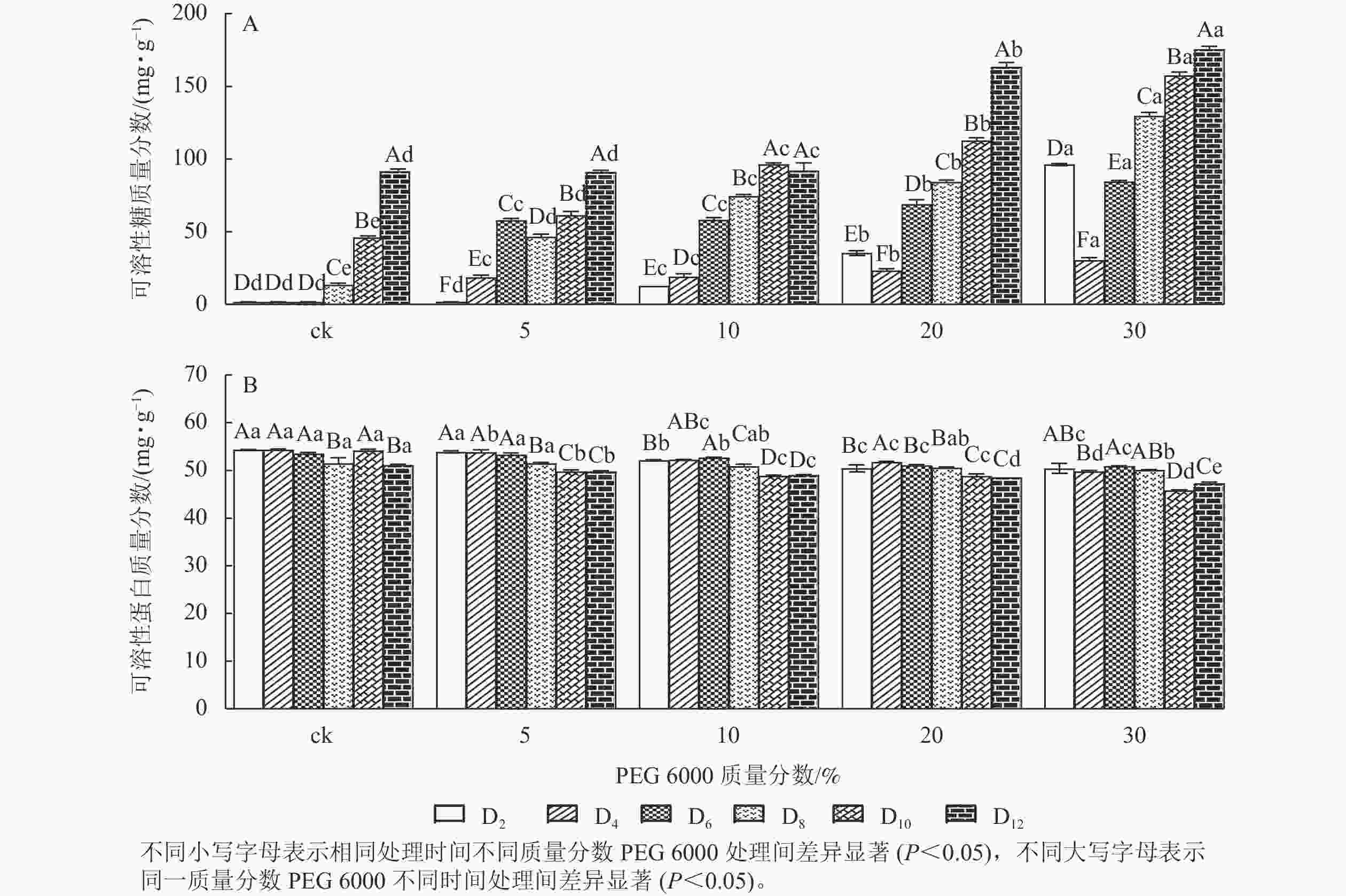
Figure 3. Changes of soluble sugar and soluble protein contents in D. candidum seedlings after PEG 6000 simulated drought stress
从图3B可知:不同质量分数PEG 6000处理对铁皮石斛幼苗的可溶性蛋白质量分数有显著影响(P<0.05),且随着PEG 6000处理时间的延长,铁皮石斛幼苗的可溶性蛋白质量分数均呈下降趋势,且在处理相同时间,PEG 6000质量分数越高,铁皮石斛幼苗的可溶蛋白质量分数越少。当PEG 6000质量分数为30%时,可溶性蛋白质量分数在第8~10天时下降。此时蛋白质分解加速,合成减少,说明铁皮石斛幼苗可在一定程度上消耗可溶性蛋白来调节适应 PEG 6000的胁迫处理,但需要一定的胁迫强度和时间。
-
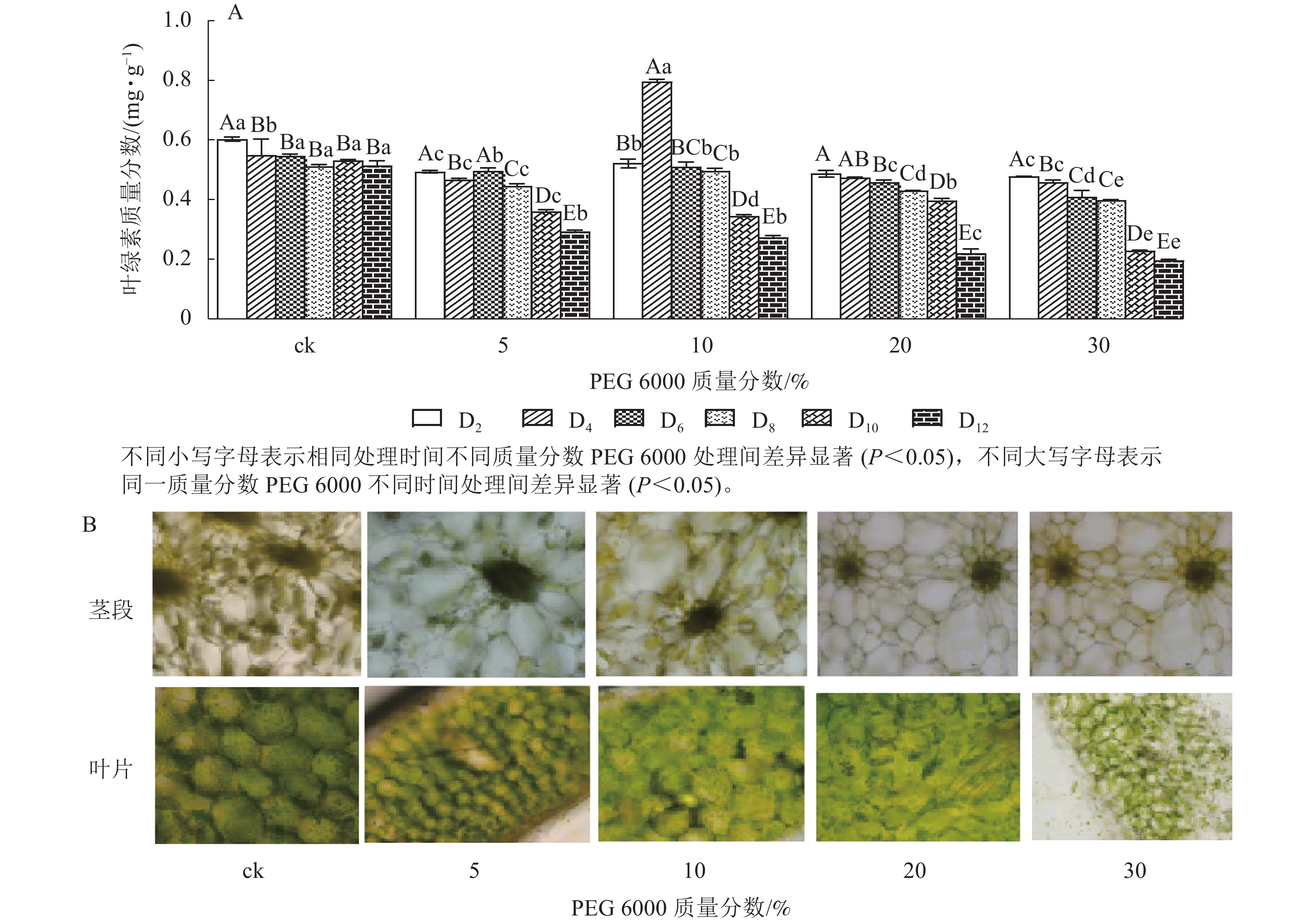
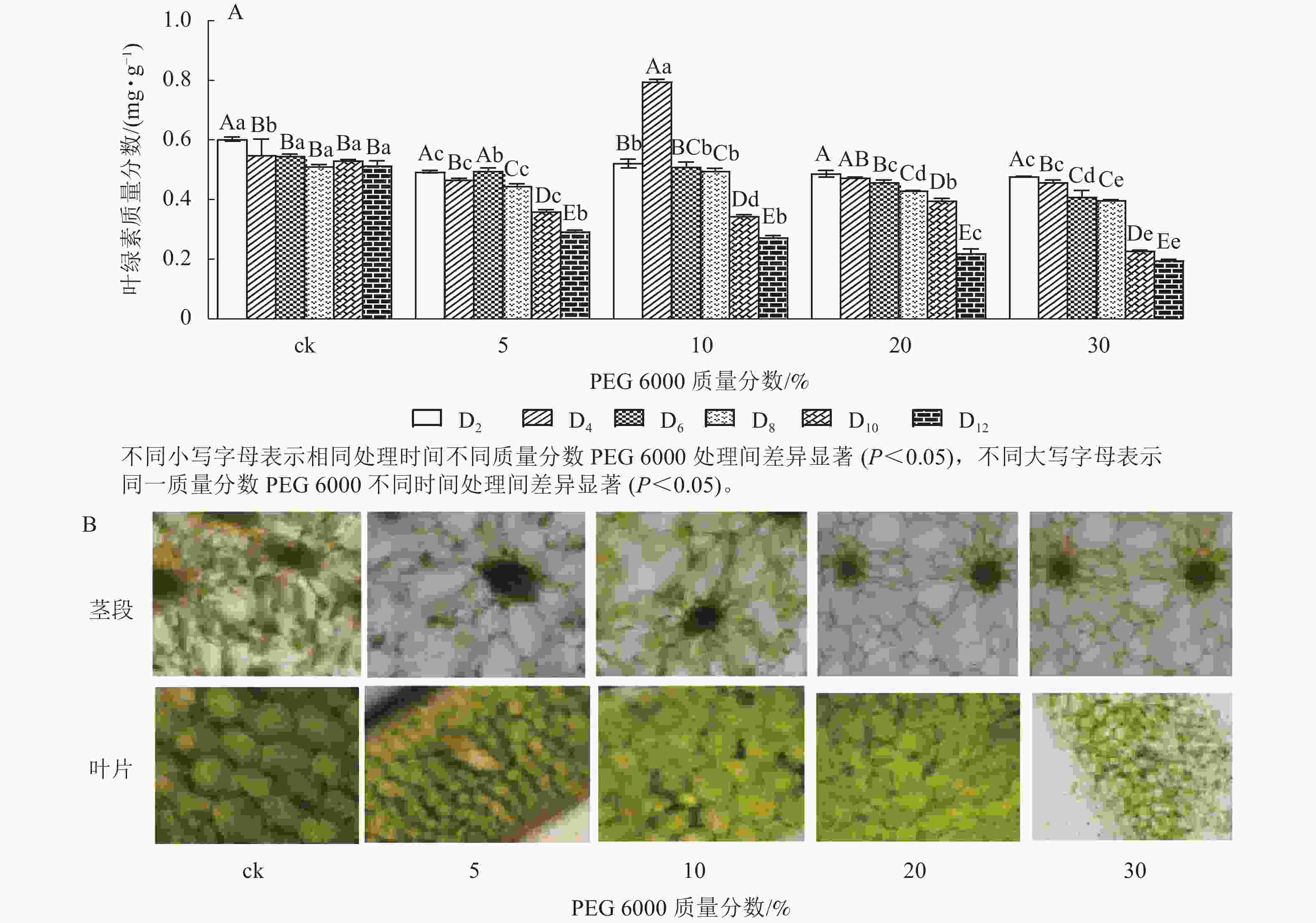
由图4A可知:PEG 6000模拟干旱胁迫对铁皮石斛幼苗茎段和叶片叶绿素质量分数有显著影响(P<0.05)。随着PEG 6000模拟干旱胁迫时间的延长,铁皮石斛幼苗叶绿素质量分数呈现逐渐减少的趋势,在PEG 6000模拟干旱处理2~8 d时,叶绿素质量分数呈现缓慢下降的趋势,到第10天时,叶绿素质量分数急剧下降,较同一时间的ck分别下降了32.01% (5%PEG 6000)、35.03% (10%PEG 6000)、44.07% (20%PEG 6000)和57.06% (30%PEG 6000),且随着PEG 6000质量分数的增加,叶绿素下降幅度明显增加。方差分析结果表明:不同质量分数PEG 6000处理对铁皮石斛幼苗叶绿素质量分数的有显著的影响(P<0.05)。对不同质量分数PEG 6000处理12 d时铁皮石斛幼苗茎段和叶片琼脂糖包埋震荡切片观察发现:铁皮石斛茎段和叶片细胞内叶绿素随着PEG 6000质量分数的增加而减少(图4B),与测得叶绿素质量分数的结果一致。
-
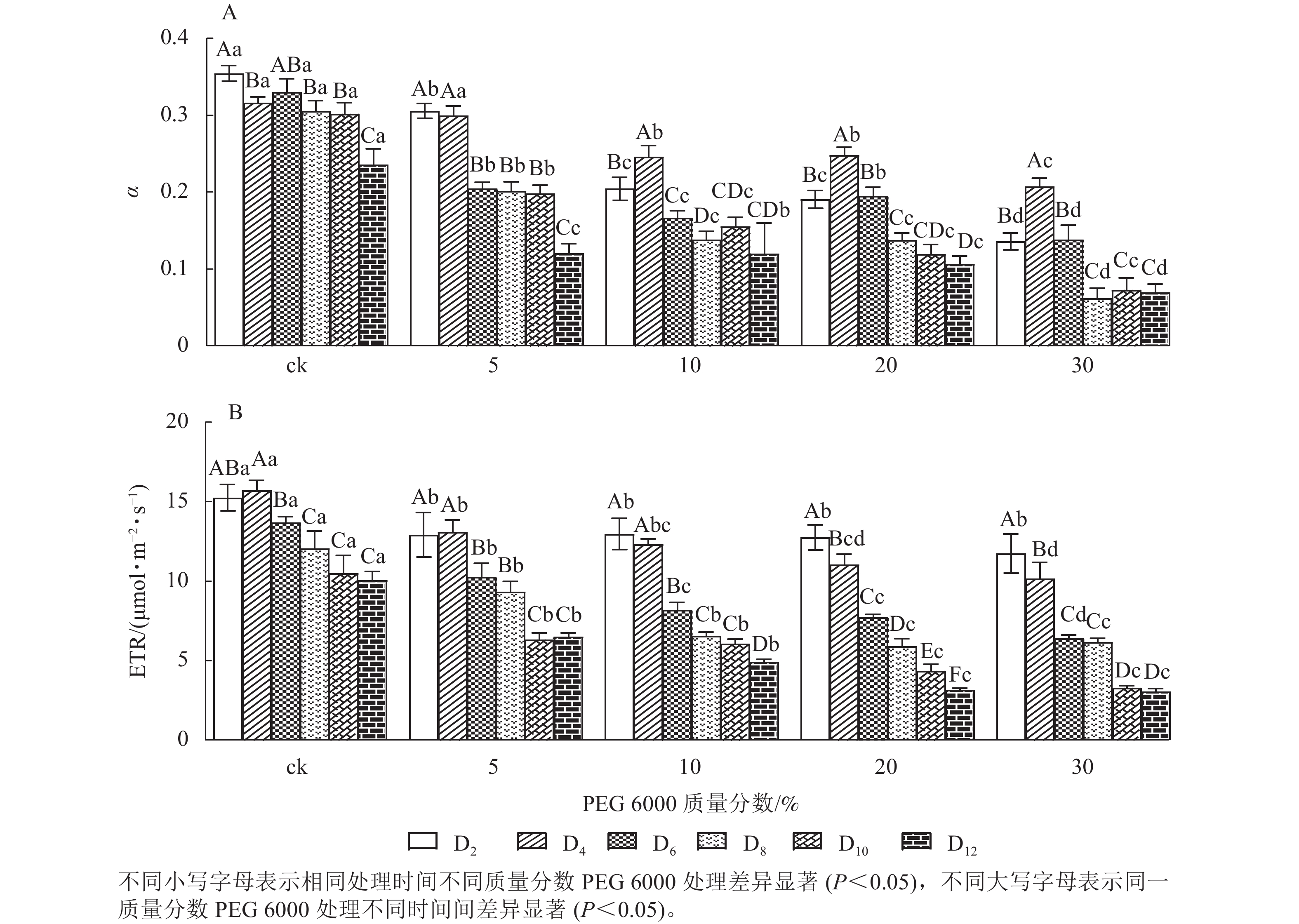
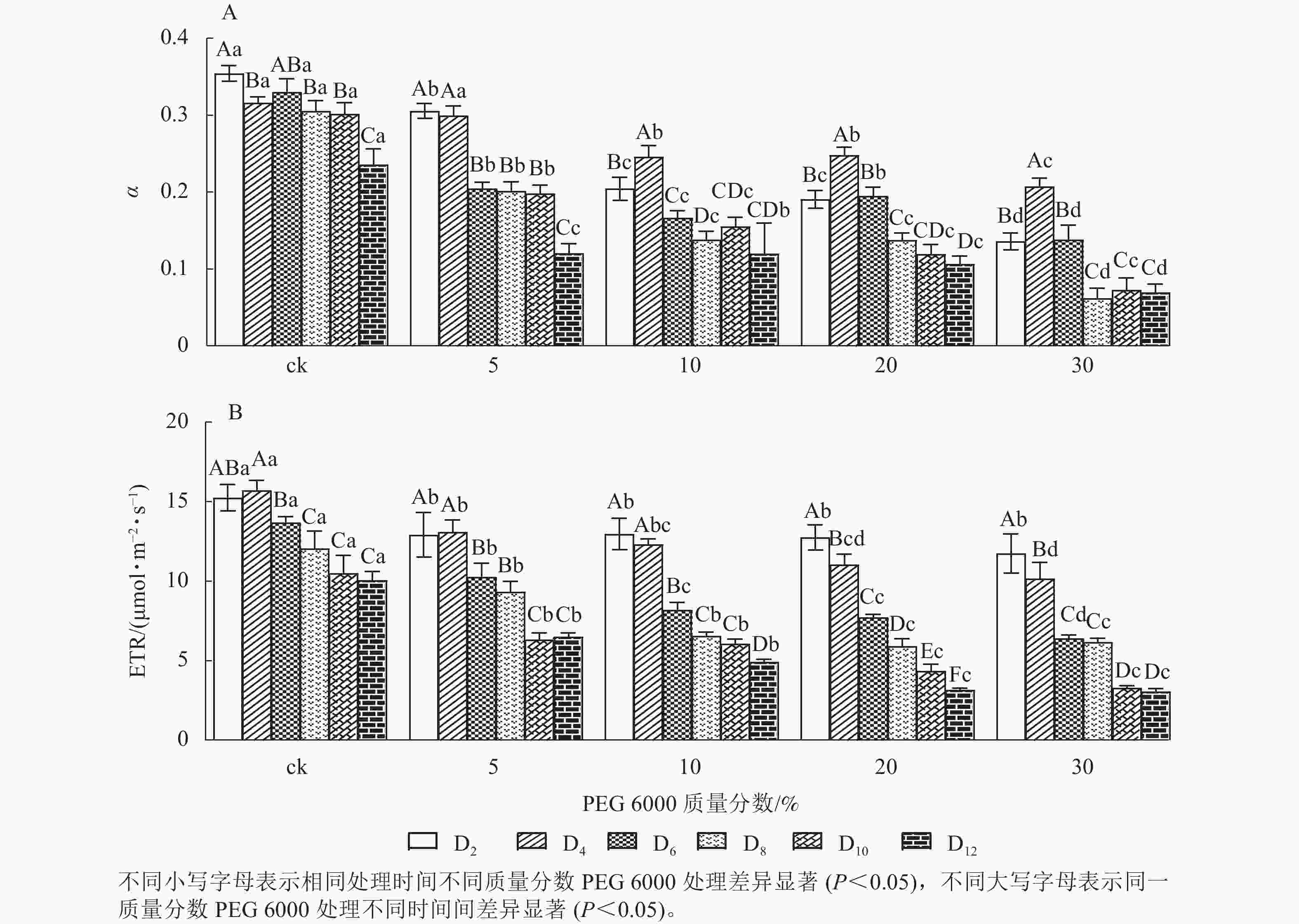
由图5A和5B可知:PEG 6000模拟干旱胁迫对铁皮石斛幼苗处理不同时间对α和ETR的影响差异显著(P<0.05)。随着PEG 6000胁迫处理时间的延长和PEG 6000质量分数的增加,α和ETR均呈下降趋势,在4~8 d时下降的幅度最大。与ck组相比,随着PEG 6000质量分数的增加,α下降幅度增大(图5A)。在PEG 6000 为30%处理时ETR下降幅度最大,PEG 6000为20%和30%处理12 d时ETR达到最低(图5B),说明PEG 6000胁迫处理会使铁皮石斛幼苗的光合作用减弱,且随着胁迫程度增加,光合作用减弱的幅度越大。这与PEG 6000胁迫处理下铁皮石斛叶绿素质量分数降低的结果一致。
-
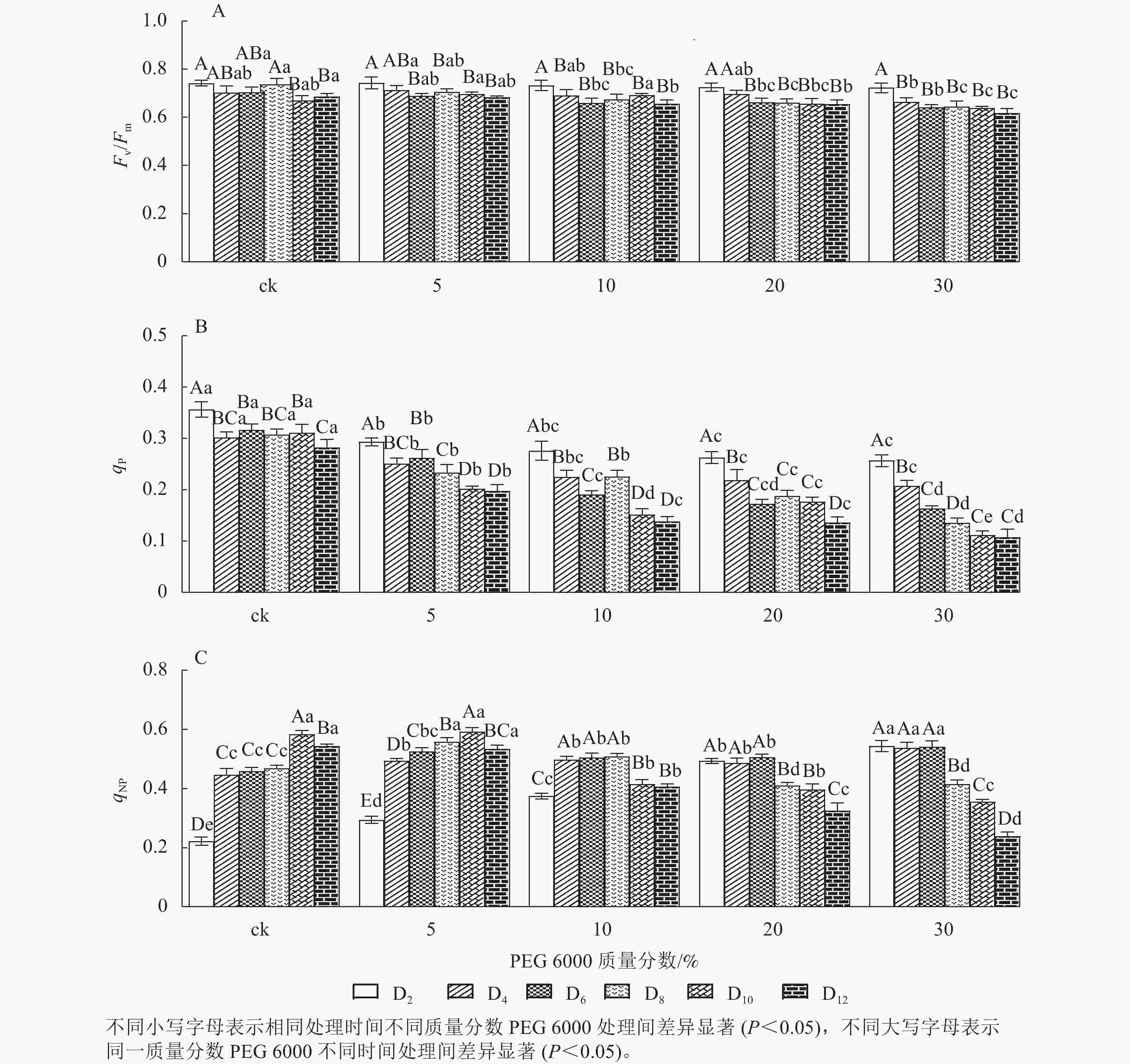
由图6A可知:Fv/Fm随着不同质量分数PEG 6000处理时间的延长呈逐渐下降的趋势,在PEG 6000处理第2天时,Fv/Fm为0.723~0.743。未添加PEG 6000处理在第2~8天时,铁皮石斛幼苗Fv/Fm为0.703~0.743,说明光能转换正常,但到第10~12天时,Fv/Fm显著下降(P<0.05),光转换效率降低,可能由于长时间液体培养会对铁皮石斛幼苗生长产生不利影响。随着PEG 6000质量分数的增加,Fv/Fm呈现显著下降趋势(P<0.05),低质量分数PEG 6000 (5%)处理后第2~8天,Fv/Fm为0.698~0.713,说明低质量分数PEG 6000对铁皮石斛幼苗PSⅡ的损伤较小,而第8~12天逐渐下降;10%~30%PEG 6000处理后第4天,Fv/Fm开始显著下降,说明铁皮石斛幼苗受到模拟干旱胁迫后,植株的最大光能转换效率降低,PSⅡ受到胁迫损伤,严重影响铁皮石斛幼苗的光合作用。
-
荧光猝灭主要有光化学猝灭和非光化学猝灭,光化学猝灭是由碳代谢酶光活化和气孔开放引起的PSⅡ电子传递速率增加,光化学猝灭系数(qP)一定程度上反映PSⅡ反应中心的开放程度;非光化学猝灭是由能量转变导致的热耗散效率增加,qNP为非光化学猝灭系数。非光化学猝灭是一种保护途径,通过热耗散消耗过剩的光能,使光合机构免受伤害[32−33]。PEG 6000模拟干旱胁迫对铁皮石斛幼苗处理不同时间对qNP和qP的影响差异显著(P<0.05)。由图6B可知:不同质量分数PEG 6000处理均使qP在第2~12天逐渐降低,且随着PEG 6000质量分数的增加而下降,说明PSⅡ反应中心的开放程度随之降低,参加光化学反应的激发能量明显降低,过剩的光能就会增加。由图6C可看出:当PEG 6000质量分数为0~10%时,铁皮石斛幼苗qNP随着处理时间的延长呈先上升后下降的趋势,PEG 6000为0和5%时,在处理的第10天qNP达最高值(0.584和0.593),PEG 6000为10%时于第8天qNP达最高值(0.509);PEG 6000为20%和30%时,铁皮石斛幼苗qNP随着处理时间的延长持续下降,说明低质量分数PEG 6000胁迫下,非光化学猝灭途径发挥了保护作用,qNP增大;但随着处理时间的延长或在高质量分数PEG 6000胁迫下,qNP自我保护机制达到一定限度,导致光合反应受到伤害,无法逆转,使qNP呈现持续下降的趋势。
-
干旱是全球性环境问题之一。植物不能逃避恶劣的自然环境条件,只能加速适应各种逆境。大多数植物可改变自身的生理生化指标来响应干旱逆境胁迫,以维持植物正常生长。
MDA是细胞膜受到逆境胁迫伤害引起的膜脂过氧化产物,MDA质量摩尔浓度是判断植物细胞膜受到伤害程度的重要指标,MDA质量摩尔浓度越高说明细胞膜受伤程度越大[28, 34−35]。本研究中,铁皮石斛幼苗MDA质量摩尔浓度随PEG 6000质量分数和处理时间不同存在差异,短时间(≤8 d) PEG 6000模拟干旱胁迫处理后铁皮石斛幼苗MDA变化不大,对细胞膜影响较小,而长时间(≥10 d)处理后MDA急剧上升,且PEG 6000质量分数越高,MDA质量摩尔浓度越高,因此,PEG 6000模拟干旱程度增加会导致细胞膜受到极大的伤害,膜脂过氧化程度也越大。这与构树Broussonetia papyrifera[36]、北美红栎Quercus rubra[37]和大豆Glycine max[38]幼苗在干旱胁迫时MDA的变化一致。
小分子渗透调节物质葡萄糖、果糖、蔗糖等可溶性糖能通过渗透调节平衡细胞渗透势,提高细胞保水能力而维持细胞的正常生理过程。同时,干旱胁迫会导致植物细胞膜受损,透性增大,引起膜脂过氧化反应,会对蛋白质造成伤害,因此细胞内可溶性糖和蛋白变化能够稳定渗透调节能力,是反映植物抗旱性的有效指标之一[14, 39]。本研究中,铁皮石斛幼苗可溶性糖质量分数随PEG 6000质量分数和胁迫处理时间的延长呈上升趋势,说明铁皮石斛幼苗细胞通过积累可溶性糖来提高细胞液浓度,降低细胞膜的渗透势,维持体内细胞的渗透压保持自身正常的生理过程。这与玉米的可溶性糖质量分数随干旱胁迫的加剧逐渐升高的变化趋势一致[40]。在处理时间相同时,PEG 6000质量分数越高,铁皮石斛幼苗可溶性蛋白质量分数越低,说明干旱环境对铁皮石斛细胞造成极大的伤害。
植物体内较低的活性氧不会对植物细胞产生伤害[41],而当植物受干旱胁迫时活性氧的代谢平衡被严重破坏,活性氧增多,攻击植物蛋白质上各种氨基酸残基,使蛋白质和叶绿素等分子解体,从而对植物细胞产生各种毒害作用。POD、CAT等保护酶能有效抑制和清除植物体内的各种活性氧和自由基[42]。本研究表明:PEG 6000模拟干旱处理铁皮石斛幼苗后,POD和CAT活性都呈现先上升后下降的趋势,POD和CAT酶活性的大幅提高大大增强对活性氧的抵抗能力和对植物光合机构的保护能力。该结果与三叶草Trifolium repens受到干旱胁迫时POD和CAT的活性变化一致[25]。因此,植物受干旱胁迫时通过提高相关保护酶活性来保持体内活性氧平衡。
-
叶绿素是光合作用最重要的色素,光合作用时叶绿素吸收能量,为CO2转变为碳水化合物提供能量;水分是叶绿素的合成原料,干旱胁迫能使叶绿素合成减缓及叶绿素解体,直接导致叶绿素质量分数降低。本研究中PEG 6000模拟干旱处理铁皮石斛幼苗后,茎段和叶片叶绿素质量分数明显降低,与PEG模拟干旱胁迫不同抗逆性棉花Gossypium hirsutum的叶绿素变化趋势一致[43]。
叶绿素荧光和植物光合作用关系密切,当强光持续照射植物时,为了有效避免植物叶绿体内吸收的光能超过光合作用中发生光化学反应的能量消耗及过量的光能灼伤植物光合作用机构,植物以荧光的方式耗尽消散掉部分光能以起重要保护作用;自然条件下叶绿素荧光和植物光合速率呈负相关,叶绿素荧光变弱时光合速率就高,反之亦然。植物遭受胁迫的程度与光合作用的叶绿素荧光特性有着密切的关系[44]。本研究中,PEG 6000模拟干旱处理铁皮石斛幼苗,Fv/Fm、qP、ETR均呈下降趋势,表明PEG 6000干旱胁迫对植物光合作用产生了伤害;PEG 6000干旱胁迫使铁皮石斛幼苗qNP呈上升趋势,说明铁皮石斛幼苗通过启动非光化学猝灭途径来大量消耗PSⅡ反应中心吸收的过剩光能。随着PEG 6000胁迫的不断加重,qNP也开始呈现明显下降的趋势,可能是由于热耗散机制受阻,无法使耗散掉吸收的过剩光能,导致光系统受到不可逆转的损伤,影响光合作用。因此,植物受到干旱胁迫时光合作用能力降低从而影响植物的正常生长。
-
通过研究对铁皮石斛幼苗进行PEG 6000模拟干旱胁迫处理后的生理和叶绿素荧光特性,发现铁皮石斛幼苗具有一定的抗旱能力,在适当干旱条件下可通过自身调节来达到正常生长的目的,但也有一定限度。在较低PEG 6000质量分数(≤5%)胁迫时,可以通过调节代谢实现自我保护,在高质量分数PEG 6000 (≥20%)时,干旱胁迫会损伤植物,影响生理过程和光合作用。20% PEG 6000胁迫10 d可作为铁皮石斛抗干旱品种筛选的标准;铁皮石斛幼苗可通过增加可溶性糖,减少可溶性蛋白,提高POD和CAT等防御酶活性抵抗和适应一定程度的干旱胁迫(≥20%PEG 6000处理≥10 d);铁皮石斛幼苗的最大光能转换效率降低,PSⅡ受到胁迫损伤,同时,铁皮石斛幼苗通过启动qNP途径来消耗PSⅡ反应中心吸收的过剩光能,维持正常的光合作用。因此,可溶性糖和可溶性蛋白、防御酶POD和CAT以及叶绿体荧光参数均可作为铁皮石斛的耐干旱指标。
Effects of PEG 6000 simulated drought stress on physiological and chlorophyll fluorescence characteristics of Dendrobium candidum seedlings
doi: 10.11833/j.issn.2095-0756.20230301
- Received Date: 2023-05-11
- Accepted Date: 2023-09-08
- Rev Recd Date: 2023-09-04
- Available Online: 2024-01-19
- Publish Date: 2024-02-20
-
Key words:
- Dendrobium candidum /
- drought stress /
- physiology and biochemistry indicators /
- chlorophyll fluorescence parameters /
- PEG
Abstract:
| Citation: | FENG Rui, ZHOU Qi, WU Lingshang, et al. Effects of PEG 6000 simulated drought stress on physiological and chlorophyll fluorescence characteristics of Dendrobium candidum seedlings[J]. Journal of Zhejiang A&F University, 2024, 41(1): 132-144. DOI: 10.11833/j.issn.2095-0756.20230301 |










 DownLoad:
DownLoad: