-
林木的遗传变异是遗传改良的前提,有效的遗传变异决定了育种过程中的改良潜力[1]。生长性状作为林木最直观的表型性状,在多数树种中存在较为丰富的变异。林木苗期生长性状是良种选育繁育和新种质创制的重要指标,苗期的生长发育状况会影响到林木后期乃至一生的生长。多数早晚性状相关回溯性实验证明[2−4]:以家系苗高或地径等生长性状为指标进行早期选择是有效的,可在提高林木良种选育效率、加速育种进程的同时,获得更高的现实和遗传增益。
南洋楹Falcataria falcata是豆科Leguminosae含羞草亚科Mimosaceae常绿高大乔木,原产马来西亚和印度尼西亚,是世界著名的热带速生固氮树种[5],其木材质轻、韧性强,纤维质量分数高达61.8%,是人造板和造纸的优良原料;其根系发达,可结瘤固氮、改良土壤,亦可运用于热带、亚热带地区生态林建设及四旁绿化[6]。在中国广东、海南等地建立的多个南洋楹引进种源及家系试验点中研究发现[7−9]:南洋楹从种源、家系、单株等不同层次表现出了丰富的遗传变异,特别表现于幼龄林和成熟林中的生长和形质性状,但其苗期生长变异以及固氮能力相关性状的遗传变异研究未见报道。本研究以南洋楹1代无性系种子园中37个半同胞家系为研究对象,对其1~6月生苗期生长性状(苗高、地径)、叶片叶绿素相对含量、生物量及根瘤数量等指标的进行测定分析,揭示南洋楹苗期苗高和地径的生长进程及家系层次的遗传变异规律,并选择出速生且固氮能力强的优良家系,为南洋楹良种选育提供依据。
-
研究区位于广东省林业科学研究院,23°11′39″N,113°22′25″E,海拔为25 m。该地区属于典型的亚热带季风气候,年平均气温为23.0 ℃,年日照时数为1 800.0~1 960.0 h,年降水量为1 677.0 mm,年平均相对湿度为69%。土壤类型为赤红壤,肥力中等。
-
参试种子来源于广东省茂名市电白区林业科学研究所“南洋楹1代无性系种子园”10~11年生37个亲本(37个半同胞家系),建园亲本均由广东省林业科学研究院选育提供。半同胞家系来源及其亲本基本情况见表1。试验研究以广东省潮州市饶平县15年生以上南洋楹母树林混合种为对照(ck)。
家系编号 亲本
基因号来源 树高/m 嫁接口径/mm 家系编号 亲本
基因号来源 树高/m 嫁接口径/mm F001 BLS952 广东博罗县 7.0 78 F036 LK01 广东江门市 7.5 94 F005 增5 广东广州市 8.5 70 F037 LK03 广东江门市 8.0 75 F007 BLS05 广东博罗县 7.5 75 F038 LY04 广东博罗县 7.5 75 F008 BLS09 广东博罗县 9.5 80 F040 LY06 广东博罗县 8.5 90 F009 BLS02 广东博罗县 10.0 78 F042 LY08 广东博罗县 9.0 90 F014 ZCS09 广东广州市 7.0 70 F043 LY10 广东博罗县 8.0 85 F015 BLS12 广东博罗县 7.0 78 F051 LY19 广东博罗县 9.0 100 F016 ZCS02 广东广州市 8.5 88 F057 LY16 广东博罗县 7.0 75 F017 ZL11 广东广州市 7.6 95 F062 ZCS28 广东广州市 8.0 90 F018 增7 广东广州市 6.5 70 F063 ZCS20 广东广州市 8.5 95 F021 BLS22 广东博罗县 8.0 90 F065 BLS31 广东博罗县 8.5 90 F023 BLS27 广东博罗县 8.5 76 F072 SB02 广东阳春市 9.8 87 F025 BLS11 广东博罗县 8.0 80 F074 SB04 广东阳春市 7.0 86 F027 BLS26 广东博罗县 7.5 75 F078 XX01 广东新兴县 8.0 85 F028 DLS06 广东东莞市 7.5 70 F081 XX05 广东新兴县 9.5 90 F029 FT01 广东博罗县 9.5 90 F083 XX07 广东新兴县 8.5 100 F030 FT02 广东博罗县 8.5 87 F086 XX10 广东新兴县 9.0 100 F031 FT04 广东博罗县 8.0 80 F087 XX12 广东新兴县 8.0 80 F033 HSS02 广东鹤山市 7.0 110 Table 1. Origin of half-sibling families and basic situation about their parent plants
-
2021年11月下旬,将当年采集的南洋楹37个半同胞家系及ck共计38份参试种子放入质量分数为 5% 高锰酸钾溶液浸泡 30 min,用清水冲洗干净后倒入85 ℃的热水浸种,待水自然冷却至常温后再继续浸种24 h,除去浮在上面的废种,再用清水漂洗1~2次。在干净培养皿中垫上湿润的滤纸,将种子均匀置于其中,并封盖放入28 ℃培养箱中暗培养,2~4 d后种子发育成芽苗[10]。
-
使用规格为6 cm×9 cm塑料容器袋,以体积比V(黄心土)∶V(泥炭土)=3∶1为基质,将38份参试种子芽苗分别点播于基质中央,1袋1株。按随机区组试验设计排列,每个家系及ck按每行10株移栽,5行小区,3次重复,即每家系及ck各150株。当年11月下旬至翌年5月下旬为育苗期,苗期管理方法统一按广东省地方标准《南洋楹育苗技术规程》[11]执行。
-
分别在苗龄 1、2、4、6月生时,对南洋楹37个半同胞家系及ck苗木的苗高(h)、地径(d)生长情况进行监测。调查样株为各家系及ck各小区中间15~24株,每个家系及ck 45~60株。6月生时,除测定苗高和地径指标外,采用SPAD-502叶绿素仪测定每样株中层的成熟叶片的叶绿素相对含量,每株测定5片,取其平均值[12]。再在每小区调查样株中选取3株长势平均的植株,每个家系及ck共计9株,取出待测样株,将根系基质冲洗干净后统计根瘤数,计算单株根瘤数的平均值,然后将地上部分和地下部分分离,用1/100电子天平称量每样株的地上部分及地下部分鲜质量。
-
采用 SAS (v 9.2)软件的 GLM 、LSR和 VARCOMP 等进行方差分析、多重比较和方差分量估计,进而估算遗传参数;相关性分析采用Pearson法,聚类分析采用CLUSTER过程平均距离法。计算家系遗传力、表型变异系数、遗传变异系数、遗传进展、现实增益、遗传增益等参数[13]。
-
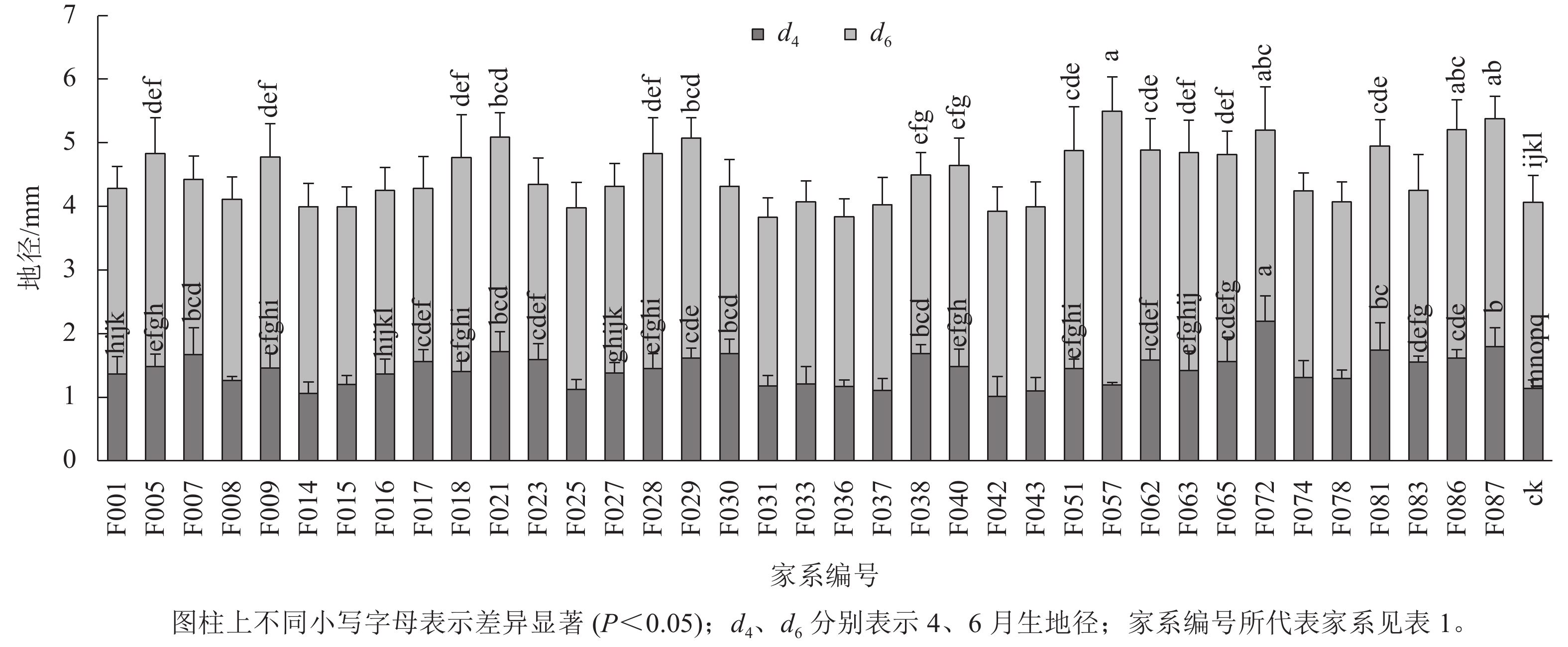
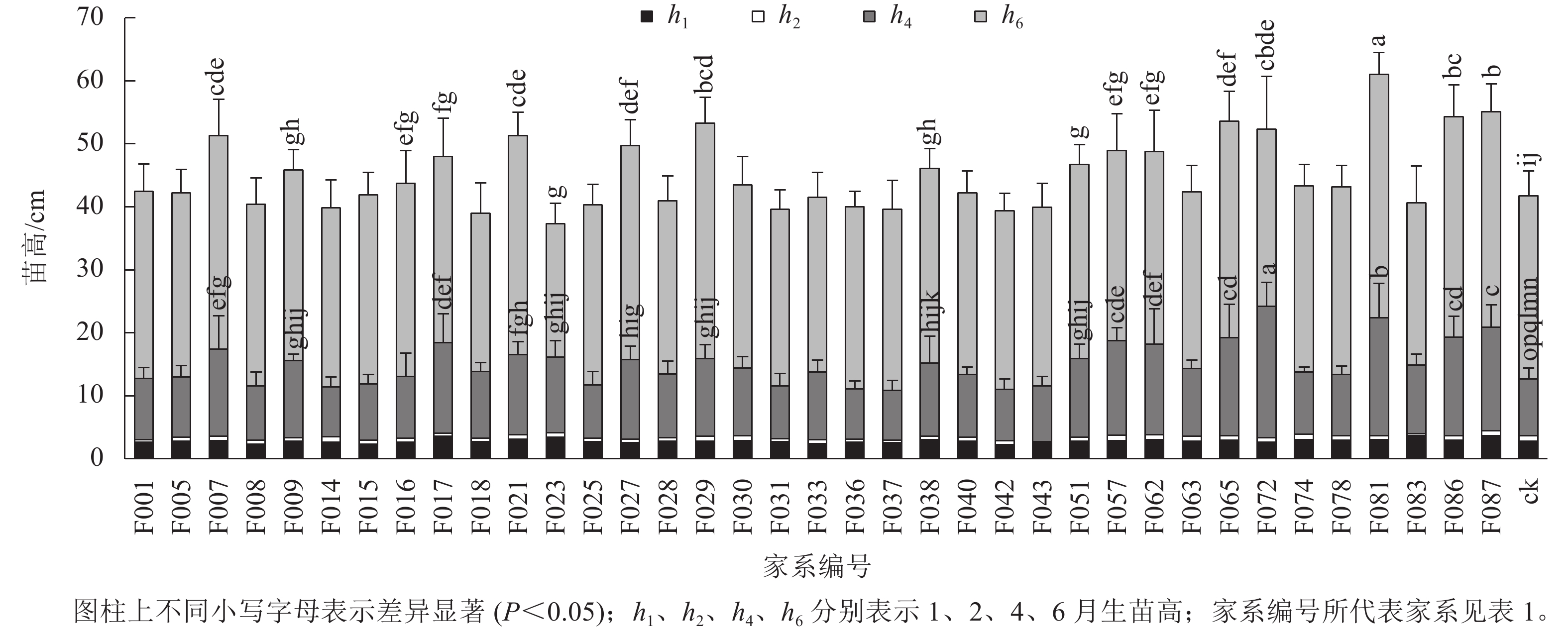
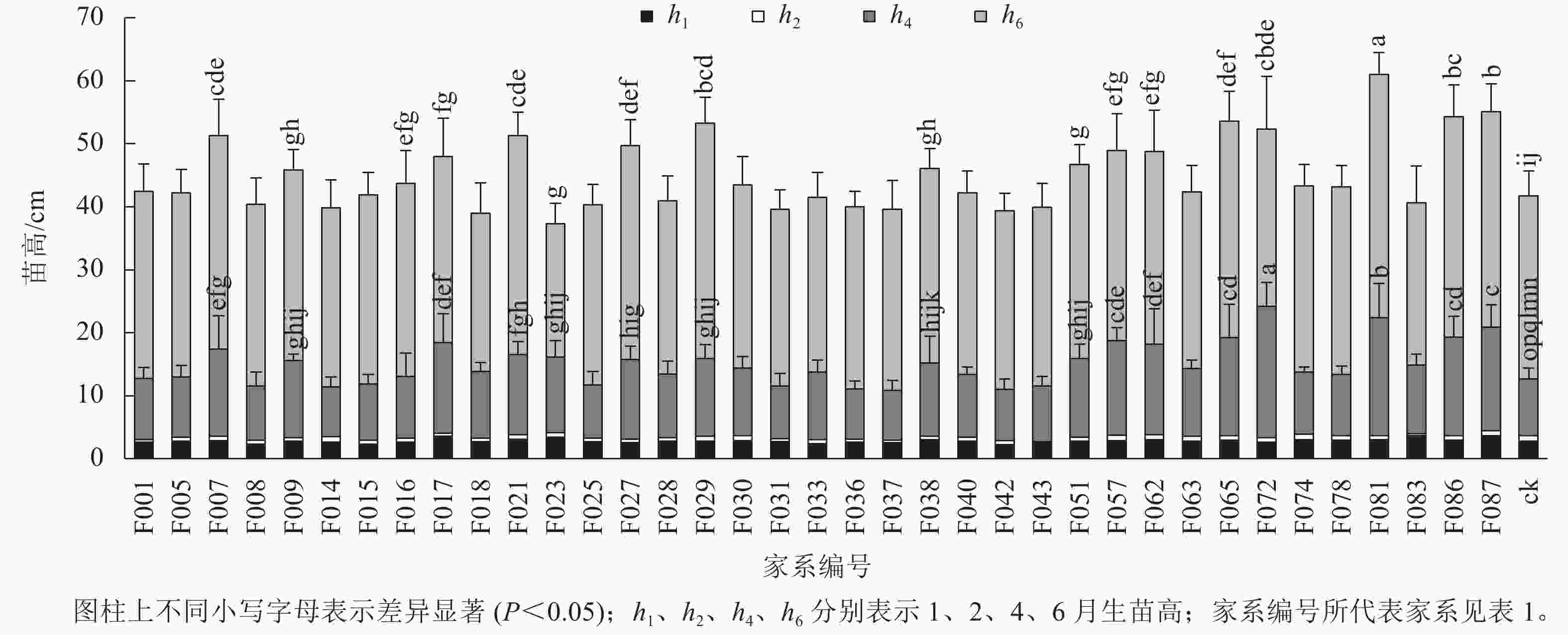
1~6月生南洋楹半同胞家系及ck的苗高和地径生长情况见表2、图1和图2。1月生时,37个半同胞家系及ck平均苗高为2.82 cm,变幅为2.23~3.63 cm,苗高生长齐整且稳定;2月生时,平均苗高3.45 cm,平均生长量仅为0.63 cm,可见,移栽后第2个月,即12月下旬至1月下旬为南洋楹蹲苗期,苗高生长几乎停滞。4月生时,平均苗高和地径分别为11.52 cm和1.43 mm,变幅分别为7.90~20.94 cm和1.01~2.19 mm。移栽后第3~4个月,即1月下旬至3月下旬,随着气温回升,南洋楹幼苗进入恢复生长期。同时,不同家系苗高、地径均出现显著差异,与ck进行Duncan多重比较结果显示:苗高大于ck的家系有28个,占比超过75%,其中有16个家系的苗高显著优于ck (P<0.05);地径大于ck的家系有32个,占比超过86%,其中有24个家系的地径显著优于ck (P<0.05)。6月生时,平均苗高和地径分别达41.85 cm和4.49 mm,长幅分别超过4月生时的260%和200%,南洋楹早期速生优势开始凸现,幼苗进入快速生长期,不同家系的生长差异性持续存在。4月生时苗高生长占优势的16个家系的依然显著优于ck (P<0.05),但地径显著优于ck的家系数量减少至17个。
苗龄/月 苗高/cm 地径/mm 均值 变幅 标准差 均值 变幅 标准差 1 2.82 2.23~3.63 0.24 2 3.45 2.74~4.42 0.31 4 11.52 7.90~20.94 2.45 1.43 1.01~2.19 0.20 6 41.85 35.71~57.38 4.47 4.49 3.83~5.49 0.41 Table 2. Growth traits of half-sib families and control
-
以南洋楹半同胞家系在4和6月生时的苗高、地径方差分析为基础,对引起家系间变异的主要因素(家系遗传效应)的方差分量和遗传参数进行估算,结果显示(表3):家系遗传力方面,不同苗龄的参试家系苗高和地径遗传力分别保持在0.89~0.90和0.84~0.86,2个生长性状均受较强且稳定的遗传控制,可为南洋楹生长性状的遗传改良提供可靠的保障。4月生时,苗高、地径的表型变异系数和遗传变异系数分别为0.212 9、0.271 7和 0.140 2、0.174 8,2个性状的遗传变异系数均略高于表型变异系数,在呈现出丰富的表型多样性的同时,具有获取优良遗传型的较大潜力,印证了这2个性状的表现主要受遗传因素控制,因此,4月生时对南洋楹苗期生长性状进行选择以达到更高的遗传增益是可靠的。但随着苗龄的增长,在6月生时,苗高和地径的变异系数均呈下降趋势。遗传进展主要由性状遗传变异系数、入选率(ρ)和选择强度(i) 决定。南洋楹半同胞家系苗高、地径的遗传进展表现为随苗龄增长而有所下降,在入选率30%时,苗高、地径的遗传进展分别为0.118 9~0.221 4和0.096 2~0.154 3,即入选群体平均苗高和地径预期高出群体均值11.89%~22.14%和9.62%~15.43%。
性状 F 遗传方差分量 家系遗传力 表型变异系数 遗传变异系数 遗传进展 ρ=0.20
i=1.40ρ=0.30
i=1.16ρ=0.40
i=0.97h4 9.85 9.796 9 0.89 0.212 9 0.271 7 0.248 0 0.221 4 0.196 0 h6 10.37 27.722 1 0.90 0.112 4 0.126 6 0.139 2 0.118 9 0.102 2 d4 6.65 0.062 1 0.84 0.140 2 0.174 8 0.179 2 0.154 3 0.132 7 d6 7.20 0.205 8 0.86 0.090 7 0.101 0 0.113 3 0.096 2 0.081 6 说明:ρ表示入选率;i表示选择强度。h4、h6、d4、d6分别为4、6月生时的苗高和地径。 Table 3. Estimation of genetic parameters for growth traits in half-sib families
-
对南洋楹半同胞家系的苗高、地径、叶绿素相对含量、地上部分生物量、地下部分生物量、根瘤数量等性状表型相关进行分析,结果显示(表4):两两性状间均存在极显著或显著正相关关系(P<0.01或P<0.05),从相关程度来看,地下部分生物量与地上部分生物量、叶绿素相对含量、苗高、根瘤数量等性状的相关系数较高(r>0.766 7),地径与根瘤数量相关系数最低(r>0.475 9)。
性状 苗高 地径 叶绿素相对含量 地上部分生物量 地下部分生物量 苗高 1 地径 0.687 2** 1 叶绿素相对含量 0.570 4* 0.694 9** 1 地上部分生物量 0.696 0** 0.664 9** 0.733 9** 1 地下部分生物量 0.772 6** 0.642 7** 0.779 6** 0.914 6** 1 根瘤数量 0.655 1** 0.475 9* 0.574 3* 0.652 6** 0.766 7** 说明:**表示在P<0.01水平上极显著相关,*表示在P<0.05水平上显著相关。 Table 4. Correlation analyses of seedling traits in half-sib families
-
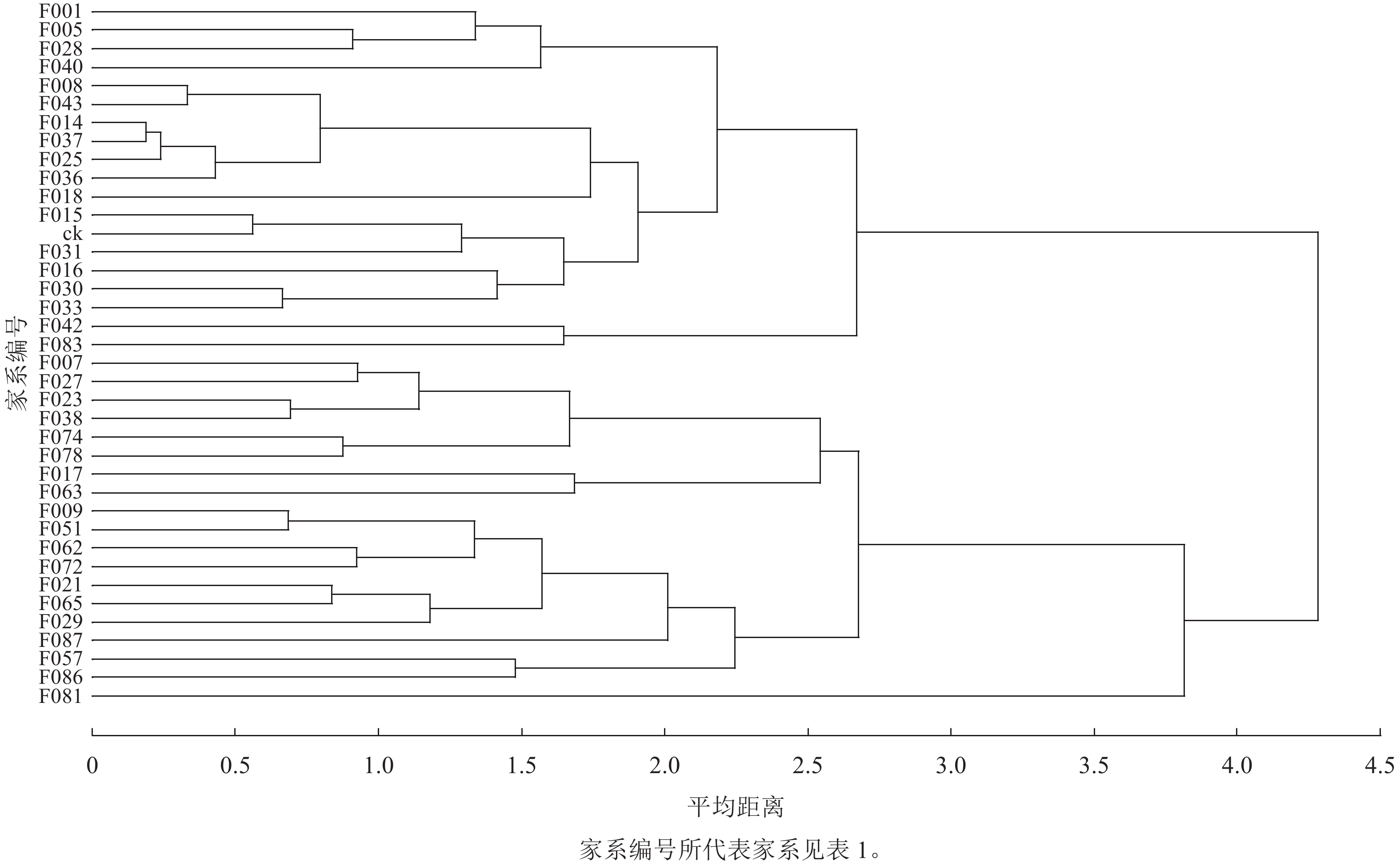
以苗高、地径、叶绿素相对含量、地上部分生物量、地下部分生物量、根瘤数量等 5个性状作为评价指标,对参试家系及ck进行聚类分析,划分类群,再分别与群体均值和ck比较计算入选家系的现实增益和遗传增益。图3显示:当平均距离为3.7时,可将参试材料划分为2大类群,第1类群包括13个家系:F009、F017、F021、F029、F051、F057、F062、F063、F065、F072、F081、F086、F087,占群体的34%,其综合表现为苗高和地径等生长指标显著优于ck,地上部分生物量、地下部分生物量和叶绿素相对含量高,自然结瘤能力强,是苗期速生固氮型优良家系。
以第1类群作为入选家系,其苗高、地径平均值及选择增益的选择结果:4月生时,入选家系平均苗高和地径分别为14.74 cm和1.61 mm。与群体平均值比较,现实增益分别为27.99%和12.73%、遗传增益分别为24.91%和10.69%;与ck比较,现实增益分别为62.47%和41.65%、遗传增益分别为55.59%和34.98%。6月生时,入选家系平均苗高和地径分别为46.92 cm和4.99 mm,与群体均值比较增益水平为9.48%~12.11%,与ck比较增益水平为19.52%~23.24%,选择增益有所下降。
-
目前,普遍认为林木遗传变异主要存在于种源间、家系间(无性系间)、个体间和个体内4个层次[14]。沈凌等[15]研究发现:无患子Sapindus saponaria优株自由授粉子代1年生的苗高和地径在优株间均存在极显著的差异,具有丰富的变异,开展家系间良种选育的空间很大;苑正赛等[16]发现:7月生美洲黑杨Populus deltoides苗期家系及单株间性状表现差异较大,可进行优良家系及单株的选择;郭飞等[17]认为:6月生马尾松Pinus massoniana 1.5代家系苗期各项生长指标存在极显著差异,家系间遗传变异丰富,选育潜力很大。本研究结果表明:南洋楹半同胞家系从4月生幼苗起,苗高和地径等生长性状发生明显遗传变异,6月生时仍保持相似差异性结果。值得注意的是,依据广东省地方标准《南洋楹育苗技术规程》,苗龄为6月生,苗高20~35 cm,地径≥0.25 mm为南洋楹实生苗木出圃合格苗。本研究参试家系群体6月生时平均苗高和地径分别达41.85 cm和4.49 mm,表明南洋楹1代无性系种子园子代群体已表现出较好的改良效果,不同南洋楹群体层次具有不同生长进程,需适时做好苗木综合管理措施和调整选育策略。南洋楹苗龄为4月生时是苗木进入速生生长的关键时期和重要的选择时期。
遗传参数估算对预期增益估计、育种策略制定和选择效果预测等方面具有指导作用[2],其中遗传力和变异系数可衡量亲本将某性状遗传给子代的能力和选择潜力,是育种者广泛关注的重要指标。不同苗龄南洋楹半同胞家系苗高和地径的遗传力分别为0.89~0.90和0.84~0.86,遗传力处于极高水平[1],表现出苗高的遗传力大于地径的规律;2个性状的遗传变异系数均略高于表型变异系数,在呈现丰富的表型多样性的同时,具有获取优良遗传型的较大潜力,印证了这2个性状主要受遗传因素控制,与苑正赛等[16]、曹昆彬等[18]的研究结果一致。本研究进一步对遗传进展进行了估算,该指标在林木遗传育种上常用于预测入选群体某性状预期高出目前群体均值的程度,其变化趋势与遗传变异系数相一致。为了尽可能获得更高遗传选择效益而不丢失优良家系,在入选率30%时,南洋楹半同胞家系苗高、地径的遗传进展分别为0.118 9~0.221 4和0.096 2~0.154 3,即入选群体平均苗高和地径预期高出群体均值11.89%~22.14%和9.62%~15.43%。
相关性分析可了解各性状指标间的相关性性质及其相关性大小[19],不同性状间的相关性体现其相互有促进或制约关系。南洋楹是豆科植物,根系易被土壤中的根瘤菌侵染形成根瘤共生体,根瘤可促进植物的生长,增加土壤有机碳、速效氮和全氮质量分数[20−21]。根瘤是豆科植物与根瘤菌共生形成的固氮器官,其结瘤率的高低与根瘤的固氮效果有密切的关系[22],根瘤的数量可反映南洋楹固氮效益的大小。叶绿素相对含量能够调控光合作用效率,较高光合效率有利于植物加强共生固氮作用的碳源和能源的供应[23],从而提高根瘤的共生固氮效率。因此,本研究除了苗高、地径、生物量等传统指标外,将不同半同胞家系的根瘤数量和叶绿素相对含量指标作为重要考察指标。结果表明:两两性状间均存在极显著或显著正相关关系,利用它们之间的相关性可对南洋楹的生长性状和固氮效能进行协同选择,选育同时具有生长优势和高效固氮的优良家系。
本研究根据平均距离聚类法,以第1类群的13个(占群体的34%)家系作为入选优良家系,其综合表现为苗高、地径等生长指标显著优于ck,生物量和叶绿素相对含量高,自然结瘤能力强,是苗期速生固氮型优良家系。与群体平均值比较,入选优良家系苗高和地径所获得的遗传增益分别为24.91%和10.69%,与入选率30%情况下遗传进展的预测结果相互印证。该13个家系的亲本主要来源于广东博罗、广东广州、广东新兴等地区,位于北回归线以南至22°41′N区域内,属于适宜南洋楹生长的南亚热带气候并同时具备静风环境条件,也是广东省南洋楹推广种植的主要地区。此外,入选家系与ck比较获得的现实增益和遗传增益均高于群体平均值,可见南洋楹37个半同胞家系群体生长水平较ck更高,南洋楹1代无性系种子园半同胞子代群体也具有较好的群体改良效果。
-
南洋楹37个半同胞家系幼苗从4月生起,苗高和地径等生长性状发生显著遗传变异,超过16个家系的苗高或地径显著优于对照,苗高和地径的家系遗传力分别保持在0.89~0.90和0.84~0.86,具有极高水平的遗传力和较好的选择潜力;在入选率为30%时,苗高和地径的遗传进展分别为0.118 9~0.221 4和0.096 2~0.154 3。苗高、地径、叶绿素相对含量、生物量、根瘤数量等性状间均呈极显著或显著正相关。据生长和固氮等性状协同分析结果,选择了13个同时具有苗期生长优势和高效固氮能力的优良家系。
Variation and selection of half-sib families of Falcataria falcata during seedling stage
doi: 10.11833/j.issn.2095-0756.20230371
- Received Date: 2023-06-20
- Accepted Date: 2023-09-18
- Rev Recd Date: 2023-09-12
- Available Online: 2024-03-21
- Publish Date: 2024-04-01
-
Key words:
- Falcataria falcata /
- half-sib family /
- genetic variation /
- seedling selection
Abstract:
| Citation: | YAN Shu, WEI Ruping, WANG Runhui, et al. Variation and selection of half-sib families of Falcataria falcata during seedling stage[J]. Journal of Zhejiang A&F University, 2024, 41(2): 306-313. DOI: 10.11833/j.issn.2095-0756.20230371 |











 DownLoad:
DownLoad: