-
氧气约占大气成分的21%,是大多数多细胞生物维持生命的必需元素,包括真菌、动物和植物。生物不仅需要氧气作为末端电子受体,以确保通过氧化磷酸化产生能量,而且在大多数代谢反应中,氧气也充当主要底物[1]。当植物生长对氧气的需求超过环境氧气的供应时,植物细胞中氧气浓度便会降低,造成缺氧。在生产中,缺氧一般是由土壤板结、淹水等因素造成的,表现为植物根系和其他器官中的氧浓度降低[2-3]。缺氧会对植物生长造成胁迫,改变植物的代谢水平,降低作物的产量[4]。缺氧会影响植物的生物量积累、激素含量、各类酶活性、细胞结构及过氧化物的产生。如ANNALISA等[5]研究发现:在缺氧条件下,拟南芥Arabidopsis thaliana细胞中活性氧(ROS)含量急剧增加,其体内过氧化物酶(POD)和过氧化氢酶(CAT)的活性降低。缺氧同时也会造成乙烯在植物根系的积累,如鹰嘴豆Cicer arietinum、蚕豆Vicia faba、水稻Oryza sativa等植物淹水后根系乙烯显著积累[6-7],参与乙烯合成的2个基因ACS1和ACO5相对表达显著增加[8]。在水稻中,脱落酸(ABA)含量会因淹水降低[9],而赤霉素(GA)含量显著升高[10],说明氧气在植物的各个生理过程中都起到重要作用。在长期进化过程中,植物体也有一定的适应手段来应对缺氧的胁迫。植物在缺氧条件下,会降低自身新陈代谢的水平,如淀粉、蛋白质和脂质等代谢[3,11]。水稻[12]、玉米Zea mays [13-14]和小麦Triticum aestivum [15]等能通过在根系形成通气组织从而适应缺氧环境,特别是水稻还可以在茎秆内形成通气组织[16], 从而在淹水条件下正常生长。水稻的通气组织是由乙烯诱导,通过细胞程序性死亡和皮层细胞溶解所形成的[17]。在淹水条件下,大气中的氧气是植物体内氧气的重要来源,如芦苇Phragmites australis [18]和水稻[19],能通过通气组织将氧气输送至地下部分,从而使得它们在长期淹水的土壤中依然能正常生长。雷竹Phyllostachys violascens属禾本科Gramineae中小型散生竹类,是一种重要的笋用竹种,喜湿润,怕积水[20]。自20世纪90年代以来,覆盖增温技术在生产中得到大面积推广应用,使雷竹笋在经济价值较高的时候大量上市,显著提高了农户的经济收入,然而长期林地覆盖经营却会引起雷竹林退化[21-22]。覆盖物的阻断加上土壤中微生物大量消耗有机物质,使得雷竹林地土壤中的氧气含量降低。有学者研究表明:竹鞭上浮可能是对覆盖技术引起的土壤缺氧的适应性反应[23]。那么氧气的缺乏会对雷竹生长造成什么的影响?雷竹为适应缺氧会做出如何的响应?对于其他禾本科植物,如水稻等,其生长与氧气之间的联系研究多且较深入[24-26],但竹子生长与氧气的关系研究鲜有报道。本研究以雷竹水培苗为材料,比较分析叶面积、生物量、光合色素质量分数和抗氧化酶活性等生长及生理指标以及根系结构变化,研究缺氧对雷竹生长的影响,初步探究雷竹对缺氧的适应性机制,为雷竹在生产实践中可能退化的原因提供理论参考。
-
2018年6月从大田中挖取雷竹小苗,带回温室进行水培驯化,待其适应水培条件后,剪取基部萌蘖生长的雷竹苗进行培养扩繁。2019年6月筛选其中长势均一的无性系雷竹水培苗作为试验材料[苗质量(1.446±0.186) g·株−1]。
-
试验于 2019 年 7−9 月,在浙江农林大学智能温室进行。以黑色圆桶(直径179 mm,高178 mm,容量为3 L)为培养容器,1/2木村B营养液(pH 5.6)为培养液,培养雷竹苗。每个容器分别装有营养液2.5 L,每盆培养6株雷竹水培苗,共3个重复。隔5 d更换1次营养液,培养时间为2个月。同时设置土培苗作为对照。于试验开展前通过流量计调节通气泵气体流速,控制营养液中溶解氧质量浓度,得出流速为0、6、27、55、400 mL·min−1,分别对应的溶解氧质量浓度约为0、2、4、6、8 mg·L−1,因此设置0、2、4、6、8 mg·L−1等5个处理组(图1)。为保证处理前营养液中溶解氧的一致性,用氮气充入营养液将其中的氧气去除,持续15 min使处理前营养液中溶解氧质量浓度为0 mg·L−1。
-
试验期间,于每日中午12:00,采用光纤式氧气测量仪(Firesting O2, Pyro Science, 德国)测定。
-
生物量增加值:实验前用吸水纸吸干根系表面的水分后,称取雷竹水培苗植株的鲜质量(精确至0.001 g),试验结束后再用吸水纸吸干根系表面的水分,称量植株鲜质量,求前后差值,重复3次。叶面积:利用统一的A4打印纸按竹叶轮廓描下成熟竹叶形状,带回实验室按轮廓剪下分别称量,记为A;另外,剪取面积为100 cm2大小的正方形A4纸张,称取其质量,记为B,每盆描9片竹叶,重复3次,叶面积(S,cm2)计算公式为:S= 100×A/B。
-
取成熟的雷竹水培苗叶片,去叶脉并剪碎,称取0.3 g,加入2 mL预冷的50 mmol·L−1的磷酸缓冲液(pH 7.8),迅速冰浴研磨成浆,并用磷酸缓冲液冲洗研钵,定容至8 mL,离心 20 min(4 ℃,8 000 r·min−1),取上清液于0~4 ℃下保存备用,供抗氧化酶活性测定。超氧化物歧化酶(SOD)采用南京建成生物工程研究所研制的SOD酶活(植物)试剂盒进行测定。过氧化氢酶(CAT)采用紫外吸收法测定[27]。过氧化物酶(POD)采用愈创木酚法测定[28]。抗坏血酸过氧化物酶(APX)参照 YOSHIYUKI 等[29]的方法进行测定。以上酶活性测定均重复3次。叶绿素质量分数测定:成熟叶片用去离子水洗净并用吸水纸擦干,去除叶脉并剪碎,称取0.1 g,置于10 mL离心管内,采用体积分数95%乙醇浸提法测定[30],重复3次。根系活力测定:取根尖样品洗净,用吸水纸吸附表面水分后称取 0.5 g,采用氯化三苯基四氮唑(TTC)法测定[31],重复3次。
-
取长度约10 cm的根系,将根系分为4段(距离根尖0.5~1.5、2.5~3.5、4.5~5.5、6.5~7.5 cm),迅速平整埋入未凝固的体积分数4%琼脂糖溶液,冷却凝固后取出置于全自动振动切片机上切取根系横截面,厚度为100 µm,利用光学显微镜观察拍摄,最终通过ZEN软件分析图像计算通气组织于根系横截面的占比。重复3次。
以上所有指标均于处理2个月后统一测定。
-
试验中所测定的原始数据采用Excel整理后经SPSS 21.0软件进行方差分析,利用Origin 2018软件进行作图。文中小写字母表示在P<0.05水平上差异显著。
-
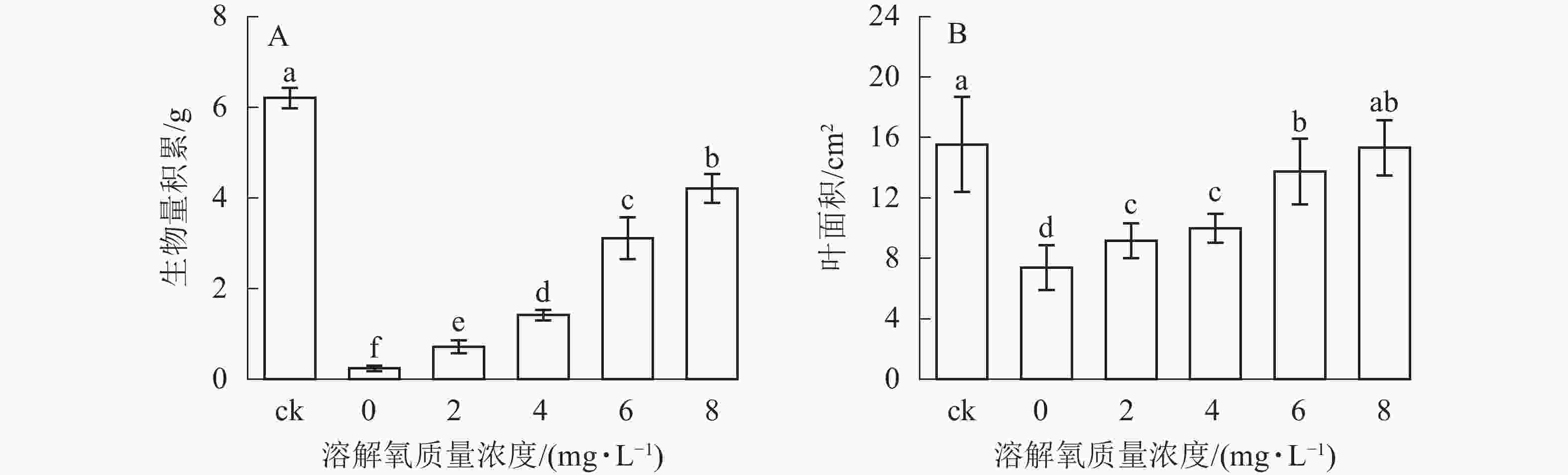
随着营养液中溶解氧质量浓度的升高,雷竹水培苗的生物量和叶面积也逐渐增加(图2)。同时 6和 8 mg·L−1处理组中的植株生物量积累显著低于对照(即土培雷竹苗) (图2A)。在0 mg·L−1处理组中,雷竹水培苗生物量积累分别仅为对照和8 mg·L−1处理下的3.9%和5.7%(图2A)。6和8 mg·L−1处理组中的植株叶面积无显著差异。0 mg·L−1处理组中的雷竹水培苗叶面积仅为对照和8 mg·L−1处理下的47.2%、48.2%(图2B)。说明溶解氧的缺乏会影响雷竹的生长,并且从生物量的积累上最直接反映出来。
-
光合作用是植物有机物合成的重要途径,光合作用的强弱可以部分从叶绿素a、叶绿素b和类胡萝卜素等光合色素质量分数反映出来。由图3可知:雷竹水培苗叶片叶绿素a、叶绿素b和类胡萝卜素质量分数均随溶解氧质量浓度的升高而增加,6、8 mg·L−1处理和对照之间叶片3种色素质量分数均无显著差异,类胡萝卜素与叶绿素b在4和6 mg·L−1处理之间也无显著差异(图3B和图3C)。0和2 mg·L−1处理组中的雷竹水培苗叶片各光合色素质量分数均显著低于其余处理组(P<0.05)(图3B和图3C)。

图 3 不同溶解氧质量浓度对雷竹水培苗根系活力和光合色素的影响
Figure 3. Effects of different dissolved oxygen concentration on the root activity and the content of photosynthetic pigments of hydroponic Ph. violascens seedlings
根系是植物吸收水分和矿质元素的主要器官。根系活力是判定植物对水分及矿质元素吸收能力的指标,可以说明植物与外界物质交换及生长等的活跃程度。雷竹水培苗根系活力随溶解氧质量浓度的升高而逐渐提高(图3D),其中土培状态下的雷竹苗根系活力最高,约为 0 mg·L−1处理组的 5.4 倍。8 mg·L−1处理组中水培苗根系活力显著高于其他处理组(P<0.05),且 6 mg·L−1处理组和4 mg·L−1处理组之间也存在显著差异(P<0.05)。当溶解氧质量浓度小于2 mg·L−1时,雷竹水培苗根系活力较低。
-
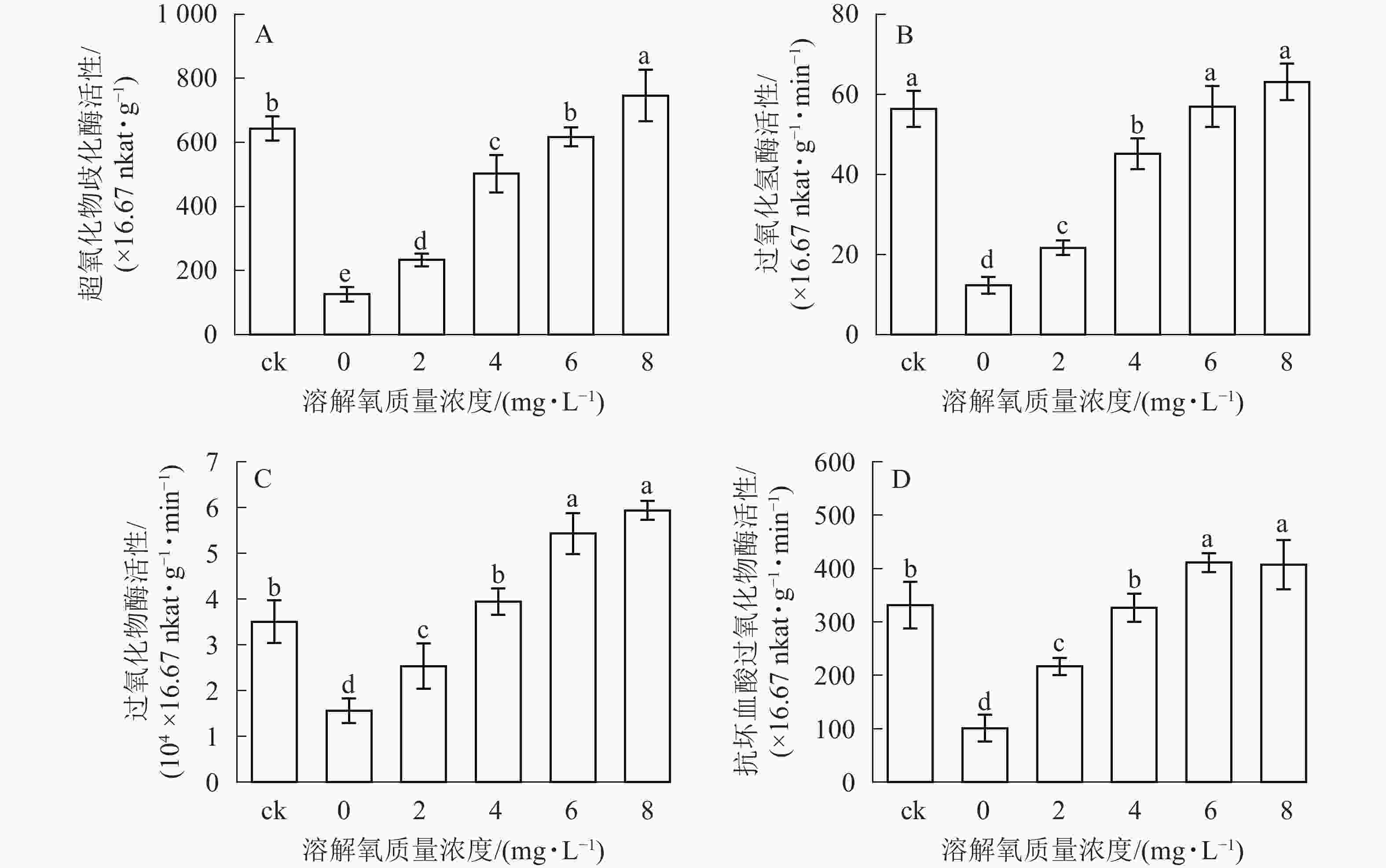
超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)、抗坏血酸过氧化物酶(APX)等抗氧化酶具有将体内产生的过氧化物转换为毒害较低或无害的物质的功效,它们可以作为植物受到某种胁迫后的生理响应指标。在处理2个月后,SOD、CAT、POD和APX活性变化趋势均保持一致(图4),即随着溶解氧质量浓度的升高,雷竹水培苗叶片内4种抗氧化酶也逐渐升高。溶解氧质量浓度为8 mg·L−1时,叶片SOD、CAT、POD和APX活性均达到了峰值,分别为0 mg·L−1处理组的5.90、5.12、3.80和4.03倍(图4)。除8 mg·L−1处理组和对照组的CAT活性没有显著差异以外(图4B),8 mg·L−1处理组其余 3 种酶活显著高于(P<0.05)对照组(图4A、图4C、图4D)。当溶解氧质量浓度高于6 mg·L−1时,水培苗叶片中4种抗氧化酶活性均显著高于(P<0.05)溶解氧质量浓度较低的处理组中的酶活性。
-
经过2个月的不同溶解氧质量浓度处理,水培苗根系距根尖10 mm以后的部位均不同程度地形成了通气组织。离根尖的距离越远,通气组织形成就越多。在距根尖70 mm处根系横截面通气组织占总面积的比例随氧气质量浓度的增加而降低,最高达到了7.1%;距根尖50 mm处也呈现相似的趋势,但2 mg·L−1处理通气组织占比要高于0 mg·L−1处理;在距根尖30 mm处却呈现了随溶解氧质量浓度升高而先升高后下降的趋势,且在4 mg·L−1处理中达到了峰值,8 mg·L−1处理组中雷竹水培苗根系在距根尖30 mm处便已没有通气组织的形成。虽然0 mg·L−1处理略低于2 mg·L−1处理,但两者之间并无显著差异;所有处理在距根尖10 mm处均无通气组织的形成,且对照处理中雷竹水培苗根系所有位置均无通气组织形成(图5A)。
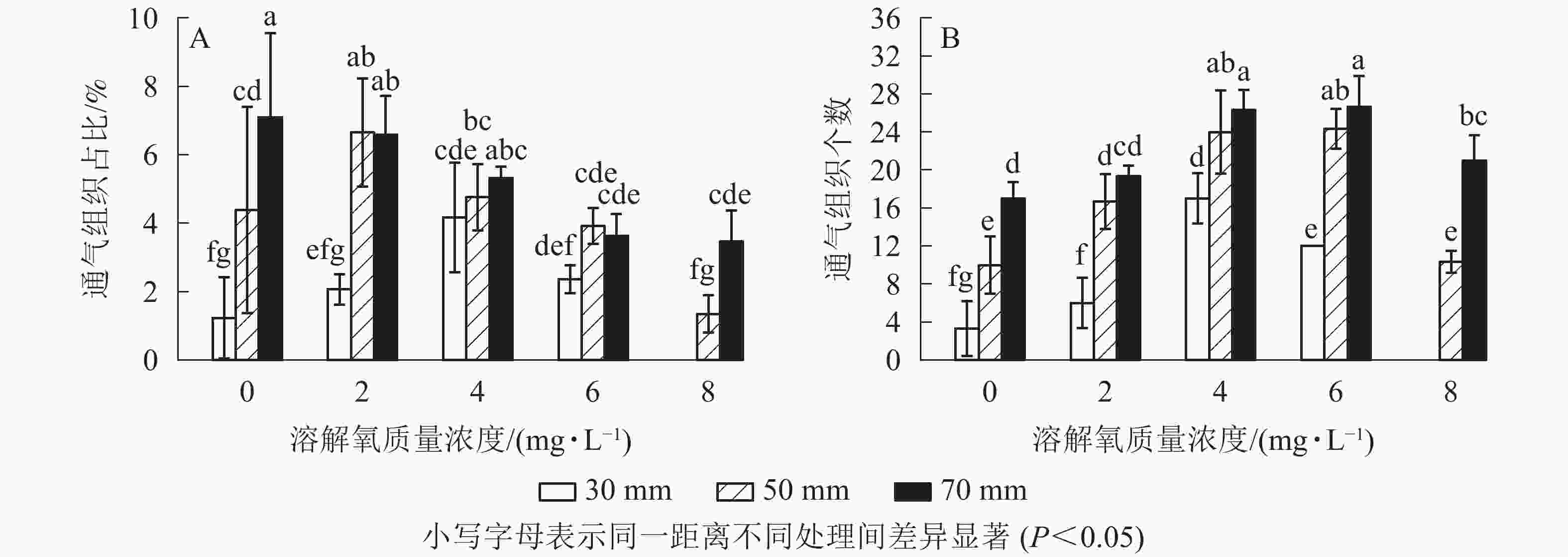
图 5 不同溶解氧质量浓度对雷竹水培苗根系距根尖10、30、50、70 mm处通气组织面积占比和形成数量的影响
Figure 5. Effects of different dissolved oxygen concentrations on the percentage and the number of aerenchyma of root cross-sectional area at 10,30,50,70 mm from the tips of roots of hydroponic Ph. violascens seedlings
通气组织数量与面积占比变化趋势不同,其在所有位置均呈现随着氧气质量浓度的升高而先升高后下降的趋势。在50和70 mm处通气组织数量随氧气质量浓度升高而增加,但在溶解氧质量浓度达到8 mg·L−1时雷竹水培苗根系中形成的通气组织数量却显著低于6 mg·L−1处理组(P<0.05),50和70 mm处,8 mg·L−1处理中根系分别仅形成10和21个;30 mm处通气组织数量随溶解氧浓度升高先增加后下降,在4 mg·L−1处理组中达到峰值(17个),后在8 mg·L−1处理中降为0(图5B)。
-
在植物进行正常生理代谢和生长发育的过程中,氧气是必不可少的重要因素。在氧化磷酸化过程中氧是呼吸链电子传递的最终受体,它驱动能量物质的合成以供植物生长发育所需[31]。当外界氧气供应不足时,植物的生理生态会受到直接影响。国内外有许多学者已经对氧气与各类作物生长的关系进行了较深入的研究,如在水稻和玉米中,均发现缺氧对植株生长造成了不同程度的负面影响[32-34]。在缺氧处理后,番茄Solanum lycopersicum植株的株高、根系长度、根系数量、生物量积累及光合色素含量均低于正常生长的番茄幼苗,而增氧处理则会促进植株的生长,甚至高于正常培养的番茄植株[33,35]。在对小麦[36]和水稻[19]的研究中有相似的结果。低氧使得植物生物量积累降低, 高氧提高生物量积累。本研究发现:随着营养液中溶解氧质量浓度的升高,雷竹水培苗生物量积累、叶面积和根系活力均升高。在溶解氧质量浓度较低时,雷竹水培苗中的这几项指标均在较低的水平。植物为适应低氧或缺氧环境,存在着几种不同的适应机制,其中包括降低生长速率[12]。雷竹水培苗在缺氧状况下光合色素质量分数显著减少,光合能力的降低,最终导致生物量积累的减少,可见缺氧抑制了雷竹的生长。此外,在0 mg·L−1溶解氧处理中雷竹水培苗各项指标都降至较低的水平,可见尽管在此实验中所采用的雷竹水培苗经过水培驯化,竹鞭退化,根系大量生长于茎秆基部,具备了一定的耐淹水能力,但缺氧对水培苗依旧造成了不利的影响。水培试验持续时间为2个月,若处理时间继续延长,则缺氧处理组中的水培苗亦可能存在死亡的威胁。
在 8 mg·L−1处理组中的雷竹水培苗生物量积累和根系活力均显著低于正常土培的雷竹苗。而水稻等作物中,在对照组的基础上再增加氧气浓度却能促进水稻植株的生长。此类情况在雷竹中并未发现,这可能是由于水稻本就属于耐水淹物种,其正常生长的环境本就处于低氧甚至缺氧的状态。有学者研究表明:缺氧环境中, 水稻的根系以无氧呼吸为主, 其产生的能量仅为氧气供应充足时的3%~5%[36-38],所以提高水稻根际的氧浓度可以大大促进水稻的生长。而雷竹正常生长时,土壤中的氧气可达10%~15%[39]。大气中空气的密度为1.293 g·L−1,可换算得雷竹正常生长于土壤时(包括覆盖及无覆盖),氧气质量浓度为129.3~193.95 mg·L−1,处于不缺氧的状态,其对氧气的需求远远大于水稻等植物。8 mg·L−1的溶解氧质量浓度可能仍然不是雷竹水培苗生长的最适溶解氧质量浓度。但即便在水中溶解氧质量浓度可以达到土壤中的氧气质量浓度水平,雷竹水培苗的生长亦可能弱于正常生长于土壤中的雷竹苗,因为气体的扩散速度取决于周围的介质。大气中的扩散速度是水中扩散速度的1万倍[40],也因此在水下的气体交换阻力是大气中的1万倍。在水培条件下,即使营养液中的溶解氧质量浓度达到与土壤中相等的水平,但由于水分的存在,氧气扩散速度大大降低,其进入根系的速率也随之降低,导致雷竹水培苗根系对氧气的吸收利用率下降,无法达到与在未淹水土壤中相等的水平。
在植物体中SOD、CAT、POD和APX负责清除ROS[5,41-42]。叶绿体是植物细胞中主要的ROS产生区域,而叶绿体缺少CAT,但存在几种APX同工酶。在叶绿体中,对过氧化氢的清除作用显然以APX最为关键,因为卡尔文循环中某些酶对过氧化氢是高度敏感的,微摩尔浓度的过氧化氢便会强烈抑制它们活性,而这些酶负责了二氧化碳的固定,这也说明了APX在控制过氧化氢水平中的关键作用[43]。有研究也发现,缺氧及低氧会抑制植物体内二氧化碳的固定[32]。在本研究中虽然未检测处理后短时间内的抗氧化酶活性变化,但在8 mg·L−1处理组中CAT、SOD、POD和APX的活性均较高,间接说明在淹水雷竹水培苗体内产生了过量的ROS。低溶解氧处理组中的雷竹水培苗叶片中的ROS出现堆积的情况,影响了雷竹水培苗的生长发育。有研究表明:ROS的积累会导致植株叶绿体的减少,出现叶片褪绿的情况,直接抑制了其光合能力[44]。亦有学者研究表明:ROS会抑制植物根系的生长[45]。可见,ROS的积累也是抑制雷竹水培苗生长的关键因子。
ROS在植物体中的作用是双面的。在水稻和芦苇等耐水淹植物中,即使根际氧浓度处于较低的水平,它们依旧能够正常生长,这主要是由于这些植物具有较强的通气能力。EVANS[17]明确定义了通气组织的概念,它是一种通过促进根与外界环境之间的气体扩散,提高植物的耐淹水能力的植物组织。有学者在水稻和玉米的根系中发现溶生型通气组织的形成,而这都是细胞程序性死亡的结果[17,46]。有研究表明:ROS诱导通气组织的形成[14,47]。可见ROS可能也参与调节雷竹水培苗中的通气组织的形成过程。本研究结果显示:雷竹不定根中通气组织最高仅占根系横截面的7.10 %,然而在水稻根系距离根尖50 mm处可达到30%~40%[48],玉米中可达到15%~20%[49]。研究表明:不定根中氧气的运输可以大大提高陆生植物在淹水下的存活率[50],而雷竹水培苗根系中的通气组织面积与水稻及其他植物之间的差距可能便是雷竹并不能如水稻等植物一样高度耐低氧的一部分原因。
-
水培条件下,溶解氧质量浓度越高,雷竹水培苗长势越好。在本研究中8 mg·L−1溶解氧处理组中的雷竹水培苗生长最佳,但雷竹水培苗对氧气的需求仍大于8 mg·L−1。缺氧抑制了雷竹水培苗的生长。缺氧诱导了雷竹水培苗根中溶生型通气组织的形成,溶生型通气组织的面积仍然达不到水稻等耐水淹物种的水平。这是雷竹不具备高耐缺氧能力的部分原因。
Effects of different dissolved oxygen concentration on growth, physiology and biochemistry of hydroponic Phyllostachys violascens seedlings
-
摘要:
目的 了解溶解氧质量浓度对雷竹Phyllostachys violascens水培苗生长、生理指标及根系结构的影响,初步探究雷竹水培苗对缺氧的适应性机制。 方法 以雷竹水培苗为材料,设置0、2、4、6、8 mg·L−1不同溶解氧质量浓度进行处理,分析不同溶解氧质量浓度对雷竹水培苗生物量积累、叶面积、根系活力、抗氧化酶活性、光合色素质量分数及根系结构的影响。 结果 雷竹水培苗生物量积累、叶面积、根系活力、叶片抗氧化酶活性和光合色素质量分数均随溶解氧质量浓度的升高而显著升高(P<0.05)。8 mg·L−1处理组中雷竹水培苗叶片超氧化物歧化酶、过氧化氢酶、过氧化物酶和抗坏血酸过氧化物酶活性达到峰值,分别为746.13×16.67 nkat·g−1、63.13×16.67 nkat·g−1·min−1、59 395.45×16.67 nkat·g−1·min−1和407.46 ×16.67 nkat·g−1·min−1。水培条件下,雷竹水培苗根系中形成溶生型通气组织,其面积占根系横截面面积的百分比随溶解氧质量浓度降低而显著升高(最高达到7.1%),通气组织个数变化趋势则相反(P<0.05)。 结论 水培条件下,溶解氧质量浓度越高,雷竹水培苗长势越好,且雷竹水培苗生长对氧气的需求大于8 mg·L−1。水培条件下,缺氧会诱导雷竹根中形成溶生型通气组织,但不足以使其具有高度耐水淹的能力。图5参50 Abstract:Objective This study aims to investigate the effects of dissolved oxygen concentration on growth, physiological indexes and root structure of hydroponic Phyllostachys violascens seedlings, and to explore the adaptive mechanism of hydroponic P. violascens seedlings to hypoxia. Method The hydroponic P. violascens seedlings were used as materials, and the effects of different dissolved oxygen concentrations (0, 2, 4, 6, 8 mg·L−1) on biomass accumulation, leaf area, root activity, antioxidant enzyme activity, photosynthetic pigment content and root structure were analyzed. Result The biomass accumulation, leaf area, root activity, leaf antioxidant enzyme activity and photosynthetic pigment content of hydroponic seedlings significantly increased with the increase of dissolved oxygen concentration(P<0.05). The activities of SOD, CAT, POD and APX of hydroponic seedling leaves treated with 8 mg·L−1 dissolved oxygen reached the peak, which were 746.13×16.67 nkat·g−1, 63.13×16.67 nkat·g−1·min−1, 59 395.45×16.67 nkat·g−1·min−1 and 407.46×16.67 nkat·g−1·min−1 respectively. Under hydroponic conditions, lysigenous aerenchyma was formed in the roots of hydroponic seedlings, and its percentage of root cross-sectional area significantly increased with the decrease of dissolved oxygen concentration (up to 7.1%), while the number of lysigenous aerenchyma changed in the opposite direction (P<0.05). Conclusion Under hydroponic conditions, the higher the dissolved oxygen concentration is, the better the growth of hydroponic seedlings can be. The oxygen requirement for the growth of hydroponic seedlings is greater than 8 mg·L−1 . Hypoxia can induce the formation of lysigenous aerenchyma in the root of hydroponic seedlings, but it is not enough to make it highly resistant to flooding.[Ch, 5 fig. 50 ref.] -
Key words:
- silviculture /
- Phyllostachys violascens /
- hydroponic /
- dissolved oxygen /
- hypoxia /
- growth /
- aerenchyma
-
图 5 不同溶解氧质量浓度对雷竹水培苗根系距根尖10、30、50、70 mm处通气组织面积占比和形成数量的影响
对照雷竹根系及各处理中雷竹水培苗根系距根尖10 mm处无通气组织形成,故图中不显示数据
Figure 5 Effects of different dissolved oxygen concentrations on the percentage and the number of aerenchyma of root cross-sectional area at 10,30,50,70 mm from the tips of roots of hydroponic Ph. violascens seedlings
-
[1] DAIMS H, WAGNER M. Nitrospira [J]. Trends Microbiol, 2018, 26(5): 462 − 463. [2] GEIGENBERGER P. Response of plant metabolism to too little oxygen [J]. Curr Opin Plant Biol, 2003, 6(3): 247 − 256. [3] DREW M C. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia [J]. Ann Rev Plant Physiol Plant Mol Biol, 1997, 48(1): 223 − 250. [4] BAILEY-SERRES J, FUKAO T. Plant responses to hypoxia–is survival a balancing act? [J]. Trends Plant Sci, 2004, 9(9): 449 − 456. [5] ANNALISA P, SOFIA C, ANTONELLA L, et al. ROS production and scavenging under anoxia and re-oxygenation in Aarabidopsis cells: a balance between redox signaling and impairment [J]. Front Plant Sci, 2016, 7: 1803. [6] MUNIR R, KONNERUP D, KHAN H A, et al. Sensitivity of chickpea and faba bean to root-zone hypoxia, elevated ethylene and carbon dioxide: root responses to low O2, ethylene, or high CO2 [J]. Plant Cell Environ, 2019, 42(1): 85 − 97. [7] HATTORI Y, NAGAI K, FURUKAWA S, et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water [J]. Nature, 2009, 460(7258): 1026 − 1030. [8] YAMAUCHI T, YOSHIOKA M, FUKAZAWA A, et al. An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions [J]. Plant Cell, 2017, 29(4): 775 − 790. [9] BAILEY-SERRES J, FUKAO T, GIBBS D J, et al. Making sense of low oxygen sensing [J]. Trends Plant Sci, 2012, 17(3): 129 − 138. [10] YAMAUCHI T, SHIONO K, NAGANO M, et al. Ethylene biosynthesis is promoted by very-long-chain fatty acids during lysigenous aerenchyma formation in rice roots [J]. Plant Physiol, 2015, 169(4): 180 − 193. [11] BAILEY-SERRES J, VOESENSK L A C J. Flooding stress: acclimations and genetic diversity [J]. Ann Rev Plant Biol, 2008, 59(1): 313 − 339. [12] SHIONO K, OGAWA S, YAMAZAKI S, et al. Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths [J]. Ann Bot, 2011, 107(1): 89 − 99. [13] HE C, FINLAYSON S A, DREW M C, et al. Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia [J]. Plant Physiol, 1996, 112(4): 1679 − 1685. [14] RAJHI I, YAMAUCHI T, TAKAHASHI H, et al. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses [J]. New Phytol, 2011, 190(2): 351 − 368. [15] THOMSON C J, ARMSTRONG W, WATERS I, et al. Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat [J]. Plant Cell Environ, 2010, 13(4): 395 − 403. [16] STEFFENS B, GESKE T, SAUTER M. Aerenchyma formation in the rice stem and its promotion by H2O2 [J]. New Phytol, 2011, 190(2): 369 − 378. [17] EVANS D E. Aerenchyma formation [J]. New Phytol, 2003, 161(1): 35 − 49. [18] ARMSTRONG J, ARMSTRONG W. Phragmites australis: a preliminary study of soil-oxidizing sites and internal gas transport pathways [J]. New Phytol, 1988, 108(4): 373 − 382. [19] SURALTA R R, YAMAUCHI A. Root growth, aerenchyma development, and oxygen transport in rice genotypes subjected to drought and waterlogging [J]. Environ Exp Bot, 2008, 64(1): 75 − 82. [20] 陈龙飞. 笋用雷竹高产栽培[J]. 安徽林业科技, 2000(5): 15. CHEN Longfei. High-yield cultivation of bamboo shoots [J]. J Anhui For Sci Technol, 2000(5): 15. [21] 周国模, 金爱武, 何钧潮, 等. 覆盖保护地栽培措施对雷竹笋用林丰产性能的影响[J]. 中南林学院学报, 1999, 19(2): 52 − 54. ZHOU Guomo, JIN Aiwu, HE Junchao, et al. The influence of cultivation techniques used in covered protected plots on the high-yield property of Lei bamboo plantation for edible shoots [J]. J Cent South For Univ, 1999, 19(2): 52 − 54. [22] 董林根, 吴伟根, 郑钢, 等. 经营干扰对雷竹叶面积指数的影响[J]. 经济林研究, 1999, 17(2): 14 − 16. DONG Lingeng, WU Weigeng, ZHENG Gang, et al. Decline in bamboo leaf area index as affected by management disturbance [J]. Econ For Res, 1999, 17(2): 14 − 16. [23] XU Mengjie, ZHUANG Shunyao, GUI Renyi. Soil hypoxia induced by an organic-material mulching technique stimulates the bamboo rhizome up-floating of Phyllostachys praecox[J]. Sci Rep, 2017, 7(1): 14353. doi: 10.1038/s41598-017-14798-8. [24] NILSSON R L K, MAGNESCHI L, LORETI E, et al. Transcript profiling of the anoxic rice coleoptile [J]. Plant Physiol, 2007, 144(1): 218 − 231. [25] 张岚清, 沈德魁. 含氧气氛下的稻杆低温烘焙研究[J]. 北京化工大学学报(自然科学版), 2017, 44(5): 27 − 32. ZHANG Lanqing, SHEN Dekui. Oxidative torrefaction of rice straw under different oxygen concentrations [J]. J Beijing Univ Chem Technol Nat Sci, 2017, 44(5): 27 − 32. [26] YOSHINAO M, YUSUKE K, MASAYA K, et al. Diel O2 dynamics in partially and completely submerged deepwater rice: leaf gas films enhance internodal O2 status, influence gene expression and accelerate stem elongation for ‘Snorkelling’ during submergence [J]. Plant Cell Physiol, 2019, 60(5): 973 − 985. [27] JIANG Yiwei, HUANG Bingru. Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses [J]. J Exp Bot, 2001, 52(355): 341 − 349. [28] 李合生. 植物生理生化实验原理和技术[M]. 北京: 高等教育出版社, 2006. [29] YOSHIYUKI N, KOZI A. Spinach chloroplasts scavenge hydrogen peroxide on illumination [J]. Plant Cell Physiol, 1980, 21(7): 1295 − 1307. [30] 高俊凤. 植物生理学实验指导[M]. 北京: 高等教育出版社, 2006. [31] 袁晓华, 杨中汉. 植物生理生化实验[M]. 北京: 高等教育出版社, 1983. [32] BJÖRKMAN O. The effect of oxygen concentration on photosynthesis in higher plants [J]. Physiol Plant, 2006, 19(3): 618 − 633. [33] CHÉRIF M, TIRILLY Y, BELANGER R R. Effect of oxygen concentration on plant growth, lipidperoxidation, and receptivity of tomato roots to pythium F under hydroponic conditions [J]. Eur J Plant Pathol, 1997, 103(3): 255 − 264. [34] QUEBEDEAUX B, HARDY R W F. Reproductive growth and dry matter production of Glycine max (L.) Merr in response to oxygen concentration [J]. Plant Physiol, 1975, 55(1): 102 − 107. [35] 郑小兰, 侯亚兵, 王瑞娇, 等. 根际氧浓度对番茄幼苗生长发育的影响[J]. 华北农学报, 2017, 32(4): 208 − 214. ZHENG Xiaolan, HOU Yabing, WANG Ruijiao, et al. Effects of oxygen concentration in rhizosphere on the growth of tomato seedlings [J]. Acta Agric Boreal-Sin, 2017, 32(4): 208 − 214. [36] ALMEIDA A M, VRIEZEN W H, van der STRAETEN D. Molecular and physiological mechanisms of flooding avoidance and tolerance in rice [J]. Russ J Plant Physiol, 2003, 50(6): 743 − 751. [37] STOIMENOVA M, IGAMBERDIEV A U, GUPTA K J, et al. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria [J]. Planta, 2007, 226(2): 465 − 474. [38] VISSER E J W, VOESENEK L A C J, VARTAPETIAN B B, et al. Flooding and Plant Growth[M]. New York: Academic Press, 1984. [39] 郭益昌, 庄舜尧, 胡昱彦, 等. 埋管通气对雷竹林土壤氧气体积分数的影响[J]. 浙江农林大学学报, 2020, 37(1): 69 − 75. GUO Yichang, ZHUANG Shunyao, HU Yuyan, et al. Soil oxygen content of Phyllostachys violascens with pipe-buried aeration [J]. J Zhejiang A&F Univ, 2020, 37(1): 69 − 75. [40] ARMSTRONG W D. Aeration in higher plants [J]. Adv Bot Res, 1980, 7: 225 − 332. [41] 焦彦生, 郭世荣, 李娟, 等. 钙对根际低氧胁迫下黄瓜幼苗活性氧代谢的影响[J]. 西北植物学报, 2006, 26(10): 2056 − 2062. JIAO Yansheng, GUO Shirong, LI Juan, et al. Effect of low rhizospere oxygen stress on reactive oxygen species metabolism in cucumber seedlings [J]. Acta Bot Boreal-Occident Sin, 2006, 26(10): 2056 − 2062. [42] LI Pengxia, ZHANG Xuan, HU Huali, et al. High carbon dioxide and low oxygen storage effects on reactive oxygen species metabolism in Pleurotus eryngii [J]. Postharvest Biol Technol, 2013, 85: 141 − 146. [43] de GARA L. Class Ⅲ peroxidases and ascorbate metabolism in plants [J]. Phytochem Rev, 2004, 3(1): 195 − 205. [44] ZHAN Gaomiao, LI Rongjun, HU Zhiyong, et al. Cosuppression of RBCS3B in Arabidopsis leads to severe photoinhibition caused by ROS accumulation [J]. Plant Cell Rep, 2014, 33(7): 1091 − 1108. [45] 吕文静. H2S介导镉调控ROS平衡抑制不结球白菜根生长机理研究[D]. 南京: 南京农业大学, 2016. LÜ Wenjing. Mechanism of H2S Mediated Cadmium Regulation ROS Inhibites Non-heading Chinese Cabbage Root Growth[D]. Nanjing: Nanjing Agricultural University, 2016. [46] GIBBS D J, LEE S C, ISA N M, et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants [J]. Nature, 2011, 479(7373): 415 − 418. [47] YAMAUCHI T, RAJHI I, NAKAZONO M. Lysigenous aerenchyma formation in maize root is confined to cortical cells by regulation of genes related to generation and scavenging of reactive oxygen species [J]. Plant Signaling Behav, 2011, 6(5): 759 − 761. [48] SOSIŃSKA A, MALESZEWSKI S. Effect of high oxygen concentration on photosynthesis in rape plants pretreated in low temperature [J]. Zeitschriftfür Pflanzenphysiologie, 1982, 108(5): 397 − 399. [49] ZABALZA A, van DONGEN J T, FROEHLICH A, et al. Regulation of respiration and fermentation to control the plant internal oxygen concentration [J]. Plant Physiol, 2009, 149(2): 1087 − 1098. [50] AYI Q, BO Zeng, LIU Jianhui, et al. Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants [J]. Ann Bot, 2016, 118(4): 675 − 683. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20200286







 下载:
下载: