-
根系是连接植物地上部分与土壤的纽带,是植物与土壤进行物质运移置换的关键工具[1]。资源分配假说认为:萌生更新能力取决于母树地上器官和地下器官的资源储存,地上部分损失后,根系的资源储存量对树木萌生具有重要意义[2]。根系的资源储存不仅受根系内在生理特性的影响,同时也会因外部条件的改变而受到影响,除了土壤、气候条件外,平茬也会对其产生影响。平茬是将苗木地上部分的枝条按照一定高度全部截去,以刺激根系及萌生枝生长而采取的一项重要的技术措施,它能够更好地促进植株生长[3]。植株地上部分被破坏后,地上叶面积大幅度减少,使光和同化产物向根系的分配减少,从而导致植株根冠比失衡,使植株在此时进行补偿性生长[4]。研究表明:平茬处理使柠条Caragana korshinskii的根系和根量明显增加,可有效提高根系活力[5]。
云南松Pinus yunnanensis为常绿针叶乔木,生长迅速,树干通直,且耐干旱瘠薄[6],广泛分布于23°00′~29°00′N,98°30′~106°00′E的中国西南地区,是西南地区乡土造林的主要树种[7]。云南松不仅可以保持水土、防风固沙,还用于树脂、造纸、烤胶和医疗等领域,在生态建设和经济建设方面具有重要作用[8−9]。然而,80%的云南松人工林是同龄纯林,林分稳定性较差、质量低劣,且生物多样性低[10]。人为砍优留劣、毁林开荒,以及生物入侵、病虫害等自然因素的影响[11],使大部分云南松林出现不同程度的退化,优良基因资源减少,出现低矮、弯曲、扭曲等不良个体[12]。云南松多为种子繁殖,会导致基因重组,母株遗传性状发生变异。因此,对云南松进行遗传改良从而培育优良苗木的工作迫在眉睫[13]。无性繁殖可以挑选优良的种质资源进行,既能缩短苗木出圃时间,又能较好地保持亲本性状[14]。针叶树种属于较难扦插生根的树种,对云南松进行扦插繁殖需要获得大量优质幼化的穗条。平茬可以有效去除顶端优势,促进休眠芽和不定芽的萌蘖,使植物产生较多的新生萌蘖枝条,从而为云南松扦插繁殖提供优良幼态化的穗条。平茬后影响云南松萌蘖的因素较多,但对根系的探讨较少。有研究发现:平茬可以促进云南松根系的生长发育,且不同平茬高度和不同平茬季节都会对云南松的根系产生影响[15−16],然而不同平茬苗龄对云南松根系形态变化的研究未见报道。鉴于此,本研究对云南松不同苗龄的苗木进行平茬,分析不同苗龄苗木根系生长随时间的动态变化,探究云南松苗木根系生长对不同苗龄平茬的响应规律,以期为云南松适宜平茬苗龄的确定提供依据。
-
研究地设置在云南省昆明市西南林业大学苗圃(25°04′00″N,102°45′41″E)。该地海拔约1 945 m,年平均气温为14.7 ℃,绝对最高气温为32.5 ℃,绝对最低气温为−9.0 ℃。降水集中在5—10月,月最大降水量为208.3 mm,日最大降水量为153.3 mm,年平均相对湿度为68%,年降水量为700.0~1 100.0 mm。年均日照时数为2 445.6 h,年均蒸发量为1 856.4 mm。基质为红土与腐质土(体积比为1∶2),土壤为酸性低磷土壤。苗木盆统一采用红黑双色苗圃塑料软花盆,口径、底径、高分别为24、16、20 cm。
-
于2017年12月在弥渡云南松无性系种子园健壮母树上采集结实多、籽粒饱满的当年生成熟球果。将球果做好标记带回实验室风干,待球果开裂后取出发育良好的种子。先用体积分数为0.5%高锰酸钾溶液消毒0.5 h,取出用清水冲洗后于50 ℃温水中浸泡24 h。分别于2018年1月20日(平茬时为30月龄,记为M30),2019年1月20日(18月龄,M18),2019年5月20日(14月龄,M14),2019年9月20日(10月龄,M10),2020年1月20日(6月龄,M6)播种育苗,株行距为5 cm×10 cm。将育苗盆整齐摆放在苗床上,有5个苗龄的苗木,3个重复,共15个小区,每个小区育苗48株,总计720株。地面铺设地膜。
-
种子播种后根据天气情况于9:00前或17:00后浇水,并定期除草。在2020年7月20日对不同苗龄的苗木进行平茬,统一留茬高度5 cm,得到不同苗龄的平茬苗木,即M6 (6月龄)、M10 (10月龄)、M14 (14月龄)、M18 (18月龄)、M30 (30月龄)。在平茬之前对苗木进行1次摸底调查,主要标记苗木的分枝数,并对苗木的枝条(侧枝)用绑扎带圈记,以此区分新旧萌条。平茬后在切口处涂抹油漆,以防水分流失和病虫害侵入。
-
平茬后每隔60 d (即分别在平茬后60、120、180、240、300、360、420、480 d)采集1次云南松苗木,测定根系形态,即从2020年9月开始,从每个重复试验组中随机选择3株苗木,重复3次,一直持续至2021年11月,共计8次测定。将苗木整株挖出,用直尺测量样株的萌条长,精确到0.1 cm,用游标卡尺测量萌条基径,精确到0.01 mm,并将萌条取下,统计其数量。将样株分为地上部分和地下部分,地上部分作其他分析用。地下部分去除根系中附着的土壤和杂质,然后将根系放在透明塑料袋中用清水清洗干净,再用根系扫描仪进行根系扫描。扫描后用WinRHIZO分析软件获得总根长、根表面积、根平均直径、根总体积等根系形态指标。然后放入温度为80 ℃烘箱烘干,用精度为0.001 g的电子天平称量至恒量,即为根生物量。通过获得的根系形态指标和生物量指标,计算出比根长(总根长/根生物量)、根比表面积(根表面积/根生物量)、根组织密度为(根生物量/根体积)、根细度(总根长/根体积) 等根系构型指标[17]。
-
应用SPSS 26.0进行单因素完全方差分析,用Duncan法进行显著性检验(α=0.05)。云南松生物量的异速生长关系用方程y=axb来描述[18],线性转化为:lgy=lga+blgx。其中:y与x为根系形态特征(根平均直径、总根长、根体积、根表面积)与根生物量;a为性状关系的截距;b为异速生长指数,即线性关系的斜率,并把斜率b与1进行差异显著性比较,斜率b与1无显著差异时,为等速生长;斜率b与1有显著差异时,为异速生长[19]。此外,还对不同苗龄苗木间的斜率b进行多重比较,若不同苗龄间的斜率b差异显著,则表明异速生长轨迹发生改变,有表型可塑性。异速生长方程的计算及方差分析均由统计软件R中的Smatr包完成[20]。
-
由图1A可知:除M30和M14外,不同苗龄平茬苗木的根平均直径随时间推移变化趋势一致,平茬后60~120 与240~300 d时呈现下降趋势,其余时间呈现增长趋势,可能与所属的季节有关。除平茬后240 d外,M18和M30的根平均直径均大于其余平茬苗龄,平茬后240 d时M10的根平均直径较大。在平茬后480 d时,M6的根平均直径为0.825 mm,M30的根平均直径为1.302 mm。从整体来看,M30的根平均直径一直有较好的长势,且根平均直径表现为随着苗龄增大而增大的生长趋势。
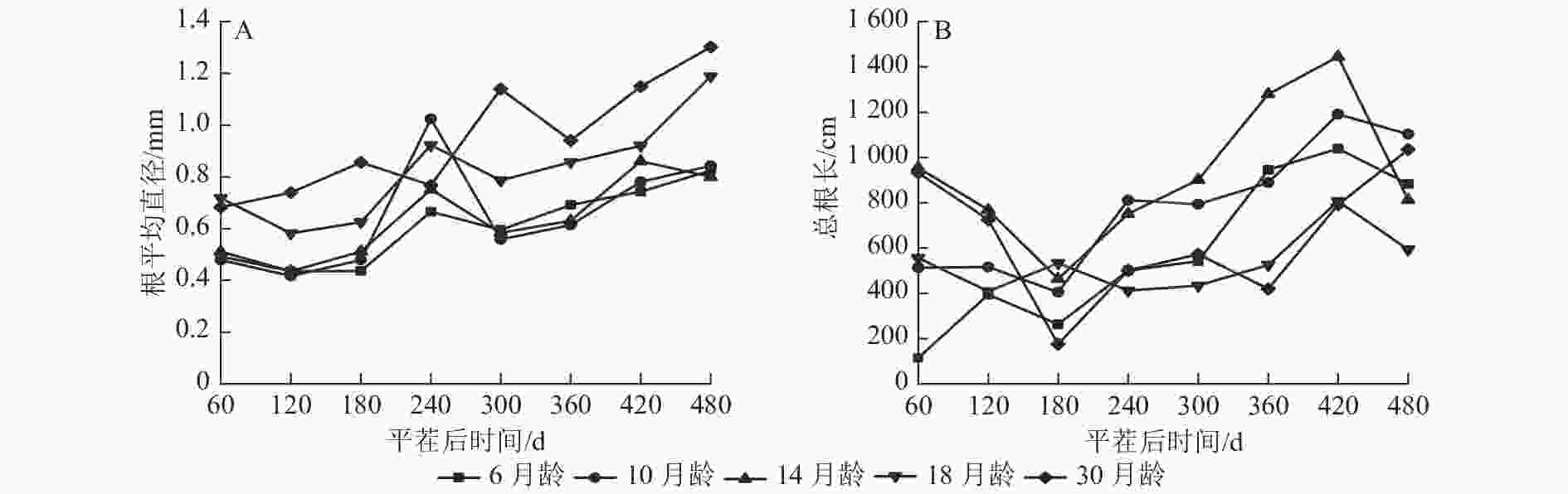
Figure 1. Variation of average root diameter and total root length with time in different seedling ages of P. yunnanensis stumping seedlings
由图1B可知:不同苗龄平茬苗木的总根长随时间推移变化趋势总体一致。除M6外,其余平茬苗龄苗木表现为总根长在平茬后60~180 d时降低,180~420 d总根长又呈增加的趋势, M6和M10的总根长在平茬后180 d前较短,随后增长变快。总体来看,总根长表现为随着苗龄增加先增加后减小的生长趋势,M6和M10的初始总根长较短,随着时间推移总根长生长快,M18和M30的初始总根长较长,随着时间推移总根长生长慢。
-
由图2A可知:不同苗龄平茬苗木的根体积随时间推移变化趋势总体一致。在平茬后60~180 d时根体积有所减小,随着时间的推移根体积有所增加。平茬后420 d时,M30的根体积增加变快,M14下降趋势明显。在平茬后360 d时,M30的根体积呈现增长加快的趋势,在平茬后60、120、420和480 d时均是M30的根体积大于其余苗龄。M6的根体积总体小于其余平茬苗龄。在480 d时,M6的根平均体积为4.435 cm3,M30的根平均体积为16.282 cm3。从整体来看,M30的根体积一有较好的长势,且根体积表现为随着苗龄增大而增大的生长趋势。

Figure 2. Variation of root volume and surface area of P. yunnanensis stumping seedlings with time under different seedling ages
由图2B可知:不同苗龄平茬苗木的根表面积随时间推移总体呈现先降低后增加的趋势。除M18外,其余苗龄平茬苗木在平茬后180 d以前的根表面积有所减小,随着时间的推移逐渐增加。在平茬后60、120、480 d时均是M30的根表面积大于其余苗龄。在平茬后180 d时,M18的根表面积最大,平茬后240~420 d时,M14的根表面积最大。在平茬后60、120、180、240 d时,均是M6的根表面积最小,平茬后360 d时,M30的根表面积最小。
-
从表1可见:除平茬后300和480 d外,从M6到M30的比根长随苗龄逐渐减小,在平茬后300 d时,M6、M10的比根长以及平茬后360 ~480 d时M6、M10和M14的比根长均显著大于其余苗龄的比根长(P<0.05),且苗龄间比根长差异不显著。从整体来看,M6的比根长一直有较好的长势,且比根长表现为随着苗龄增加而减小的生长趋势,随时间推移,M6、M10、M14之间比根长的差异有所减小。
平茬后时间/d 不同苗龄苗木的比根长/(cm·g−1) M6 M10 M14 M18 M30 60 2 365.007±901.510 a 1 556.480±559.489 b 1 209.910±737.060 b 332.793±167.766 c 241.239±71.996 c 120 1 457.954±588.839 a 1 028.822±393.701 b 687.435±182.464 c 263.379±81.094 d 286.997±289.058 d 180 768.183±202.630 a 579.952±269.778 b 371.361±86.468 c 262.487±79.542 c 83.081±7.998 d 240 1 039.679±318.141 a 746.030±364.945 ab 727.551±293.785 b 403.004±288.571 c 383.563±272.744 c 300 773.558±197.134 a 958.900±407.788 a 608.661±533.015 b 278.183±160.656 ab 633.522±718.033 ab 360 960.036±667.643 a 726.070±186.374 a 718.169±207.428 a 271.822±70.555 b 164.293±50.870 b 420 560.087±139.406 a 535.467±211.748 a 507.365±217.268 a 273.619±96.297 b 107.175±42.225 c 480 315.741±108.602 a 298.487±49.132 a 322.064±117.352 a 146.113±75.098 b 65.156±26.157 c 说明:表中数据为平均值±标准差。同行不同字母表示不同苗龄间差异显著(P<0.05)。M6. 6月龄,M10. 10月龄,M14. 14月龄,M18. 18月龄,M30. 30月龄。 Table 1. Variation of specific root length of P. yunnanensis stumping seedlings with time under different seedling ages
-
从表2可见:在平茬后60、120 d时,M6的根比表面积显著大于其余苗龄平茬苗木(P<0.05)。平茬后180 d时,M6和M10的根比表面积显著大于其余苗龄平茬苗木(P<0.05)。平茬后240~480 d时,M6、M10、M14之间没有显著差异。从整体来看,M6的根比表面积一直有较好的长势,且根比表面积表现为随着苗龄增加而减小的生长趋势。随时间推移,M6、M10、M14的根比表面积差异有所减小。
平茬后时间/d 不同苗龄苗木的根比表面积/(cm2·g−1) M6 M10 M14 M18 M30 60 358.667±123.753 a 233.679±89.001 b 192.197±107.299 b 69.791±26.223 c 52.336±11.692 c 120 204.351±96.262 a 134.660±53.384 b 93.906±21.808 bc 48.066±13.609 c 61.294±62.338 c 180 102.509±19.989 a 85.335±34.989 a 60.312±12.081 b 50.937±12.274 b 22.352±2.215 c 240 178.156±52.767 a 131.689±40.644 ab 159.044±67.559 ab 108.013±79.274 b 96.030±76.120 b 300 141.127±27.398 a 151.574±28.577 a 109.724±90.765 a 70.337±51.065 a 181.006±220.073 a 360 244.148±245.367 a 142.160±39.705 ab 140.305±29.155 ab 75.244±15.495 b 49.566±15.183 b 420 130.657±27.856 a 130.527±52.918 a 134.490±51.033 a 78.937±26.838 b 37.461±9.030 c 480 80.196±21.316 a 79.214±15.089 a 80.978±24.451 a 51.823±19.919 b 26.740±6.611 c 说明:表中数据为平均值±标准差。同行不同字母表示不同苗龄间差异显著(P<0.05)。M6. 6月龄,M10. 10月龄,M14. 14月龄,M18. 18月龄,M30. 30月龄。 Table 2. Variation of root specific surface area with time in different seedling ages of P. yunnanensis stumping seedlings
-
从表3可见:M6的根组织密度始终显著大于其余苗龄平茬苗木(P<0.05)。在平茬后60~360 d时,M14、M18和M30的根组织密度没有显著差异。在平茬后420和480 d时,M18和M30的根组织密度显著小于其余苗龄(P<0.05)。从整体来看,M6的根组织密度一直有较好的长势,且根组织密度表现为随着苗龄增大而减小的生长趋势。
平茬后时间/d 不同苗龄苗木的根组织密度/(g·cm−3) M6 M10 M14 M18 M30 60 2 365.007±901.510 a 778.240±279.744 b 403.303±245.687 bc 83.198±41.942 c 48.248±14.399 c 120 1 457.954±588.839 a 514.411±196.851 b 229.145±60.821 c 65.845±20.274 c 57.399±57.812 c 180 768.183±202.630 a 289.976±134.889 b 123.787±28.823 c 65.622±19.886 c 16.616±1.600 c 240 1 039.679±318.141 a 373.015±182.472 b 242.517±97.928 c 100.751±72.143 c 76.713±54.549 c 300 773.558±197.134 a 479.450±203.894 b 202.887±177.672 c 69.546±40.164 c 126.704±143.607 c 360 960.036±667.643 a 363.035±93.187 b 239.390±69.143 bc 67.955±17.639 bc 32.859±10.174 c 420 560.087±139.406 a 267.733±105.874 b 169.122±72.423 c 68.405±24.074 d 21.435±8.445 d 480 315.741±108.602 a 149.244±24.566 b 107.355±39.117 b 36.528±18.775 c 13.031±5.231 c 说明:表中数据为平均值±标准差。同行不同字母表示不同苗龄间差异显著(P<0.05)。M6. 6月龄,M10. 10月龄,M14. 14月龄,M18. 18月龄,M30. 30月龄。 Table 3. Variation of root tissue density with time in different seedling ages of P. yunnanensis stumping seedlings
-
从表4可见:除平茬后180、240和360 d外,M6、M10、M14的根细度显著大于其余苗龄(P<0.05),且3个苗龄间没有显著差异。平茬后180、240 d时,M6和M10的根细度显著大于其余苗龄(P<0.05)。从整体来看,M6的根细度一直有较好的长势,且根细度表现为随着苗龄增加而减小的生长趋势。随时间推移,M6、M10、M14的根细度差异有所减小。
平茬后时间/d 不同苗龄苗木的根细度/(cm·cm−3) M6 M10 M14 M18 M30 60 538.715±101.953 a 562.057±67.385 a 490.254±65.533 a 268.159±92.403 b 262.565±79.300 b 120 676.732±94.517 a 755.165±148.466 a 677.849±89.121 a 390.075±62.240 b 255.400±87.933 c 180 738.766±337.287 a 580.688±154.127 ab 328.577±68.932 bc 173.682±0.949 cd 475.307±103.989 d 240 432.975±92.777 a 380.991±123.399 a 261.408±110.540 b 178.308±58.188 b 221.305±50.541 b 300 376.687±96.275 a 441.787±186.435 a 357.782±64.583 a 221.037±76.707 b 231.492±153.209 b 360 257.560±80.470 b 329.895±39.174 a 308.735±76.841 ab 151.382±24.112 c 133.709±21.509 c 420 224.318±56.612 a 210.484±57.907 a 175.682±56.774 ab 149.549±24.835 b 97.803±30.956 c 480 190.122±48.450 a 180.684±39.987 a 191.692±30.004 a 92.608±27.830 b 68.164±26.017 b 说明:表中数据为平均值±标准差。同行不同字母表示不同苗龄间差异显著(P<0.05)。M6. 6月龄,M10. 10月龄,M14. 14月龄,M18. 18月龄,M30. 30月龄。 Table 4. Variation of root fineness with time in different seedling ages of P. yunnanensis stumping seedlings
-
不同苗龄苗木平茬后480 d,即平茬后萌条生长了16个月是本试验观测的最后时期,其萌条与采穗圃培育萌条的时长一致,这时的有效萌条可以采集进行扦插繁殖。研究分析此时苗木各个构件的发育情况,可以更好地了解平茬苗木的生长机制,为培育高质量萌条提供系统的研究思路。根系性状与根生物量间呈不同的异速生长关系(表5)。不同苗龄平茬苗木的根平均直径、根体积与根生物量的异速生长指数没有显著差异,并出现了共同斜率,分别为:−0.403 0、0.982 0,异速生长轨迹未发生改变。从总根长与根生物量间的异速生长关系来看,M30的斜率是负数,为负增长,总根长生长速率小于根生物量的生长速率;M14、M18的斜率为与1.0无显著差异的等速生长关系;M6、M10为显著大于1.0的异速生长关系(P<0.05),即M6、M10的总根长大于根生物量的生长速率。从根表面积与根生物量间的异速生长关系来看,M30的斜率为显著小于1.0的异速生长关系(P<0.05);M6、M14和M18的斜率为与1.0无显著差异的等速生长关系;M10为显著大于1.0的异速生长关系(P<0.05),即M10的根表面积大于根生物量的生长速率。
性状 苗龄 R2 P 斜率 F P−1.0 类型 根平均直径-根生物量 M6 0.736 0.006 −0.442 6 a 18.732 0.005 异速生长 M10 0.077 0.505 0.311 9 a 13.615 0.010 异速生长 M14 0.078 0.466 0.187 7 a 50.171 0.000 异速生长 M18 0.053 0.551 −0.414 4 a 7.381 0.030 异速生长 M30 0.661 0.008 0.501 8 a 11.460 0.012 异速生长 总根长-根生物量 M6 0.750 0.005 1.676 6 a 7.009 0.038 异速生长 M10 0.910 0.000 1.349 1 a 6.179 0.047 异速生长 M14 0.551 0.022 1.063 0 a 0.058 0.816 等速生长 M18 0.310 0.120 1.182 5 a 0.288 0.608 等速生长 M30 0.675 0.007 −0.478 9 b 13.942 0.007 异速生长 根体积-根生物量 M6 0.549 0.035 1.028 6 a 0.011 0.922 等速生长 M10 0.834 0.002 1.647 7 a 9.803 0.020 异速生长 M14 0.737 0.003 1.006 8 a 0.001 0.973 等速生长 M18 0.594 0.015 0.742 1 a 1.579 0.249 等速生长 M30 0.671 0.007 0.718 8 a 2.405 0.165 等速生长 根表面积-根生物量 M6 0.683 0.011 1.318 8 a 1.486 0.269 等速生长 M10 0.892 0.000 1.460 3 a 8.390 0.027 异速生长 M14 0.659 0.008 1.016 6 a 0.006 0.943 等速生长 M18 0.460 0.045 0.878 3 a 0.219 0.654 等速生长 M30 0.850 0.000 0.105 0 b 136.978 0.000 异速生长 说明:根平均直径、根体积与根生物量的共同斜率分别为−0.403 0、0.982 0;总根长、根表面积与根生物量无共同斜率。P−1.0 表示斜率与理论值1.0 的差异显著性;斜率中不同小写字母表示差异显著(P<0.05)。M6. 6月龄,M10. 10月龄,M14. 14月龄,M18. 18月龄,M30. 30月龄。 Table 5. Allometric growth of root traits and root biomass of P. yunnanensis stumping seedlings under different seedling ages
-
根系形态是衡量植物生产力的重要因素,在维持根系的功能中起重要作用,根系的形态及分布会影响其对土壤养分、水分的吸收,同时土壤条件的变化也会反过来影响根系的形态特性[21]。不同平茬时期对苗木有不同的影响,但苗木构件中的针叶和侧根会保持较大的生长速率,不受平茬季节的影响[15]。不同苗龄平茬处理对苗木根平均直径、总根长、根体积、根表面积有显著影响。本研究表明:不同苗龄平茬苗木根平均直径、根体积表现为随着苗龄增大而增大的趋势,不同苗龄平茬苗木总根长表现为M6和M10随着时间推移生长加快,M18和M30随着时间推移生长减慢。在本研究中不同平茬苗龄苗木的根系生长均发生了改变,但因苗龄不同,苗木所采用的生长策略不同。总根长、根体积以及根表面积在平茬后60~180和420 d后有所下降,这可能是因为此期间正好为秋冬季。有研究表明:随着冬季的到来,植物以及根系进入休眠期,活力下降[22],大部分生命较短、直径较小的根,可能会因为地下水分、温度以及碳分配的降低而减小甚至消亡[23]。从平茬后300 d (5月)开始,是不同苗龄平茬苗木的总根长生长关系产生显著变化的转折点。此时正值初夏,天气炎热,苗龄较小的苗木需要大量的水分供给。水分胁迫本身会使根系直径变小,根长增加,从而提高根系对养分的吸收速率,保证苗木在资源贫瘠的环境中也能保持正常生长发育[24]。这与沙柳Salix psammophila、骆驼刺Alhagi sparsifolia在干旱条件下增加根系长度,占有大面积的土壤来获取水分,适应胁迫环境的结果相同[25−26]。相对于苗龄较小的苗木,较大苗龄的苗木更需要根的支撑,保证植物稳定生长。根系直径变粗可以提高根系对轴向阻力的克服能力[27]。其原因可能是机械阻力改变了根尖细胞的生长,影响了根系淀粉石的沉淀,从而诱发生长素流动和分配的变化。根系阻抗还会引起根系生长深度的变化,总根长也会随之变短[28]。
不同苗龄平茬苗木根系构型随时间推移有显著性差异,比根长与根系的吸收面积和资源获取率成正比[29]。根比表面积与养分利用效率成正比,根组织密度是反映根功能情况的特征之一,因为其和根的生理活动紧密相连[30]。根细度是总根长与根体积的比值,其值变化可反映根的粗细。有研究表明:根系越细越有利于植物对土壤中营养元素的吸收[31]。本研究中M6的比根长、根比表面积、根组织密度、根细度均保持较好的长势,且表现为随着苗龄增加而减小的生长趋势。随时间推移,M6、M10、M14除了根组织密度外的其余指标差异均有所减小,表明不同苗龄苗木平茬后所采取的生态策略不同。相对于苗龄较大的苗木,苗龄较小的苗木以较大的比根长采取了低投资高收益的生态策略,苗龄较小的苗木构建了更细、更密集的根系,这有利于增加根系分布的深度和广度,从而提高对土壤养分的获取能力和利用效率[17]。对黄土高原子午岭辽东栎Quercus wutaishanica不同年龄幼苗根系构型的研究表明:随年龄的增加,不仅粗根比例逐渐增加,根系内外连接数量也在增加,说明根系的固着作用随苗龄的增加而加强[32]。这与本研究结果一致,随着平茬苗龄的增加,根系皮层组织退化,维管束逐渐发达,木质化程度高,代谢慢,养分吸收速率低,逐渐提高了运输和储藏功能。所以在本研究中苗龄较小的M6长势最好,既能为地上部分提供资源又能促进地下部分与地上部分的良性循环。
形态性状是植物长期与环境相互作用、逐渐适应形成的[33],其中根系的形态变化最为显著[34]。根平均直径、根体积与根生物量在不同苗龄平茬苗木间出现了共同斜率,即苗木间异速生长轨迹没有发生变化。表明不同苗龄平茬后,苗木根平均直径、根体积与根生物量的生长速率没有显著差异,呈现趋同进化趋势,异速生长关系较为稳定[35]。总根长、根表面积与根生物量之间没有共同斜率,且M30显著小于其他苗木,表明不同苗龄平茬对苗木根系的生长速率影响不同,随着苗龄增大,总根长、根表面积的生长速率呈逐渐降低的趋势。M6、M10与M30的总根长以及M10与M30的根表面积的异速生长关系发生了显著性差异,具有可塑性。植物根系在受到胁迫后,会采取改变代谢途径,重新分配能量物质,改变根系形态等措施来响应外界胁迫[36]。较长的根系能扩大植物吸收水分与养分的范围,能较好地适应外界环境[37]。根系表面积能够反映出根系在土壤空间中的生长状况,进而推断出根系的吸收、代谢及固结土壤能力[38]。经济型谱认为:在资源充足的环境下,植物会选择竞争型生长策略。对杨树Populus的研究表明:年龄较小的苗木为满足快速生长和对水分养分的需要,会加大根生物量的分配比例,促进构建根系生长[39],这与本研究结果一致。苗龄较小的苗木以较大的总根长、根表面积生长速率来最大限度地获取养分资源,实现资源的有效利用。植物体内的水流阻力随年龄和个体大小的增加而加强,从而阻碍了水分和养分的运输[40]。因此苗龄大的苗木选择保守型生长策略,降低总根长、根表面积生长速率,以增加自身的耐受力和持久性。虽然不同苗龄平茬苗木的根系形态生长速率不同,但苗木均需要对其投入代谢成本,两者相比苗龄较大的苗木对地下部分投入的成本更高,促进了根系生长。而庞大的根系消耗了大量的水分、养分,减少了对地上部分的能量物质输送,不利于地上部分的生长。因此在平茬苗木生长发育过程中应注意对土壤养分、水分的检测,高效利用水肥资源。
-
平茬对云南松不同苗龄苗木根系形态、根系构型生长特征以及苗木构件的异速生长规律产生了影响。根系形态指标与根系构型指标表明平茬苗龄较小的苗木构建了更细、更密集的根系。异速生长指标表明:随着平茬苗龄增加,总根长、根表面积与根生物量生长速率呈逐渐降低的趋势。综上所述,平茬苗龄较小的苗木根系对资源的获取和利用效率更高。在本研究的5个平茬苗龄中,以6月龄平茬苗木的根系生长状况较好,平茬效果较好。
Analysis of root morphological characteristics of Pinus yunnanensis seedlings at different stump-ages
doi: 10.11833/j.issn.2095-0756.20230466
- Received Date: 2023-09-11
- Accepted Date: 2023-12-04
- Rev Recd Date: 2023-12-01
- Available Online: 2024-03-21
- Publish Date: 2024-04-01
-
Key words:
- Pinus yunnanensis /
- seedling age /
- stumping /
- root morphology
Abstract:
| Citation: | CHENG Sili, WANG Dan, HE Bin, et al. Analysis of root morphological characteristics of Pinus yunnanensis seedlings at different stump-ages[J]. Journal of Zhejiang A&F University, 2024, 41(2): 322-332. DOI: 10.11833/j.issn.2095-0756.20230466 |









 DownLoad:
DownLoad:
