-
兰科Orchidaceae以其多样性化的“欺骗性传粉系统”(包括食源性欺骗、性拟态、繁殖地拟态和信息素拟态等类型)而闻名[1]。石豆兰属Bulbophyllum是兰科最具代表性的属之一,该属因其假鳞茎圆形而得名,目前全世界已知2 000多种,中国仅有105种[2−4]。近年来,由于气候变化、生境破坏、人为采集等原因,石豆兰属植物的野生种群受到不同程度的破坏,面临着生存危机[5]。莲花卷瓣兰Bulbophyllum hirundinis是一种小型附生兰科植物,具有较高的药用价值与观赏价值[6−7],主要分布于中国的浙江、安徽、广西、海南、云南、台湾等地[8],通常生长在海拔500~3 000 m山地林的树干或岩石上[9],被《世界自然保护联盟濒危物种红色名录》(IUCN)评估为“近危”(NT)物种[10]。
目前,关于莲花卷瓣兰的研究集中在新分布地和潜在适生地等方面[11−12]。有研究发现:气温是影响莲花卷瓣兰分布地的最主要因素,推测其适宜分布地在广东、海南等南部过渡带以南的地区[13]。但关于莲花卷瓣兰的繁育系统与传粉生物学还鲜见报道。大多数兰科植物具有特定的传粉者[14],这种特殊的传粉系统增加了兰科植物灭绝的风险[15],这可能也是致莲花卷瓣兰濒危的原因之一。由于低海拔区域人类活动较为频繁,该物种与其他兰科植物一样受到生境丧失的威胁[16]。长期种群监测数据显示:莲花卷瓣兰数量呈下降趋势,亟需采取保护措施。开展繁育系统与传粉生物学研究是实施有效保护的重要基础[17]。这些研究不仅能揭示莲花卷瓣兰濒危机制,更能为制定科学的保护策略提供依据[18−19],对种群的长期存续具有决定性意义。
鉴于此,本研究通过野外调查对莲花卷瓣兰的开花物候、花部形态、繁育系统和传粉昆虫进行研究,探讨了莲花卷瓣兰的繁育系统与传粉生物学特征,并量化其在自然范围内的繁殖成功率,以期为保护该物种提供科学依据。
-
研究区位于浙江省杭州市临安区清凉峰国家级自然保护区(30°04′3.42″N,118°56′56.53″E,海拔为485 m),所处群落为山核桃Carya cathayensis混交林,本研究所选的莲花卷瓣兰位于该混交林下西北坡的岩石壁上,居群数量约212株。该区域为亚热带北缘,年均气温为11.7~14.7 ℃,年平均降水量为1 862.0~2 332.0 mm。无霜期为234.0 d[20]。所选莲花卷瓣兰居群生长习性为附生,花序为伞形,单株结果蒴,喜温暖湿润、半阴环境。
-
2023和2024年的5—6月开花期,根据DAFNI等[21]的方法,记录莲花卷瓣兰整个居群的初花期、盛花期和末花期。当25%的植物开始开花时记为居群的始花期,约5月中上旬;当50%的植物处于开花高峰时,记为居群的盛花期,约5月中下旬;当95%的植物完成开花时,记为居群的末花期,约6月上旬。为了评估花的寿命,从未授粉的花和补充授粉的花开始监测,直到枯萎。使用相机对20株植物进行了蜜汁分泌评估,并通过嗅闻花来确定花香。
从处于盛花期的花序中随机选取15个花序,每个花序随机选取1朵开放的花,用游标卡尺(精度为0.01 cm)对花部进行测量,包括花萼、花瓣、唇瓣的长度及宽度,以及访花昆虫入口高度(药帽到唇瓣的距离)、入口宽度(唇瓣的宽度)和入口深度(唇瓣的长度)。
-
2023和2024年的5—6月,在莲花卷瓣兰花开放前随机选择20个单株,每株平均1个花序,每株随机选取4朵花,用细尼龙网(间隙0.5 mm×0.5 mm)进行套袋处理。当花完全开放时,在已套袋的20株中选取5株进行自花授粉后套袋(自花授粉),5株异花授粉后套袋(异花授粉),5株去雄不授粉后套袋(去雄套袋),5株维持套袋不授粉(直接套袋),以自然状态为对照(自然授粉)。开花结束后对不同处理的坐果情况进行统计。
-
2023和2024年的5—6月分别进行了访花昆虫观测。在晴天8:00—17:00随机选取花朵(10个花序共60朵花)进行观测,共观测90 h。由于花的体积较小,使用LAOWA Mini FF Ⅱ 85 mm F5.6 Macro微距镜头搭配SONY Alpha 6000相机观察昆虫对花的访问频率,使用三脚架固定相机进行拍摄,以减少观察者的气味和动作对访花昆虫的潜在干扰,并对访花昆虫的行为进行记录和拍照。同时,使用封口塑料袋捕获其他植株上的访花昆虫,经乙酸乙酯处理后用于物种鉴定,测量其体宽、体长、胸高数据以进行传粉昆虫评估。标本带回实验室后通过查询《浙江昆虫志》进行物种鉴定,然后存放于浙江农林大学林业与生物技术学院。
为了确认访花昆虫是否为传粉昆虫,每隔30 min检查被访问花的花粉团数量是否缺少。为评估夜间传粉者是否存在,同时选取15株植物个体的76朵花,每天在18:00记录每朵花的花粉团数量,并在次日7:00再次检查。
-
利用Excel 2016进行数据处理与绘制表格,Photoshop 2021进行图片制作[22]。
-
物候监测表明:莲花卷瓣兰在5月上旬至6月中旬开花,花黄色带紫红色,不同年份之间有差异。伞形花序着生3~6朵花,方便了传粉者在不同花朵之间进行转移,平均每花序为(5.3±1.5)朵,平均单花花期约10 d,平均花序花期约12 d,群体花期约30 d,多数花(60%左右)在15 d内开放,具有集中开花特性,果实7月上旬成熟。
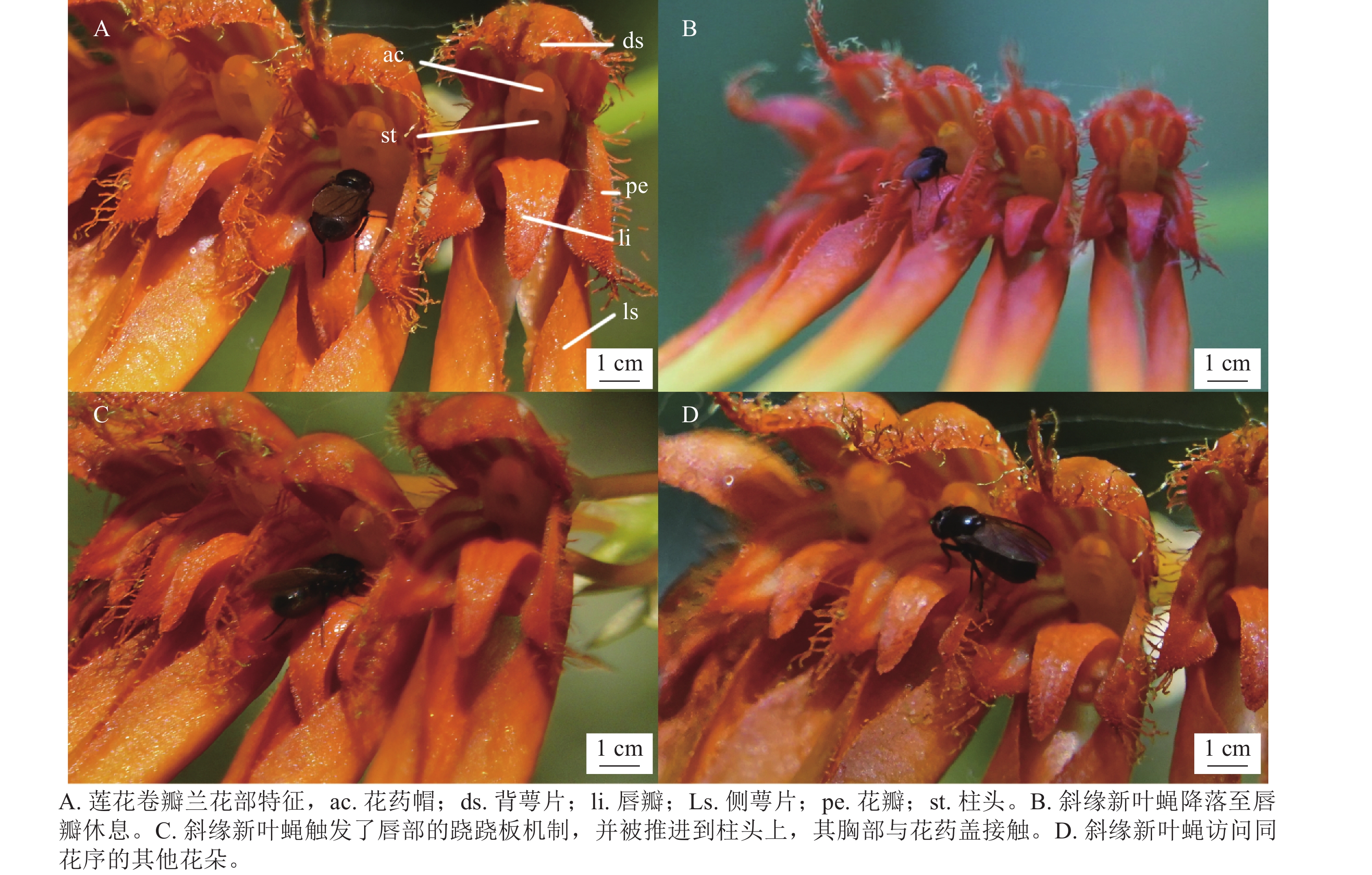
如表1所示:莲花卷瓣兰苞片披针形,长约3 mm,中萼片卵形,长约5 mm,中部宽约3 mm,先端较短,边缘具流苏状缘毛;花瓣斜卵状三角形,长约3 mm,中部宽约2 mm,先端锐尖,边缘具流苏状的缘毛,具3条脉;唇瓣呈现肉质化特征,整体呈舌形,长约2.5 mm,自中央区域向外部延伸并呈显著下弯形态。其基端具凹陷沟槽,通过与蕊柱足末端的机械偶联,构成铰链式关节结构,赋予其可动性功能。在所观察的花中没有发现蜜腺。
项目 花瓣
长/mm花瓣
宽/mm中萼片
长/mm中萼片
宽/mm唇瓣
长/mm唇瓣
宽/mm花葶
长/mm平均值 3.04 2.11 5.14 2.37 2.37 1.51 82.50 标准误 1.61 0.97 2.11 0.66 0.66 0.37 51.12 说明:样本数为15个花序。 Table 1. Floral trait of B. hirundinis
-
如表2所示:不同授粉方式下结实率有明显的区别,自花授粉和直接套袋未授粉均未见结实,说明莲花卷瓣兰自交不亲和。异花授粉处理后套袋,结实率为60.00%,说明莲花卷瓣兰异交能正常坐果。去雄处理后套袋,结实率为0,说明莲花卷瓣兰不存在无融合生殖现象。自然状态下,共记录了179朵花,结实率为21.23%。
处理 花朵数/朵 坐果数量/粒 结实率/% 自花授粉 20 0 0 异花授粉 20 12 60.00 去雄套袋 20 0 0 直接套袋 20 0 0 自然授粉 179 38 21.23 Table 2. Fruit setting rate in different pollination types of B. hirundinis
-
通过对15株植物个体的76朵花的花粉团数量统计发现:莲花卷瓣兰不存在夜间花粉移出现象,说明莲花卷瓣兰无夜间传粉者。对莲花卷瓣兰2 a的日间观测中,共观测到6种访花昆虫,分别为斜缘新叶蝇Neophyllomyza leanderi、天目山短肛䗛Baculum tianmushanense、中华驼舞虻Hybos chinensis、游举腹蚁Crematogaster vagula、黑颜单突叶蝉Lodiana brevis和管蓟马科Phlaeothripidae (仅有1种)。
其中天目山短肛䗛和黑颜单突叶蝉为植食性昆虫,啃食莲花卷瓣兰的花瓣和叶片。中华驼舞虻是周围环境中活动较为频繁的肉食昆虫,访花频率极低,在整个访花过程中仅活动在花瓣与花梗上,同时未观察到其携带花粉,属于对莲花卷瓣兰不起传粉作用的天敌昆虫。游举腹蚁从花梗处爬至花序上,从侧面进入花朵吸取花蜜或露水,多见于未完全开放的花朵,访花频率较低。访花管蓟马昆虫常停留在花萼与花瓣上,未观察到其有转移花粉的行为。如表3所示:在日间观测中斜缘新叶蝇、天目山短肛䗛、中华驼舞虻、游举腹蚁、管蓟马和黑颜单突叶蝉均为莲花卷瓣兰的访花昆虫,除管蓟马和斜缘新叶蝇外,其余访花昆虫的体宽和胸高均大于传粉通道的入口宽度与入口高度,无法进入传粉通道,并无传粉作用。管蓟马大多发现于花瓣上,亦无传粉作用。由于被观察植物花朵较小,视频拍摄受风力影响较大,难以直接清晰地拍摄到访花昆虫携带的花粉。通过每30 min观察其花粉团是否减少,结合30 min内的访花昆虫视频记录进行对比,仅发现斜缘新叶蝇是有规律的访花者,且有接触合蕊柱并将花粉从花中移出的现象。
物种 个体数/个 入口宽度或体宽/mm 入口高度或胸高/mm 入口深度或体长/mm 莲花卷瓣兰Bulbophyllum hirundinis 15 0.85±0.31 0.82±0.22 2.31±0.12 斜缘新叶蝇Neophyllomyza leanderi 10 0.55±0.04 0.56±0.07 2.12±0.12 中华驼舞虻Hybos chinensis 5 2.15±0.15 0.72±0.35 5.58±0.85 天目山短肛竹节虫Baculum tianmushanense 5 2.11±1.51 1.85±0.51 32.1±1.35 游举腹蚁Crematogaster vagula 5 1.21±0.12 1.16±0.15 3.12±0.35 黑颜单突叶蝉Lodiana brevis 3 3.80±0.73 1.20±0.15 0.72±0.35 管蓟马Phlaeothripidae 5 0.15±0.10 0.12±0.12 0.72±0.35 Table 3. Comparative size of B. hirundinis flowers and various flower-visiting insects
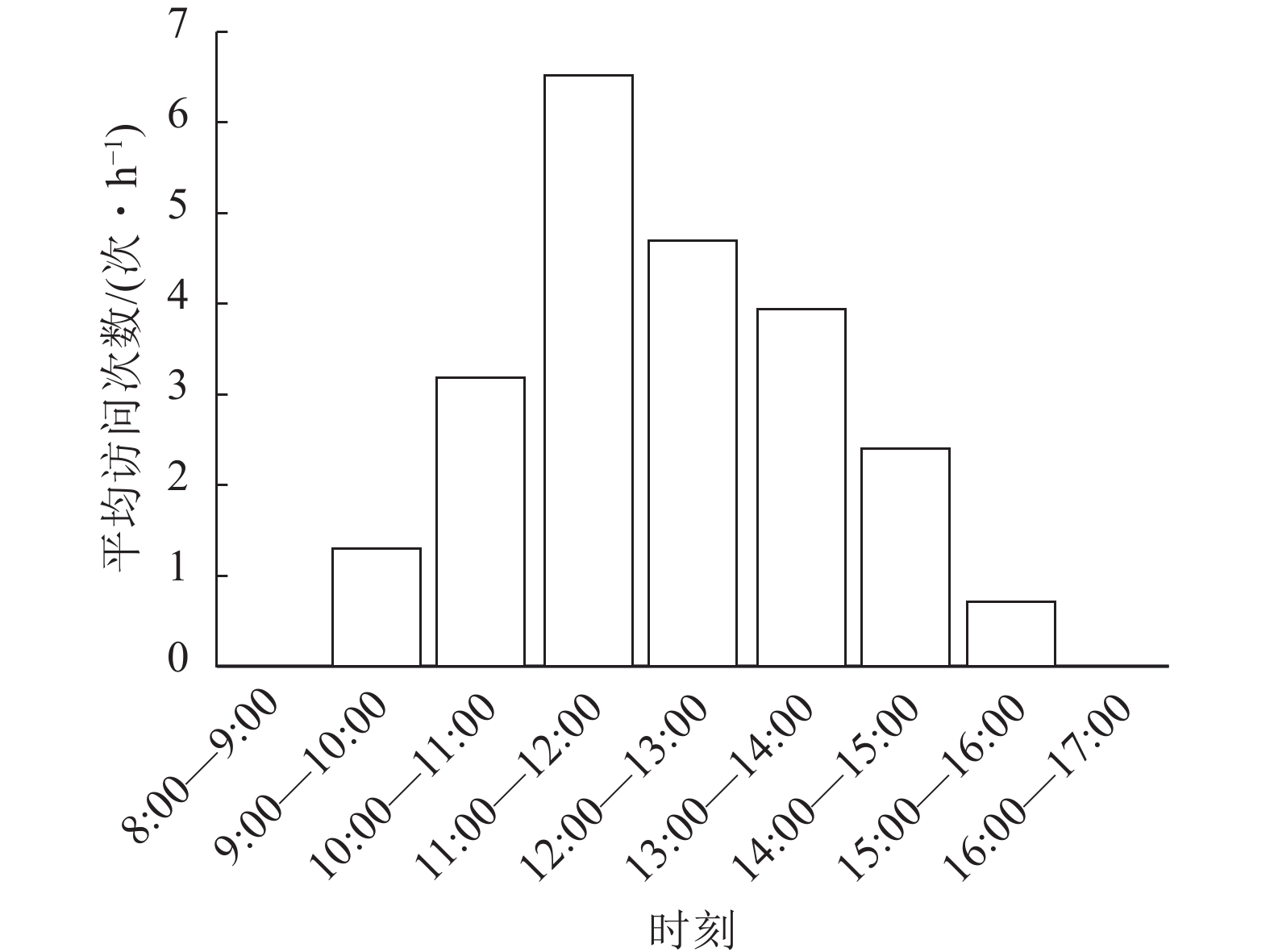
在90 h的拍摄过程中,证明斜缘新叶蝇是莲花卷瓣兰的有效传粉者(图1)。当斜缘新叶蝇飞翔降落在唇瓣前端后,其会向唇瓣基部移动,达到一个临界平衡点后,斜缘新叶蝇的质量会导致有铰链结构的唇瓣向上翘起,随着斜缘新叶蝇的继续移动,开始重复做弹簧运动,推动斜缘新叶蝇的胸部与花蕊柱接触(图1)。斜缘新叶蝇移出花粉团后在同一朵花中停留一段时间,可能会接着访问同一花序中相邻的几朵花,也可能会飞走。斜缘新叶蝇对莲花卷瓣兰的访问通常在气温为25 ℃的晴朗条件下,9:00气温开始上升,此时斜缘新叶蝇开始活动,访花行为集中在9:00—15:00,随后访花次数非常少,其访花频率在气温较高的11:00—12:00达到最高峰,平均访花次数达6次·h−1 (图2)。本研究表明:斜缘新叶蝇平均单次访花为(5.70±0.40)朵(n=40),单花停留时间为(7.41±0.45) s (n=145),单次访问平均停留时间为(41.12±7.14) s (n=40)。传粉通道的入口宽度为(0.85±0.31) mm (n=15),入口高度为(0.82±0.22) mm (n=15),都略微高于斜缘新叶蝇的胸高和体宽,入口深度为(2.31±0.12) mm,高于斜缘新叶蝇的体长。此外,本研究未观察到斜缘新叶蝇在莲花卷瓣兰上的产卵行为,也未在任何花上发现斜缘新叶蝇卵,说明莲花卷瓣兰并非通过模拟斜缘新叶蝇的产卵地诱导其传粉。
-
兰科植物大多数都是自交亲和的[22−23],但对于自交不亲和物种也有少量报道[24]。本研究发现:莲花卷瓣兰自交不亲和,与石豆兰属芳香石豆兰Bulbophyllum ambrosia不同[25],但与同组的天贵卷瓣兰Bulbophyllum tianguii和二色卷瓣兰 Bulbophyllum bicolor等相同[18, 26]。石豆兰属植物,自然结实率普遍较低,如拟泰国卷瓣兰B. nipondhii自然结实率为6.95%[27],莲花卷瓣兰自然状态下结实率较高;同时发现莲花卷瓣兰人工异花授粉植株的结实率与梳帽卷瓣兰B. andersonii相近,比自然授粉的植株明显更高,可能与花粉限制有关。因此,自花授粉不亲和可能是导致莲花卷瓣兰自然结实率低的主要原因,这对于无“欺骗性传粉系统”的兰花来说并不罕见[28]。
-
在兰科植物中,花形较小的植物大多数是由蝇类帮助授粉的,但不同植物执行的策略不同。如黄花杓兰Cypripedium flavum会散发出臭味物质吸引蝇类传粉[29];热带亚洲兜兰属Paphiopedilum和火烧兰属Epipactis大多数物种的花都有类似蚜虫的斑点,可以吸引喜欢在蚜虫群中产卵的雌性食蚜蝇前来传粉[30−31]。莲花卷瓣兰花较小,访花昆虫无法进行有效传粉。
石豆兰属植物具有弹性的唇瓣,被认为是适应虫媒传粉平行进化的结果[32]:当昆虫停留在唇瓣上活动时,唇瓣可产生反弹力,将访花昆虫弹起送到合蕊柱上带走花粉或将花粉带到柱头腔内[33]。本研究显示:莲花卷瓣兰活动的唇瓣在传粉过程中同样起到重要的作用,能够将斜缘新叶蝇多次弹起接触合蕊柱,完成带出或送入花粉的行为。同时,传粉通道与昆虫体型的匹配度也是提高传粉效率的关键因素,它可以限制许多非传粉的访花昆虫带出花粉。在对莲花卷瓣兰的长时间观测中,斜缘新叶蝇是唯一访问完一朵花后会连续访问同株其他花朵的访花昆虫,也只有它与莲花卷瓣兰传粉通道大小相近,能够进入传粉通道完成传粉。
-
现有研究表明:部分兰科植物在进化过程中与传粉昆虫形成了完美的协同进化关系,有些甚至形成了一对一的专性传粉关系[34]。而石豆兰属物种除少部分以蜜蜂为传粉媒介[25],大多数为蝇类传粉,且被认为具有典型的蝇类传粉特征[35],例如梳帽卷瓣兰、拟泰国卷瓣兰的传粉者分别为曲角杆蝇Gampsocera sp.、蚤蝇Megaselia sp. [27, 36]。石豆兰属植物的花通常因含有甲基丁香酚(methyl eugenol)和树莓酮(raspberry ketone) 2种蝇类的引诱剂而散发出难闻的气味[37],也有些因为含有姜酮而散发出“水果气味”[38]。这些气味一方面能帮助石豆兰属植物吸引到特定的传粉昆虫,如B. cheiri花内的甲基丁香酚能吸引杨桃实蝇Bactrocera carambolae、木瓜实蝇B. papayae和番石榴实蝇B. umbrosa等蝇类成为其传粉昆虫[39];另一方面也能作为材料帮助传粉昆虫合成其他物质,如姜酮常被一种雄性果蝇采集后用于合成性信息素;这种独特的互惠关系让双方都能获得有效的生殖效益[40]。莲花卷瓣兰上发现了蜘蛛和食虫虻的活动,而真叶蝇属Eupeodes成虫有典型的资源利用行为特征,其通过截获捕食者(如蜘蛛目Araneae、食虫虻科Asilidae等节肢动物)已控制的猎物资源完成能量获取[41],这可能促进了斜缘新叶蝇的访花。斜缘新叶蝇可能和其他具有访花习性的叶蝇科Milichiidae成虫一样[40],被莲花卷瓣兰的纤毛和挥发性气味吸引到唇瓣上取食分泌物。此外,石豆兰属植物的唇瓣基部通常有一个小的弹性铰链,当访花昆虫在花上活动时会促使其与花粉接触,增加传粉的效率[42]。
传粉昆虫对传粉的贡献取决于它们的数量和它们的行为。莲花卷瓣兰中萼片上的白色条纹可能对访花昆虫起到视觉信号的作用,但仅当传粉者在访问过程中完成花粉附着与转移行为时,方可将其界定为有效传粉者(effective pollinator)。本研究观察到莲花卷瓣兰的访花昆虫6种,但仅有斜缘新叶蝇为莲花卷瓣兰的有效传粉昆虫,这在石豆兰属传粉生物学研究中属首次报道。
-
莲花卷瓣兰为伞形花序,单花花期约10 d,群体花期为5月上旬至6月中旬,开放时无明显香味。莲花卷瓣兰的繁育系统是需要传粉者异交的交配系统,自然结实率较低(21.22%),唯一的传粉者是斜缘新叶蝇。
Research on the breeding system and pollination biology of Bulbophyllum hirundinis
doi: 10.11833/j.issn.2095-0756.20250150
- Received Date: 2025-02-20
- Accepted Date: 2025-09-03
- Rev Recd Date: 2025-06-30
- Available Online: 2025-11-26
- Publish Date: 2025-12-20
-
Key words:
- Bulbophyllum hirundinis /
- breeding system /
- pollination biology /
- Neophyllomyza leanderi
Abstract:
| Citation: | HU Kaida, GUO Rui, LONG Chengpeng, et al. Research on the breeding system and pollination biology of Bulbophyllum hirundinis[J]. Journal of Zhejiang A&F University, 2025, 42(6): 1184−1191 doi: 10.11833/j.issn.2095-0756.20250150 |









 DownLoad:
DownLoad: