-
核桃Juglans regia又名胡桃、羌桃,为胡桃科Juglandaceae胡桃属Juglans 植物。核桃作为重要的木本油料作物及经济树种,有助于推动中国经济落后地区发展,实现乡村振兴战略。2019年中国核桃种植面积占世界核桃栽植总面积的48.4%,产量占世界总产量的56.1%,出口量约3 564 t,出口收益高达2 900万美元,是中国农民重要的经济来源[1]。
核桃为喜阳植物,对水分需求较高。中国核桃主要种植区为干旱或半干旱地区,水资源供应不足,严重影响植株的生长发育,因此干旱是制约中国核桃产业发展的重要因素[2]。干旱严重威胁到植株形态、光合作用、气孔开度、细胞膜稳定性等。植物受到胁迫信号后,触发自身恢复机制,例如调控气孔开度、植物激素、渗透物质以及抗氧化酶保护系统来减少胁迫对植物的伤害,因此,干旱胁迫条件下,植株生长形态可直观反映其抗干旱能力[3],植株光合色素、丙二醛(MDA)、过氧化氢(H2O2)、超氧化物歧化酶(SOD)及过氧化物歧化酶(POD)等作为研究植株抗旱性强弱的主要生理指标,对植株抗旱性评价具有指示作用[4]。
植物内源激素在植物响应干旱胁迫过程中发挥着重要作用,赤霉素(gibberellins, GAs)能与其他内源激素交互作用,共同调节植物的生长发育,因此,赤霉素的合成、代谢与信号转导对植物生长及应对不同环境至关重要。其中,2-ODDs 家族基因是赤霉素合成的关键基因,其家族包含GA20ox、GA3ox、GA2ox。GA3ox作为合成酶参与赤霉素合成通路最后一步,可催化GA20转化为具有生物活性的GA1,调节植物体内的赤霉素水平。研究表明:GA3ox在器官内表达会影响赤霉素稳态和GA1水平,从而影响植株生长[5]。GA3ox基因影响拟南芥Arabidopsis thaliana、玉米Zea mays、白杨Populus tomentosa等植物的伸长,其过表达植株具有更长的节间,能延迟开花,增加顶端分生组织寿命,并改变维管发育。还有研究表明:减少植物体内赤霉素可以增强植物抗性[6]。DELLA蛋白是赤霉素信号的负调控因子。当植株受到胁迫时,赤霉素显著降低后 DELLA蛋白迅速积累,抵抗胁迫过程中出现的活性氧等物质,增强植株对胁迫的抵抗力。拟南芥中,AtGA20ox表达量上调,与FTL/DDF转录因子互作调控植株的耐旱性[7]。杨树Populus tremula ×P. alba的PtGA20ox表达上调,引起植物色素积累,提高植株的抗旱能力[8]。葡萄Vitis vinifera的VvGA3ox6、VvGA20ox1、VvGA20ox6、VvGA20ox7表达下调能提高植株的抗旱性[9]。目前,关于GA3ox基因的研究集中在调控植株生长、节间伸长等方面,对于抗性等生物功能研究较少。
本研究以核桃JrGA3ox基因过表达及干扰植株为材料,选用聚乙二醇 8000 (PEG 8000)模拟干旱胁迫处理,探究不同核桃植株在干旱胁迫条件下的形态变化、生理和分子响应机制,为核桃抗旱品种选育提供理论依据。
-
材料为浙江农林大学森林食物资源挖掘与利用全国重点实验室培育的野生型植株(WT)和JrGA3ox基因过表达植株(OE)及干扰植株(RNAi),其中JrGA3ox-OE基因构建于 PC1300-GFP植物表达载体,JrGA3ox-RNAi基因构建于PTCK303干扰载体,载体抗性为卡那霉素(kanmycin,Kan),利用根癌农杆菌Agrobacterium tumefaciens菌株 GV3103介导将构建好的35S::JrGA3ox::GFP 过表达载体及PTCK303-JrGA3ox干扰载体转化到核桃野生型体细胞胚中,植物筛选标记为潮霉素 (hygromycin,Hyg)。本研究所用的核桃组培苗为野生型体胚、JrGA3ox过表达体胚及干扰阳性体胚经脱水萌发获得,温室苗由上述组培苗经过生根、驯化获得。
-
分别选取核桃野生型植株、过表达植株、干扰植株在Driver&Kunivuki&McGranahan (DKW)培养基[10]中于组培室(培养条件:温度为25 ℃,湿度为75%~80%,光照强度为1 500~2 000 lx,光照周期为16 h光照/8 h黑暗)培养14 d,观察植株的生长状态、株高及节间长变化。设置3组生物学重复,每组3株。
-
分别选取核桃野生型植株、过表达植株、干扰植株培养至健壮,采用2步生根法对组培苗诱导驯化:第1步,将生长健壮的苗通过添加10 mg·L−1吲哚丁酸钾(K-IBA)在黑暗环境中培养7 d诱导根原基的发生;第2步,将暗培养结束的植株转移至无菌瓶中(蛭石与DKW培养基体积比为50∶46),放置于25 ℃,湿度为75%~80%,光照强度为1 500~2 000 lx,光照周期为16 h光照/8 h黑暗的组培室中培养21~28 d。不定根形成后,采用流水冲洗根部,洗净培养基与蛭石的残留,使用多菌灵浸泡1 min左右,转移至排水良好的盆栽土壤中[V(泥炭)∶V(珍珠岩)∶V(蛭石)=2∶1∶1],在温度为25℃,湿度为90%的驯化室内驯化培养。
-
对核桃野生型植株、过表达植株、干扰植株使用天根RNA试剂盒提取植株RNA,反转录得到cDNA,利用实时荧光定量PCR (RT-qPCR)测定植株体内JrGA3ox基因的相对表达量。设置3组生物学重复,每组3株。
-
选取生长状态一致的核桃野生型植株、过表达植株、干扰植株,放置在DKW-5%PEG 8000 (体积分数)[11]培养基中模拟干旱胁迫,于组培室培养28 d,每隔14 d拍照1次,观察植株形态变化。设置3次生物学重复,每组3株。
-
采用指甲油印迹法,取野生型植株、过表达植株、干扰植株分别胁迫0~14 d的同一部位叶片,并将指甲油均匀涂于叶片下皮层,待其自然风干,用镊子撕下成膜的指甲油,置于玻片上,使用光学显微镜观察气孔形态并拍照,统计气孔的长度与宽度。每个样品设置3次生物重复。
-
选取干旱胁迫 0~14 d的野生型植株、过表达植株、干扰植株叶片进行氯化硝基氮蓝四唑(NBT)及3, 3-二氨基联苯胺(DAB)化学染色分析,方法参照文献[12]。设置3次生物学重复,每组3株。
-
分别选取干旱胁迫 0~28 d的野生型植株、过表达植株、干扰植株叶片测定生理指标。用丙酮浸取法测定叶绿素质量分数;用愈创木酚法测定过氧化物酶活性;用紫外分光光度法测定过氧化氢酶活性;采用硫代巴比妥酸法,参照文献[12],测定超氧化物歧化酶活性、活性氧质量分数、丙二醛质量摩尔浓度。
-
分别选取干旱胁迫 0~28 d的野生型植株、过表达植株、干扰植株叶片进行抗旱基因RT-qPCR分析,方法及基因筛选参考文献[11] 。通过Primer 3 input在线软件设计引物(表1)。
表 1 RT-qPCR引物
Table 1. Primers for RT-qPCR analysis
引物 序列(5′→3′) 用途 引物 序列(5′→3′) 用途 Actin-F GCCGAACGGGAAATTGTC 内参 QJrPOD-R AGAGACGGTCGTTGAAGGAG RT-qPCR Actin-R AGAGATGGCTGGAAGAGG 内参 QJrLEA-F CAGCATCACCGACGTTGATT RT-qPCR QJrSOD-F TTGGAGCCACATATGAGCCA RT-qPCR QJrLEA-R TCAACAATATGCTGTGCGGC RT-qPCR QJrSOD-R CCTGTCCTGCGTTGTTGAAA RT-qPCR QJrGAI -F TGTTCTGGTTGATTCGCACG RT-qPCR QJrPOD-F TCAAGCGAAATAGAGGCCCA RT-qPCR QJrGAI -R TAAGTGGCGACCTTTCCCAT RT-qPCR -
使用SPSS 25进行单因素方差分析(P<0.05)和多重比较,利用GraphPad Prism 7绘图。
-
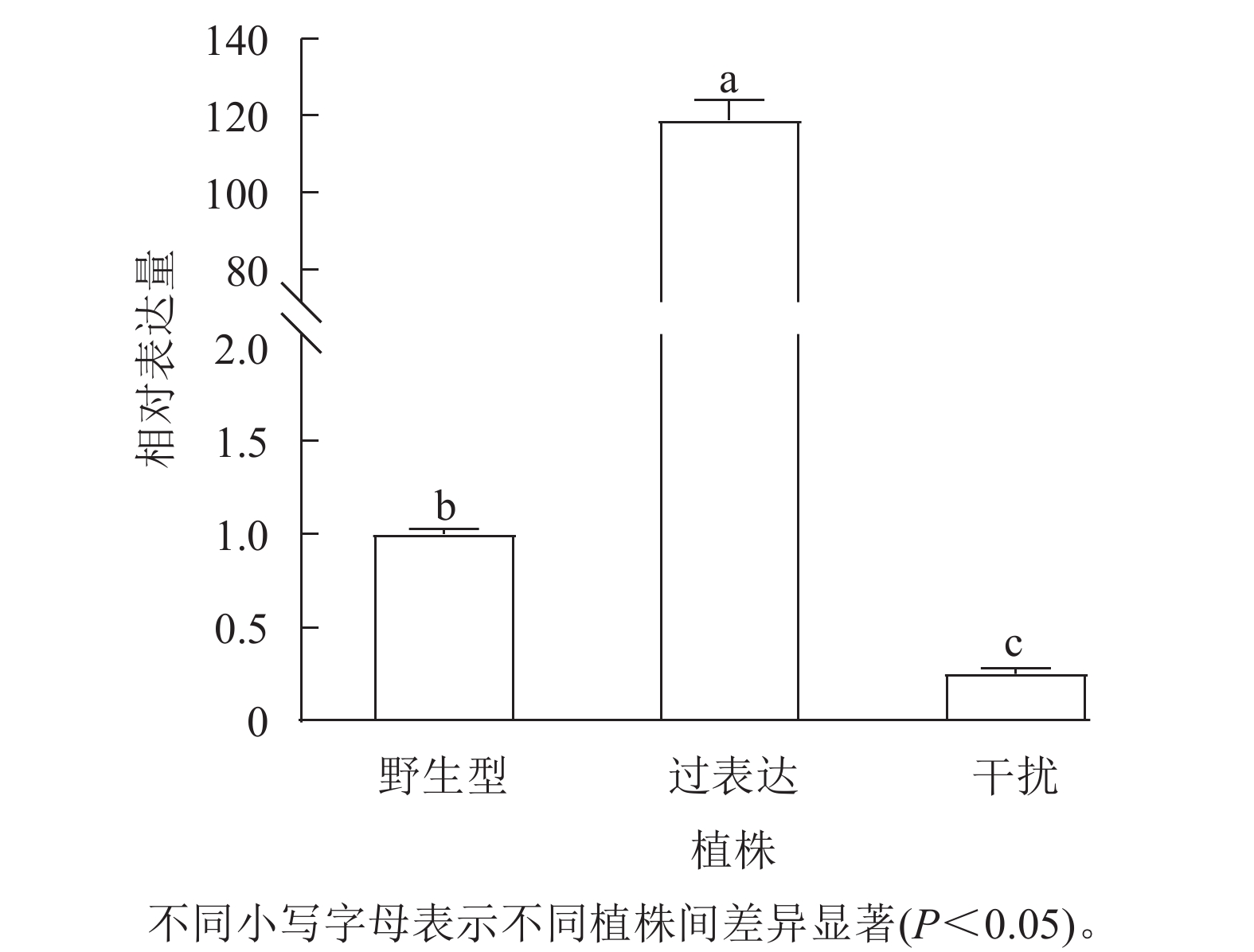
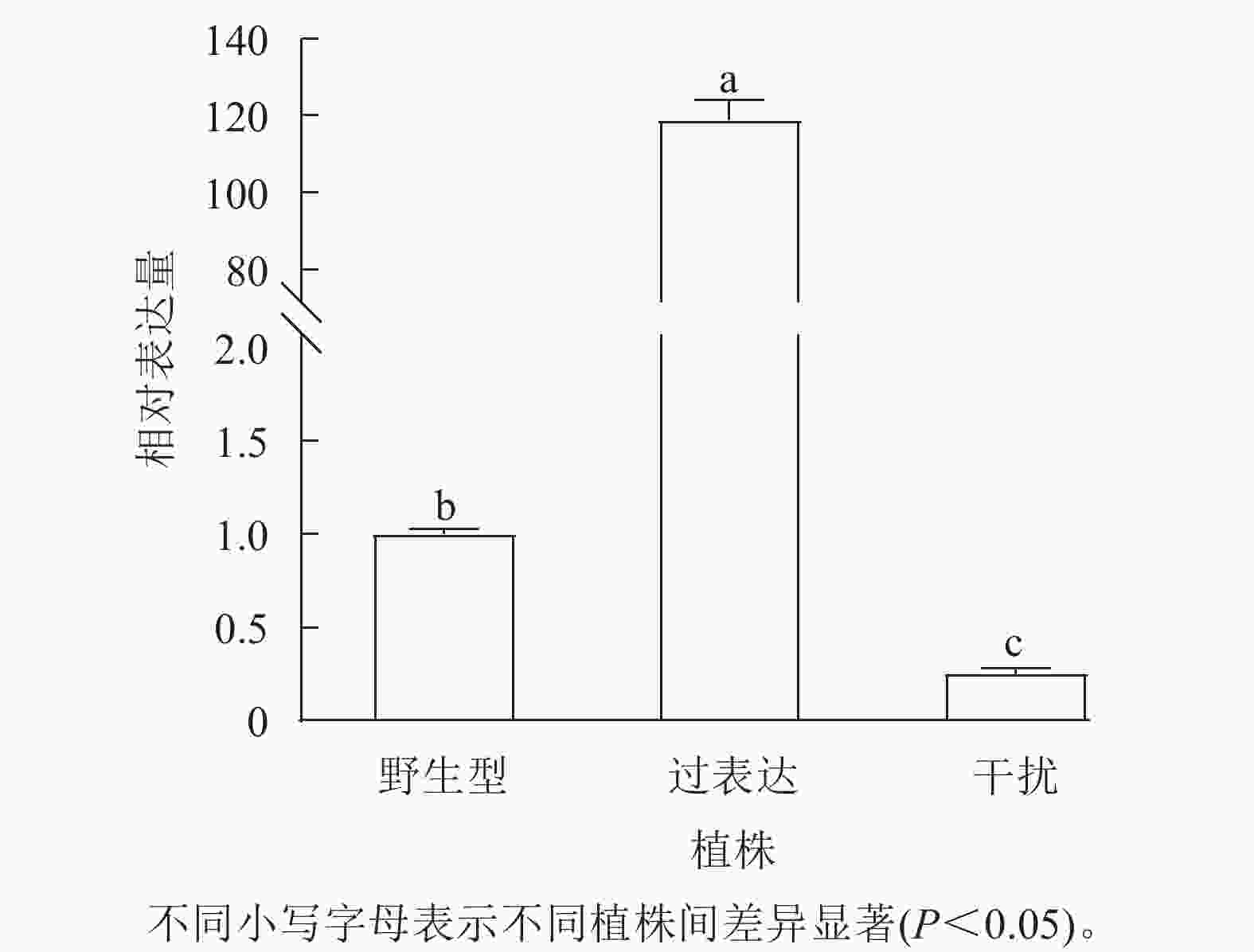
对核桃野生型植株、过表达植株、干扰植株的JrGA3ox基因相对表达量进行RT-qPCR分析。将野生型植株的JrGA3ox基因相对表达量定义为1.0,结果表明:过表达植株相对表达量显著高于野生型植株(P<0.05),是野生型植株的120.0倍。干扰植株的JrGA3ox基因相对表达量显著低于野生型植株,是野生型植株的0.3倍。3种植株的PCR阳性鉴定结果参照魏广利等[13]的研究。表明过表达植株和干扰植株均为阳性植株(图1)。
-
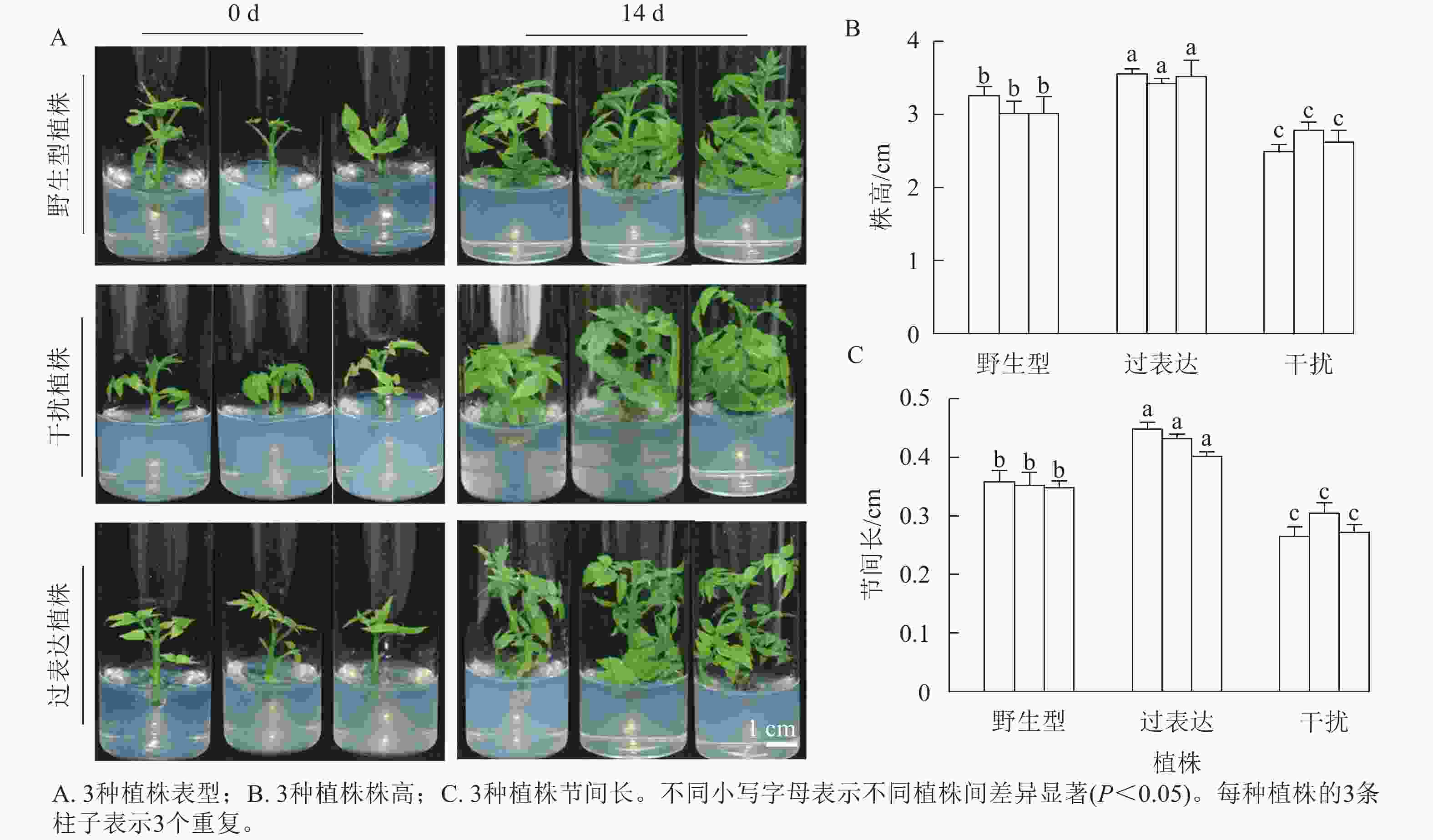
分别选取野生型植株、过表达植株、干扰植株培养14 d,结果表明:3种植株的株高和节间长有显著差异(图2A)。其中野生型植株的平均株高为3.21 cm,3株过表达植株的株高分别为3.57、3.47、3.63 cm,分别是野生型植株的1.11、1.08和1.13倍,显著高于野生型植株(P<0.05)。3株干扰植株的株高分别为2.50、2.80和2.73 cm,显著低于野生型植株(P<0.05),分别是野生型植株的0.78、0.87和0.85倍(图2B)。野生型植株的平均节间长为0.36 cm,3株过表达植株的节间长分别为0.45、0.43、0.40 cm,显著高于野生型植株(P<0.05),分别是野生型植株的1.25、1.19和1.11倍。3株干扰植株的节间长分别为0.28、0.31和0.27 cm,显著低于野生型植株(P<0.05),分别是野生型植株的0.78、0.86和0.35倍(图2C)。综上所述,JrGA3ox基因正调控核桃植株的生长发育。
-
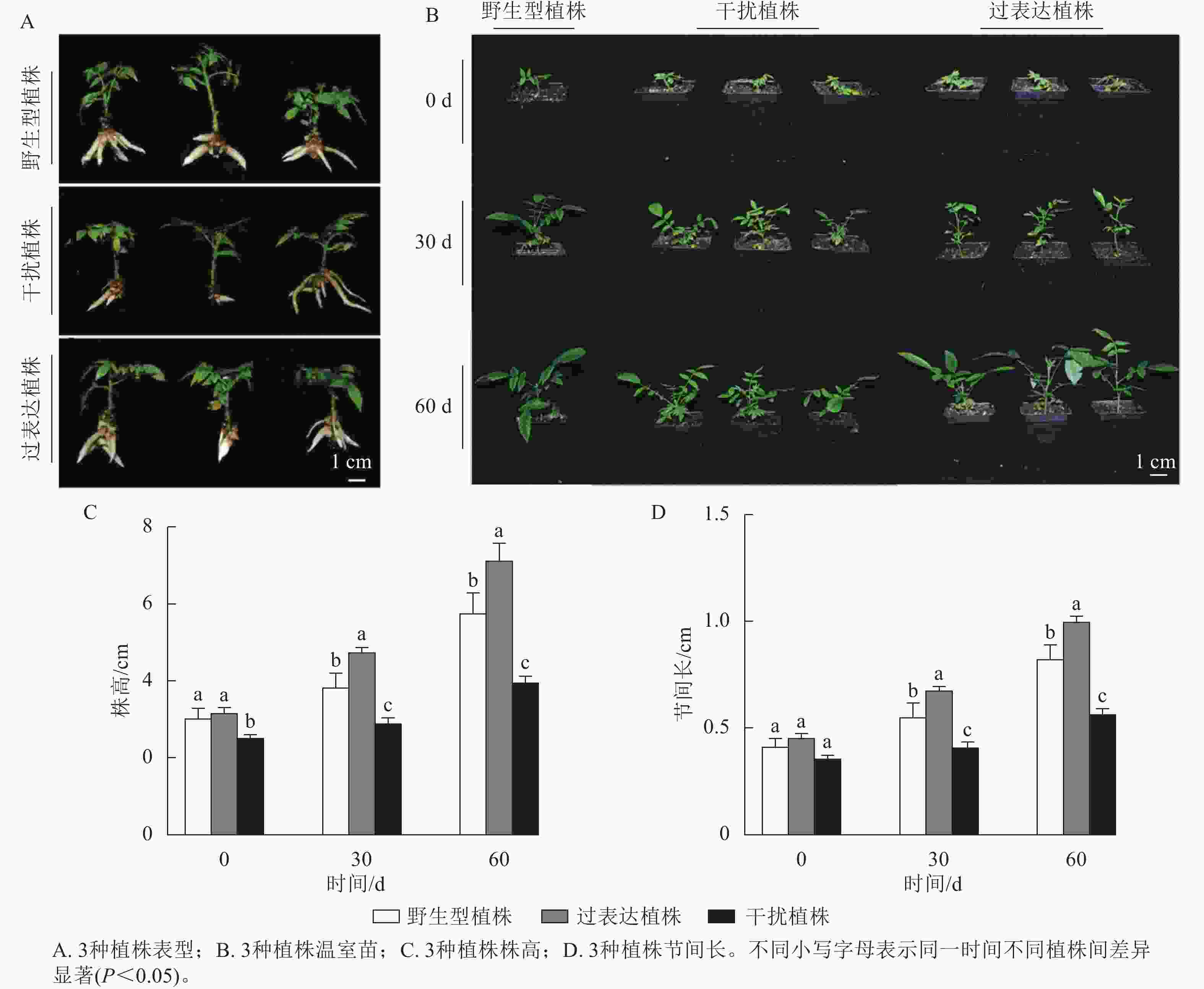
对核桃野生型植株、过表达植株、干扰植株不定根生成(图3A)后进行移栽驯化,获得温室苗(图3B)。定植培养30 d时,3种植株间差异显著(P<0.05),过表达植株温室苗株高及节间长分别是野生型植株的1.85、1.89倍,干扰植株温室苗的株高及节间长分别是野生型植株的0.66、0.71倍。定植培养60 d时,过表达植株温室苗株高及节间长显著高于野生型植株(P<0.05),分别是野生型植株的5.16、1.61倍,干扰植株温室苗的株高及节间长显著低于野生型植株(P<0.05),分别是野生型植株的0.64、0.65倍(图3C~D)。综上所述,与野生型植株相比,过表达植株、干扰植株的株高及节间长具有显著变化(P<0.05),进一步证明JrGA3ox基因正调控核桃的生长发育。
-
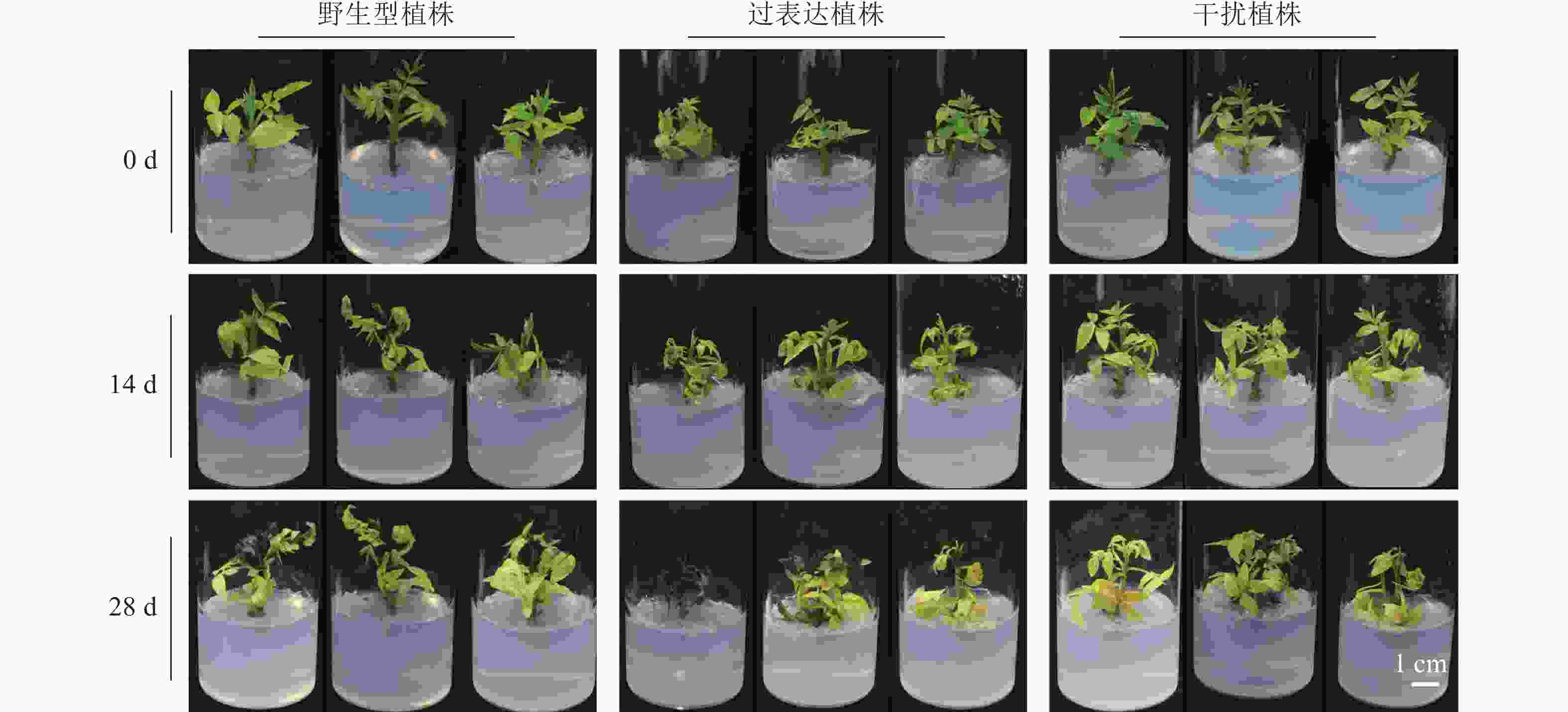
选取生长状态一致的核桃野生型植株、过表达植株、干扰植株,培养于DKW+5%PEG 8000 (体积分数)培养基中,观察植株表型变化。0 d时,3种植株的生长状况良好,叶色深绿。干旱胁迫14 d时,3种植株间存在明显差异。过表达植株叶片发黄脱落及萎焉程度明显高于野生型植株,干扰植株生长状态较好,叶片萎焉发黄程度明显低于野生型植株。28 d时,3种植株均出现生长停滞(图4)。初步判断干扰植株的耐旱性强于野生型植株,过表达植株耐旱性最弱。
-
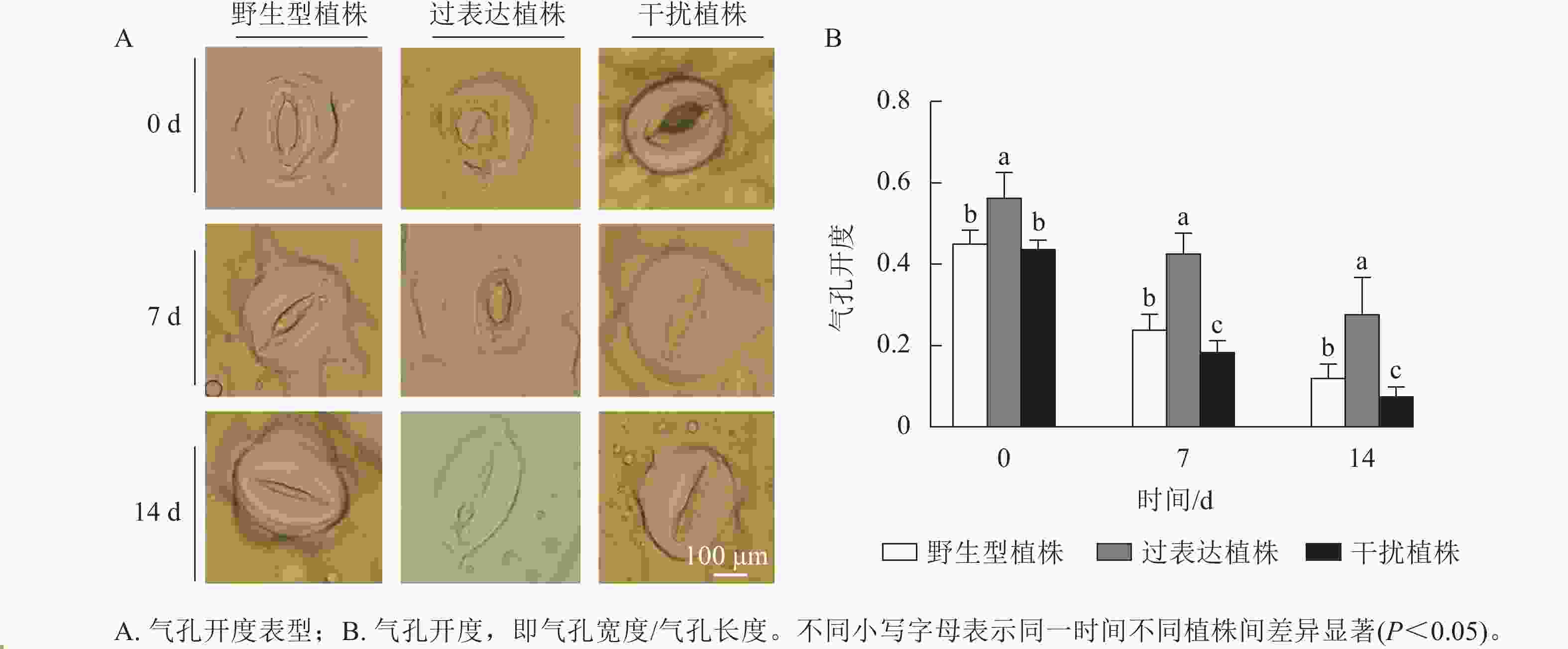
叶片气孔是植物与土壤、大气之间的重要物质交换通道,平衡植物光合作用与水分利用[14]。气孔能快速感知干旱[15],并通过控制气孔开度调控植株的失水率。对干旱胁迫下3种植株的气孔开度进行测定,结果显示:随着干旱胁迫时间的延长,3种植株的气孔开度逐渐下降。干旱胁迫7~14 d时,过表达植株的气孔开度始终显著高于野生型植株(P<0.05),分别是野生型植株的1.82、2.27倍;干扰植株的气孔开度始终显著低于野生型植株(P<0.05),分别是野生型植株的0.82、1.86倍(图5)。综上所述,在干旱胁迫下干扰植株的气孔开度较小,水分蒸腾速率较慢。
-
植物有氧代谢过程伴随活性氧物质积累,例如超氧阴离子自由基(${\mathrm{O}}_2^{\cdot -} $)、过氧化氢(H2O2)和羟自由基(·OH)等[16]。在正常生长过程中,机体内活性氧的产生与清除处于动态平衡。当植物受到干旱胁迫时,该平衡被破坏,引起活性氧物质的过度积累,导致细胞出现损失甚至死亡现象。
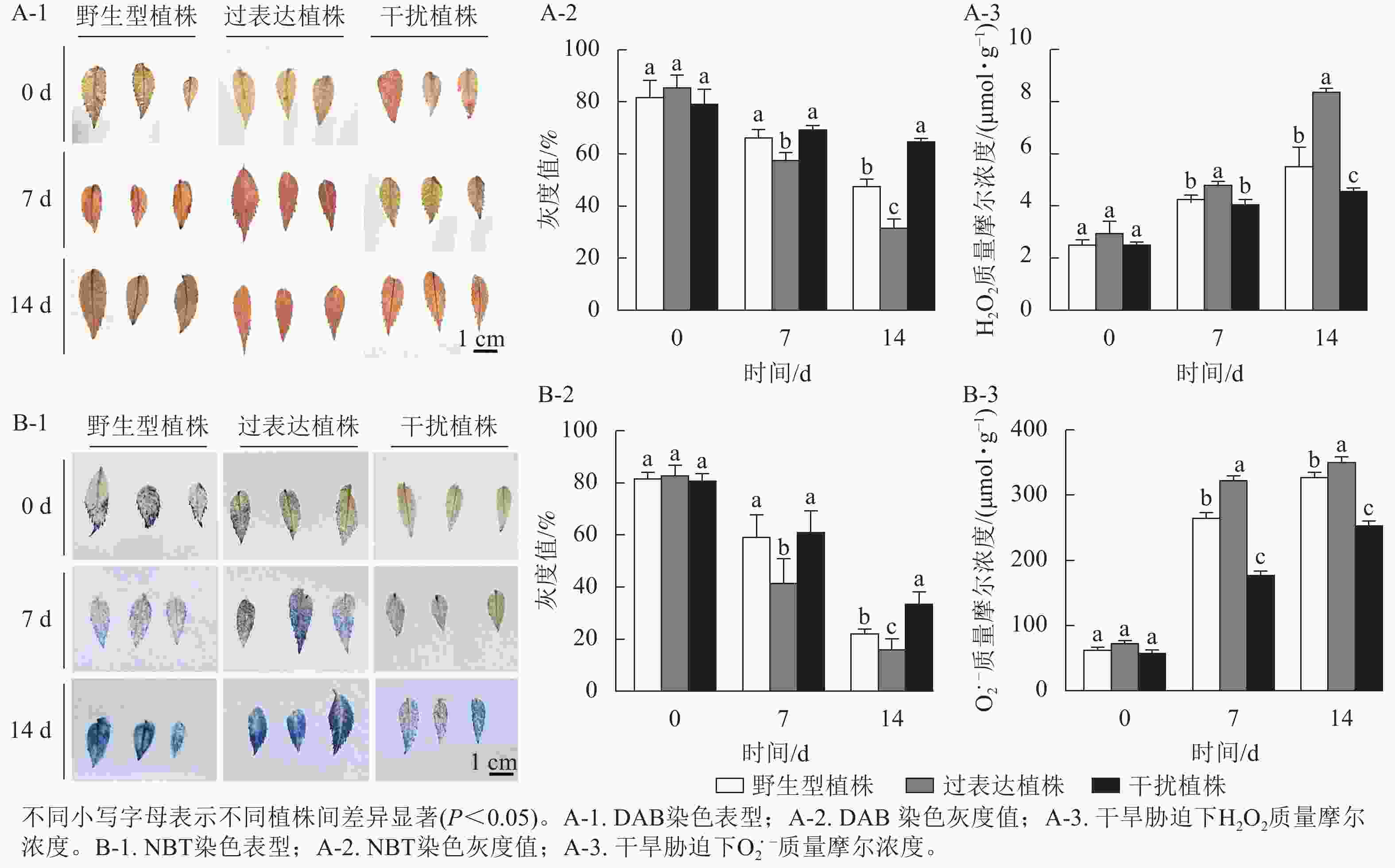
0 d时,3种植株染色后叶片DAB、NBT颜色无显著差异,随着胁迫时间的延长,染色程度逐渐加深,14 d时过表达植株染色程度较深,干扰植株染色较浅(图6A-1,B-1)。对其颜色进行灰度值(反应色彩深浅程度,颜色越深,数值越小)测定,胁迫14 d时,野生型植株、过表达植株、干扰植株的DAB染色灰度值分别为47.67%、32.00%、65.00%,干扰植株显著高于野生型植株(P<0.05),是野生型植株的1.36倍,过表达植株灰度值显著低于野生型植株(P<0.05),是野生型植株的0.67倍(图6A-2);野生型植株、过表达植株、干扰植株的NBT灰度值分别为22.33%、16.33%、33.67%,干扰植株灰度值显著高于野生型植株(P<0.05),是野生型植株的1.50倍;过表达植株灰度值显著低于野生型植株(P<0.05),是野生型植株的0.73倍 (图6B-2)。进一步对干旱胁迫下不同时间叶片的H2O2及$ {\mathrm{O}}_2^{\cdot -}$进行测定,发现随着干旱胁迫时间的延长,H2O2及$ {\mathrm{O}}_2^{\cdot -} $质量摩尔浓度逐渐上升,植株受活性氧伤害程度逐渐加重。0 d时,野生型植株的H2O2和$ {\mathrm{O}}_2^{\cdot -} $质量摩尔浓度分别为2.56、63.37 μmol·g−1,过表达植株的H2O2和$ {\mathrm{O}}_2^{\cdot -} $质量摩尔浓度分别为2.98、73.56 μmol·g−1,干扰植株的H2O2和$ {\mathrm{O}}_2^{\cdot -} $质量摩尔浓度分别2.54、58.65 μmol·g−1,3种植株间无显著差异。在干旱胁迫14 d时达到最大值,差异显著(P<0.05)。野生型植株、过表达植株、干扰植株的H2O2质量摩尔浓度分别为5.54、8.40、4.60 μmol·g−1,过表达植株的H2O2质量摩尔浓度显著高于野生型植株(P<0.05),是野生型植株的1.52倍;干扰植株显著低于野生型植株(P<0.05),是野生型植株的0.83倍(图6A-3)。14 d时野生型植株、过表达植株、干扰植株的$ {\mathrm{O}}_2^{\cdot -} $质量摩尔浓度分别为328.61、352.11、253.49 μmol·g−1,过表达植株的$ {\mathrm{O}}_2^{\cdot -} $质量摩尔浓度显著高于野生型植株(P<0.05),是野生型植株的1.07倍,干扰植株显著低于野生型植株(P<0.05),是野生型植株的0.77倍(图6B-3)。综上所述,随着干旱胁迫时间的延长,3种植株受活性氧侵害程度从轻到重依次为干扰植株、野生型植株、过表达植株,表明干扰植株的抗旱性较强。
-
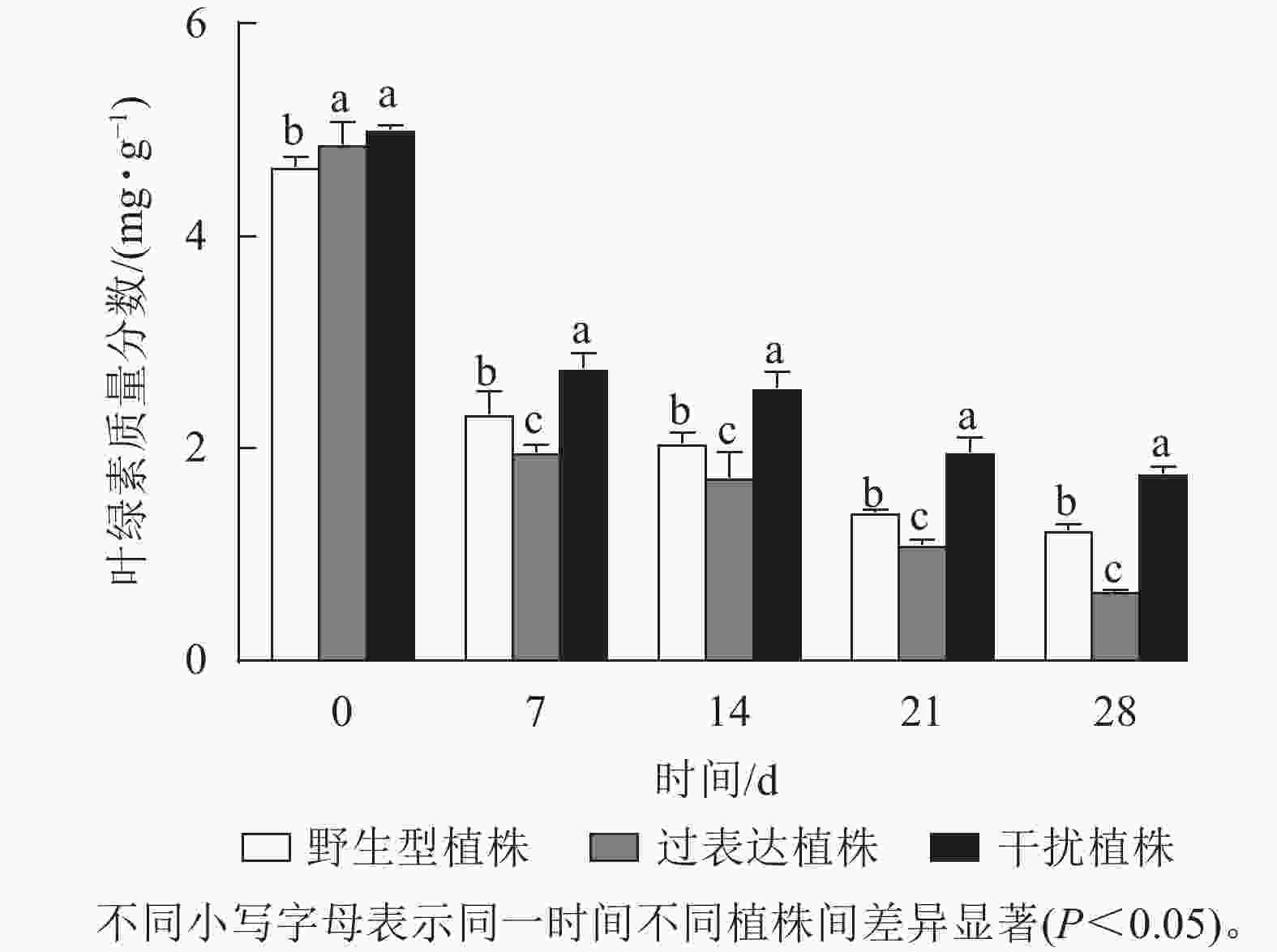
叶绿素质量分数稳定能避免产生过多自由基对植物造成损伤[17]。对干旱胁迫下核桃野生型植株、过表达植株、干扰植株叶绿素质量分数进行测定。结果显示:0 d时,3种植株间叶绿素质量分数差异不显著。随着干旱胁迫时间的延长,叶绿素质量分数逐渐下降。干旱胁迫7 d时野生型植株、过表达植株、干扰植株叶绿素质量分数分别为2.34、1.96、2.76 mg·g−1。与野生型植株相比,过表达植株叶绿素质量分数较低,干扰植株较高。干旱胁迫14 d时,野生型植株与过表达植株叶绿素质量分数差异显著,干扰植株显著高于野生型植株(P<0.05),是野生型植株的1.60倍。干旱胁迫21 d时,野生型植株、过表达植株、干扰植株的叶绿素质量分数分别为1.40、1.10、1.97 mg·g−1。过表达植株叶绿素质量分数是野生型植株的0.79倍,显著低于野生型植株(P<0.05),干扰植株是野生型植株的1.40倍,显著高于野生型植株(P<0.05)。28 d时,过表达植株叶绿素质量分数为0.65 mg·g−1,显著低于野生型植株(P<0.05),是野生型植株的0.53倍;干扰植株叶绿素质量分数为1.76 mg·g−1,显著高于野生型植株(P<0.05),是野生型植株的1.43倍(图7)。综上所述,干扰JrGA3ox基因表达能减少叶绿素的降解,增强植物光合作用,从而在一定程度上提高核桃的抗旱能力。
-
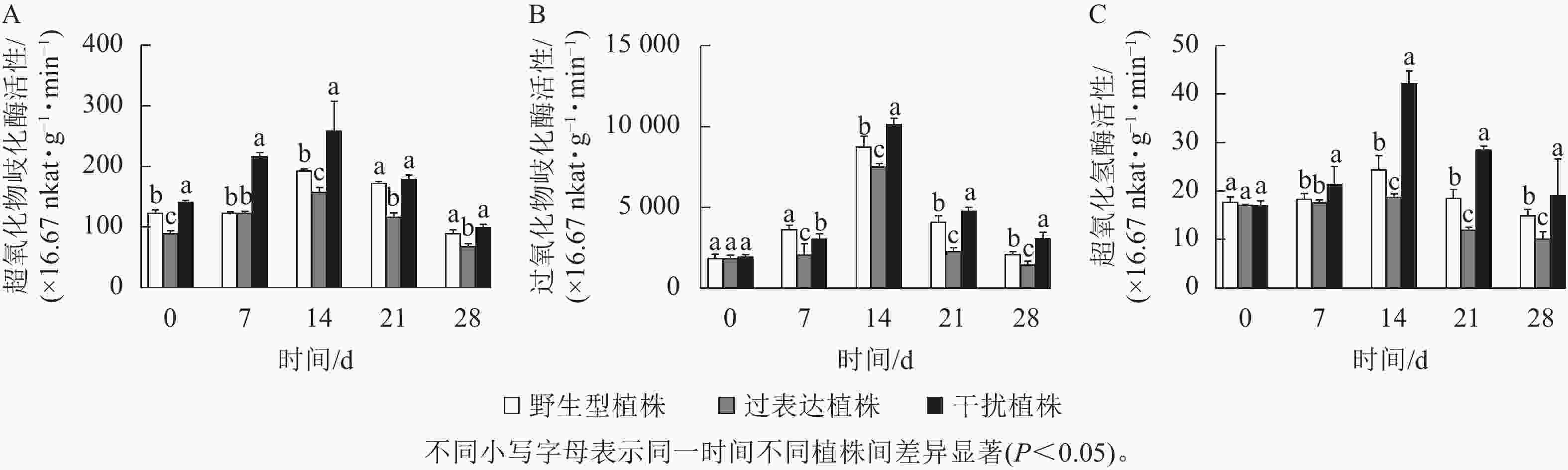
植物在胁迫时产生大量的活性氧物质,机体则利用抗氧化酶系统来抵御活性氧对植物造成的伤害。随着干旱胁迫时间的延长,过氧化物歧化酶、超氧化物歧化酶、过氧化氢酶呈先上升后下降的趋势(图8)。0 d时,野生型植株的超氧化物歧化酶、过氧化物歧化酶、过氧化氢酶活性分别为112.42×16.67、1 833.33×16.67、17.99×16.67 nkat·g−1·min−1,过表达植株分别为89.69×16.67、1 873.00×16.67、19.01×16.67 nkat·g−1·min−1,干扰植株分别为140.93×16.67、1 966.67×16.67、19.01×16.67 nkat·g−1·min−1,3种植株间无显著差异。在干旱胁迫14 d时,氧化酶活性均达到最高值且差异显著(P<0.05)。此时野生型植株、过表达植株、干扰植株超氧化物歧化酶活性分别为192.63×16.67、157.24×16.67、259.36×16.67 nkat·g−1·min−1,3种植株间存在显著差异(P<0.05),过表达植株超氧化物歧化酶活性是野生型植株的0.81倍,干扰植株是野生型植株的1.65倍(图8A);野生型植株、过表达植株、干扰植株过氧化物歧化酶活性分别为8 760.00×16.67、7 546.67×16.67、10 186.67×16.67 nkat·g−1·min−1,过表达植株显著低于野生型植株(P<0.05),是野生型植株的0.86倍,干扰植株是野生型植株的1.16倍,显著高于野生型植株(P<0.05,图8B);野生型植株、过表达植株、干扰植株的过氧化氢酶活性分别为22.81×16.67、18.86×16.67、42.30×16.67 nkat·g−1·min−1,其中干扰植株的过氧化氢酶活性显著高于野生型(P<0.05),是野生型植株的1.85倍,而过表达植株显著低于野生型植株(P<0.05),是野生型植株的 0.83倍(图8C)。干扰植株的氧化酶活性在干旱胁迫7~28 d时活性始终较高。此结果表明:干旱胁迫下JrGA3ox基因负调控相关酶活性的表达,进而提高植株的抗旱性。
-
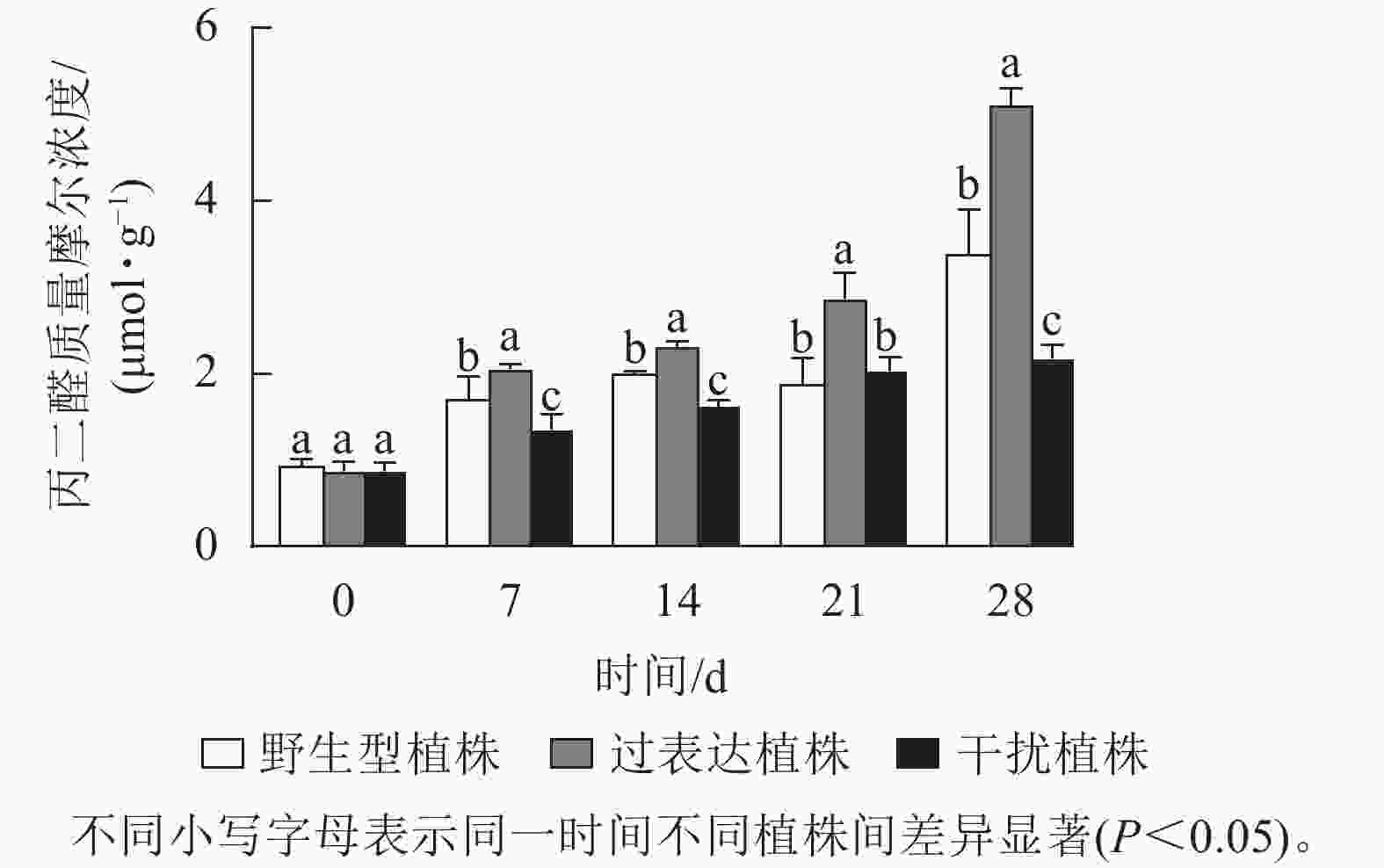
植物在胁迫时引起体内活性氧积累,发生膜脂过氧化作用,产生丙二醛,对机体造成严重伤害。0 d时,3种植株的丙二醛质量摩尔浓度差异不显著,为0.9 μmol·g−1。随着胁迫时间的延长,各植株丙二醛质量摩尔浓度逐渐上升,在胁迫28 d时达到最高值,且差异显著(P<0.05),野生型植株、过表达植株、干扰植株的丙二醛质量摩尔浓度分别为3.38、5.10、2.18 μmol·g−1,过表达植株的丙二醛质量摩尔浓度显著高于野生型植株(P<0.05),是野生型植株的1.51倍;干扰植株的丙二醛质量摩尔浓度显著低于野生型植株(P<0.05),是野生型植株的0.72倍(图9)。结果表明:过表达植株的丙二醛质量摩尔浓度始终较高,其受干旱胁迫程度较大,说明JrGA3ox基因过表达降低了核桃抗干旱能力,而干扰JrGA3ox的表达能提高植株的抗旱能力。
-
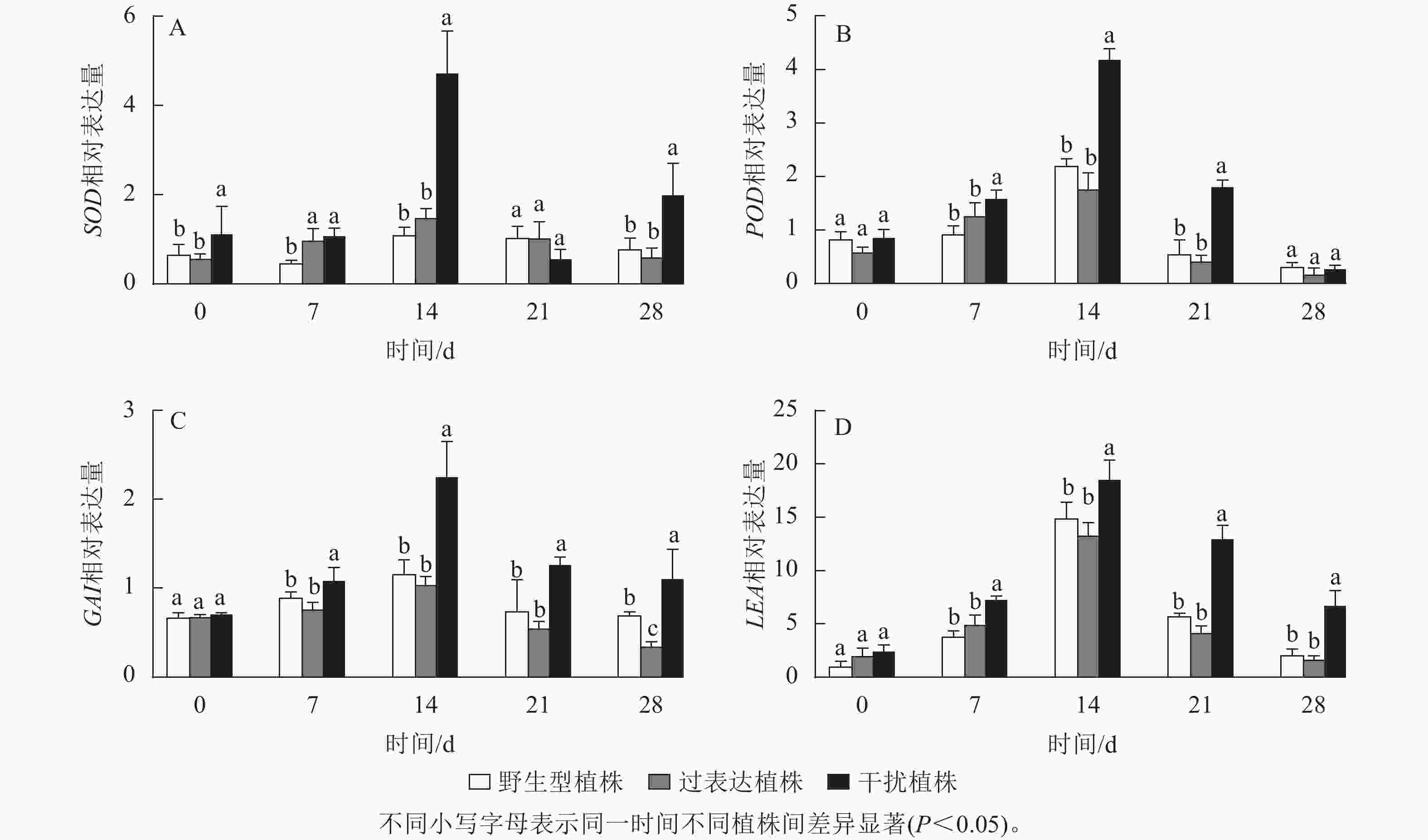
SOD、POD是编码活性氧的抗氧化酶基因。逆境胁迫时引起LEA基因表达,参与调控抗旱性。GAI基因是编码DELLA蛋白的关键基因,而DELLA蛋白是抗逆境胁迫中多种植物激素的调控中心。以上基因的表达可以反映植株在干旱环境中的抗性。对野生型植株、过表达植株、干扰植株在干旱胁迫0~28 d时SOD、POD、LEA、GAI基因进行RT-qPCR检测(图10),结果显示:各植株 SOD、POD、GAI、LEA基因的相对表达量随干旱胁迫时间的延长呈先上升后下降趋势,在干旱胁迫14 d时相对表达量最高,将野生型植株的各基因相对表达量定为1.00,干扰植株SOD、POD、GAI、LEA的相对表达量显著高于野生型植株(P<0.05),分别为野生型植株的3.95、1.91、1.94和1.24倍;过表达植株SOD、POD、LEA、GAI相对表达量与野生型植株无显著差异。在21 d时,SOD、POD、GAI 、LEA基因相对表达量开始下降,干旱胁迫28 d时相对表达量达最低,过表达植株SOD、POD、LEA基因相对表达量与野生型植株差异不显著,GAI基因相对表达量显著低于野生型植株(P<0.05),是野生型植株的0.50倍;干扰植株与野生型植株相比POD基因相对表达量差异不显著,SOD、GAI、LEA基因相对表达量显著高于野生型植株(P<0.05),分别是野生型植株的2.52、1.78、3.13倍。综上所述,JrGA3ox基因负调控SOD、POD、LEA、GAI基因的表达,提高植株的耐旱能力。
-
赤霉素通过刺激细胞伸长促进植株节间生长,从而引起植株高度改变。过表达CcGA3ox基因的山核桃Carya cathayensis植株高度显著增加,节间明显伸长[13];水稻Oryza sativa的OsGA3ox基因在不同细胞内合成内源赤霉素,调控植株生长发育[18]。在本研究中,对核桃JrGA3ox基因表达植株进行表观形态验证,结果表明:核桃JrGA3ox基因过表达植株的株高及节间长显著高于野生型,JrGA3ox基因干扰植株呈现矮化状态,其株高及节间长显著低于野生型。这与前人研究结果相似。
-
本研究对核桃野生型植株、过表达植株及干扰植株干旱胁迫处理后的植株表型、生理生化指标和相关抗性基因进行测定,结果表明:干旱胁迫时过表达植株长势较弱,其叶片发黄萎焉程度明显高于野生型植株,而干扰植株生长状态良好。
在干旱胁迫下,植物通过控制气孔开闭调控叶片的蒸腾速率[19]。植物受到胁迫时会促进气孔关闭,减慢蒸腾速率,保持植株含水量。本研究对3种植株干旱胁迫时气孔开度进行测定,结果表明:干扰植株气孔开度显著低于野生型植株,这与小麦Triticum aestivum[20]、银腺杨Populus alba ‘Berolinensis’[21]和油茶Camelia oleifera[22]在受到干旱胁迫时的气孔变化一致,表明较低气孔开度的植株具有较强的耐旱性。研究表明:叶绿素质量分数直接影响植株光合作用[23]。在干旱环境中叶绿素质量分数稳定可以增强植株适应干旱的能力[24]。本研究中,3种植株的叶绿素质量分数随干旱胁迫时间的增加都出现了降低态势,其中干扰植株的叶绿素质量分数始终显著高于其他2种植株,说明干扰植株的叶绿素质量分数较稳定,该植株的抗旱能力较强。植物在非生物胁迫时过量积累活性氧,产生氧化胁迫反应,此时机体通过引起酶系统(超氧化物歧化酶、过氧化物歧化酶、过氧化氢酶)和非酶物质的增多来清除多余的活性氧,维持植株稳定性[25]。本研究检测结果表明:随着干旱胁迫持续时间的增长,过表达植株的活性氧物质增加明显超过野生型植株,说明过表达植株在干旱胁迫中更易受到伤害,产生较多的活性物质;而抗氧化酶活性随着干旱时间延长,整体呈现先上升后下降的趋势,且干扰植株体内的抗氧化酶活性始终高于野生型植株,说明干扰植株的抗旱性优于野生型植株。丙二醛质量摩尔浓度是植物遭受逆境时的常用指标。有研究表明:麻栎Quercus acutissima、皂荚Gleditsia sinensis、白蜡Fraxinus chinensis等植物在干旱胁迫下丙二醛质量摩尔浓度均上升,其中麻栎的丙二醛质量摩尔浓度变化更为稳定,变化幅度较小,说明麻栎相比皂荚、白蜡,具有更好的抗旱性[26]。本研究发现随着胁迫时间的增加,3种植株的丙二醛质量摩尔浓度均呈现上升趋势,其中干扰植株较为稳定,与前人研究结果一致,因此,推测干扰植株抗旱性较强。
DELLA蛋白是GAs 信号通路中的抑制因子,参与赤霉素合成,影响植物的非生物胁迫。有研究表明:植物GAs降低由DELLA蛋白(GAI、RGA、RGL1、RGL2、RGL3[27])介导完成。GID1感知GAs信号后形成GAs-GID1复合体,促使EL1 (Earlier flowering1)蛋白和SPY (SPINDLY)蛋白磷酸化,激活DELLA蛋白活性后与GA-GIDI复合物结合,形成GA-GID1-DELLA三聚体,诱发26S蛋白酶降解阻遏DELLA蛋白,进而调控相关基因,刺激植物生长[28]。植物处于逆境时可促进编码保护蛋白的LEA基因、编码DELLA蛋白的GAI基因以及编码抗氧化酶的相关基因SOD、POD等表达。因此,推测在干旱胁迫下,赤霉素氧化酶基因表达能调控植株体内的 GAs,使DELLA 不断积累,并参与脱落酸逆境调控,调节下游相关抗性基因。本研究通过RT-qPCR检测了核桃野生型植株、过表达植株、干扰植株在干旱胁迫下相关抗性基因 GAI、SOD、POD、LEA的相对表达量,结果表明:JrGA3ox 基因负调控相关抗旱基因表达,从而调控植株的抗旱性。
-
核桃JrGA3ox基因负调控植株抗旱性。在干旱胁迫下,核桃JrGA3ox基因干扰植株的叶绿素质量分数稳定,气孔开度降低,丙二醛质量摩尔浓度变化幅度较小,活性氧产生较少, 过氧化物歧化酶、超氧化物歧化酶和过氧化氢酶等防御酶活性提高。研究结论为核桃育种应用提供了理论依据,为改变基因型以提高植株抗旱性提供了参考依据。
Response of JrGA3ox gene expression to growth and drought stress in Juglans regia
-
摘要:
目的 研究赤霉素氧化酶基因JrGA3ox对核桃Juglans regia品种改良、生长发育及抗旱品质具有重要意义。 方法 以核桃野生型植株(WT)、JrGA3ox过表达植株(OE)及干扰植株(RNAi)为材料,利用体积分数为5%的聚乙二醇8000 (PEG 8000)模拟干旱处理,探究干旱胁迫下植株表型、生理生化指标及抗旱基因表达水平,明确JrGA3ox基因抗旱机制。 结果 ①经实时荧光定量PCR验证,JrGA3ox基因在过表达植株中表达量是野生型植株的120.0倍,在干扰植株中是野生型植株的0.3倍。②对植株生长表型分析,过表达植株的株高、节间长显著高于野生型植株,干扰植株的株高及节间长显著低于野生型植株(P<0.05)。③与野生型植株相比,干旱胁迫0~28 d时,干扰植株长势良好,过表达植株长势较弱。④气孔开度、叶绿素质量分数随干旱胁迫时间的延长呈逐渐下降趋势,干扰植株的气孔开度显著低于野生型植株, 过表达植株显著高于野生型植株(P<0.05),且干扰植株的叶绿素质量分数始终显著高于过表达植株和野生型植株(P<0.05)。⑤干旱胁迫后,过表达植株活性氧及丙二醛质量摩尔浓度显著高于野生型植株,干扰植株显著低于野生型植株(P<0.05)。⑥抗氧化酶活性及相关抗性基因表达量在胁迫过程中呈先上升后下降趋势,在干旱胁迫14 d时达到最大值,且干扰植株显著高于野生型植株,过表达植株显著低于野生型植株(P<0.05)。 结论 核桃JrGA3ox基因正调控植株的株高及节间长,负调控植株的气孔开度、光合作用、抗氧化酶活性等,从而提高植株抗旱能力。图10表1参28 Abstract:Objective The objective is to study the gibberellin oxidase JrGA3ox gene, which is of great significance for improvement, growth and development, and drought resistance of Juglans regia varieties. Method J. regia wild-type (WT), JrGA3ox overexpression (OE) and interference (RNAi) plants were used as experimental materials. Drought treatment with 5% PEG 8000 volume fraction was simulated to investigate the plant phenotype, physiological and biochemical indexes and expression level of drought resistance gene under drought stress, and clarify the drought resistance mechanism of JrGA3ox gene. Result (1) Real-time fluorescent quantitative PCR verification showed that the expression level of JrGA3ox gene in OE plants and RNAi plants were 120.0 and 0.3 times that of WT plants, respectively. (2) Plant growth phenotype analysis showed that plant height and internode length of OE plants were significantly higher than those of WT plants, while those of RNAi plants were significantly lower than those of WT plants (P<0.05). (3) Compared with WT plants, under drought stress for 0−28 days, RNAi plants showed better growth, while OE plants showed weaker growth. (4) Stomatal opening and chlorophyll mass fraction decreased gradually with the extension of drought stress time. Stomatal opening of RNAi plants were significantly lower than that of WT plants (P<0.05), while stomatal opening of OE plants were significantly higher than that of WT plants. The chlorophyll mass fraction of RNAi plants were always significantly higher than that of OE plants and WT plants (P<0.05). (5) After drought stress, the mass molar concentration of reactive oxygen species and malondialdehyde in OE plants were significantly higher than that in WT plants, while that in RNAi plants were significantly lower than that in WT plants (P<0.05). (6) The activity of antioxidant enzymes and expression level of related resistance genes firstly increased and then decreased during the stress process, reaching their maximum value at 14 days of drought stress, and those in RNAi plants were significantly higher than those in WT plants, while those in OE plants were significantly lower than those in WT plants (P<0.05). Conclusion JrGA3ox gene in J. regia can positively regulate plant height and internode length, and negatively regulate stomatal opening, photosynthesis, antioxidant enzyme activity of plants, thus improving plant drought resistance. [Ch, 10 fig. 1 tab. 28 ref.] -
Key words:
- Juglans regia /
- JrGA3ox /
- drought stress /
- physiological characteristics /
- stress resistance gene
-
表 1 RT-qPCR引物
Table 1. Primers for RT-qPCR analysis
引物 序列(5′→3′) 用途 引物 序列(5′→3′) 用途 Actin-F GCCGAACGGGAAATTGTC 内参 QJrPOD-R AGAGACGGTCGTTGAAGGAG RT-qPCR Actin-R AGAGATGGCTGGAAGAGG 内参 QJrLEA-F CAGCATCACCGACGTTGATT RT-qPCR QJrSOD-F TTGGAGCCACATATGAGCCA RT-qPCR QJrLEA-R TCAACAATATGCTGTGCGGC RT-qPCR QJrSOD-R CCTGTCCTGCGTTGTTGAAA RT-qPCR QJrGAI -F TGTTCTGGTTGATTCGCACG RT-qPCR QJrPOD-F TCAAGCGAAATAGAGGCCCA RT-qPCR QJrGAI -R TAAGTGGCGACCTTTCCCAT RT-qPCR -
[1] 苗庆选, 刘春晓, 怀婷婷, 等. 我国核桃研究文献的统计分析[J]. 安徽农业科学, 2020, 48(6): 223−226. MIAO Qingxuan, LIU Chunxiao, HUAI Tingting, et al. Statistical analysis of walnut research literatures in China [J]. Journal of Anhui Agricultural Sciences, 2020, 48(6): 223−226. [2] 牛选明. 干旱胁迫对薄皮核桃果实品质的影响[J]. 山东林业科技, 2018, 48(5): 61−63. NIU Xuanming. Effects of drought stress on fruit quality of thin-skinned walnut [J]. Journal of Shandong Forestry Science and Technology, 2018, 48(5): 61−63. [3] XU Zhenzhu, JIANG Yanling, ZHOU Guangsheng. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants [J/OL]. Frontiers in Plant Science, 2015, 6 : 701[2024-03-29]. DOI: 10.3389/fpls.2015.00701. [4] de la FUENTE J L, ZUNZUNEGUI M, BARRADAS M C D. Physiological responses to water stress and stress memory in Argania spinosa [J/OL]. Plant Stress, 2023, 7 : 100133[2024-03-29]. DOI: 10.1016/j.stress.2023.100133. [5] REINECKE D M, WICKRAMARATHNA A D, OZGA J A, et al. Gibberellin 3-oxidase gene expression patterns influence gibberellin biosynthesis, growth, and development in pea [J]. Plant Physiology, 2013, 163(2): 929−945. [6] XUE Dawei, ZHANG Xiaoqin, LU Xueli, et al. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance [J/OL]. Frontiers in Plant Science, 2017, 8 : 621[2024-03-29]. DOI: 10.3389/fpls.2017.00621. [7] KANG H G, KIM J, KIM B, et al. Overexpression of FTL1/DDF1, an AP2 transcription factor, enhances tolerance to cold, drought, and heat stresses in Arabidopsis thaliana [J]. Plant Science, 2011, 180(4): 634−641. [8] ZAWASKI C, BUSOV V B. Roles of gibberellin catabolism and signaling in growth and physiological response to drought and short-day photoperiods in Populus trees [J/OL]. PLoS One, 2014, 9 (1): e86217[2024-03-29]. DOI: 10.1371/journal.pone.0086217. [9] 何红红. 葡萄赤霉素氧化酶基因GA2ox、GA3ox和GA20ox家族的鉴定与GA2ox7的耐盐性功能分析[D]. 兰州: 甘肃农业大学, 2021. HE Honghong. Identification of Grape Gibberellin Oxidase Genes GA2ox, GA3ox and GA20ox Family and Analysis of Salt Tolerance Function of GA2ox7 [D]. Lanzhou: Gansu Agricultural University, 2021. [10] DRIVER J A, KUNIYUKI A H. In vitro propagation of paradox walnut rootstock [J]. HortScience, 1984, 19(4): 507−509. [11] 魏广利. 核桃赤霉素2-ODDs家族氧化酶基因的克隆与功能分析[D]. 杭州: 浙江农林大学, 2021. WEI Guangli. Cloning and Functional Analysis of Oxidase Gene of Gibberellin 2-ODDs Family in Walnut [D]. Hangzhou: Zhejiang A&F University, 2021. [12] 高俊山, 蔡永萍. 植物生理学实验指导[M]. 北京: 中国农业大学出版社, 2018. GAO Junshan, CAI Yongping. Experimental Guidance of Plant Physiology [M]. Beijing: China Agricultural University Press, 2018. [13] 魏广利, 梁璧, 张佳琦, 等. 山核桃赤霉素氧化酶基因CcGA3ox的克隆和功能分析[J]. 果树学报, 2019, 38(1): 13−28. WEI Guangli, LIANG Bi, ZHANG Jiaqi, et al. Cloning and functional analysis of CcGA3ox gene from hickory (Carya cathayensis) [J]. Journal of Fruit Science, 2019, 38(1): 13−28. [14] CASSON S A, HETHERINGTON A M. Environmental regulation of stomatal development [J]. Current Opinion in Plant Biology, 2010, 13(1): 90−95. [15] LI Shuang, LIU Junming, LIU Hao, et al. Role of hydraulic signal and ABA in decrease of leaf stomatal and mesophyll conductance in soil drought-stressed tomato [J/OL]. Frontiers in Plant Science, 2021, 12 : 653186[2024-03-29]. DOI: 10.3389/fpls.2021.653186. [16] KERCHEV P I, van BREUSEGEM F. Improving oxidative stress resilience in plants [J]. The Plant Journal, 2022, 109(2): 359−372. [17] DMITRIEVA V A, TYUTEREVA E, VOITSEKHOVSKAJA O. Singlet oxygen in plants: generation, detection, and signaling roles [J/OL]. International Journal of Molecular Sciences, 2020, 21 (9): 3237[2024-03-29]. DOI: 10.3390/ijms21093237. [18] 郝小花, 胡爽, 赵丹, 等. OsGA3ox通过合成不同活性GA调控水稻育性及株高[J]. 遗传, 2019, 45(9): 845−855. HAO Xiaohua, HU Shuang, ZHAO Dan, et al. OsGA3ox genes regulate rice fertility and plant height by synthesizing diverse active GA [J]. Hereditas, 2019, 45(9): 845−855. [19] SPERRY J S, VENTURAS M D, ANDEREGG W R L , et al. Predicting stomatal responses to the environment from the optimization of photosynthetic gain and hydraulic cost [J]. Plant Cell & Environment, 2017, 40(6): 816−830. [20] 刘德政, 卢山, 高珅奥, 等. 大田和旱棚环境下小麦旗叶气孔性状变异及其与光合参数的关系[J]. 麦类作物学报, 2024, 44(3): 360−369. LU Dezheng, LU Shan, GAO Shen’ao, et al. Variations of flag leaf and stomatal traits of wheat in field and drought shed environment [J]. Journal of Triticeae Crops, 2024, 44(3): 360−369. [21] 王质璞, 李卓蓉, 罗志斌, 等. PagAPY1基因调控银腺杨耐旱性的作用机制研究[J]. 南京林业大学学报(自然科学版), 2023, 47(6): 105−112. WANG Zhipu, LI Zhuorong, LUO Zhibin, et al. Mechanisms of PagAPY1 in regulating drought tolerance in Populus alba×P. glandulosa [J]. Journal of Nanjing Forestry University (Natural Sciences Edition), 2023, 47(6): 105−112. [22] 何小三, 周文才, 邱凤英, 等. 不同油茶品种对干旱胁迫的响应及其抗旱性综合评价[J]. 中南林业科技大学学报, 2019, 43(9): 1−14. HE Xiaosan, ZHOU Wencai, QIU Fengying, et al. Responses of different Camellia oleifera varieties to drought stress and the comprehensive evaluation of their drought resistance [J]. Journal of Central South University of Forestry & Technology, 2019, 43(9): 1−14. [23] 冯蕊, 周琪, 吴令上, 等. PEG 6000模拟干旱胁迫对铁皮石斛幼苗生理和叶绿素荧光特性的影响[J]. 浙江农林大学学报, 2024, 41(1): 132−144. FENG Rui, ZHOU Qi, WU Lingshang, et al. Effects of PEG 6000 simulated drought stress on physiological and chlorophyll fluorescence characteristics of Dendrobium candidum seedlings [J]. Journal of Zhejiang A&F University, 2024, 41(1): 132−144. [24] 董斌, 蓝来娇, 黄永芳, 等. 干旱胁迫对油茶叶片叶绿素含量和叶绿素荧光参数的影响[J]. 经济林研究, 2020, 38(3): 16−25. DONG Bin, LAN Laijiao, HUANG Yongfang, et al. Effects of drought stress on photosynthetic pigments and chlorophyll fluorescence characteristics in leaves of Camellia oleifera [J]. Non-wood Forest Research, 2020, 38(3): 16−25. [25] MITTLER R, VANDERAUWERA S, SUZUKI N, et al. ROS signaling: the new wave? [J]. Trends in Plant Science, 2011, 16(6): 300−309. [26] 张清航, 张永涛. 植物体内丙二醛(MDA)含量对干旱的响应[J]. 林业勘查设计, 2019(1): 110−112. ZHANG Qinghang, ZHANG Yongtao. Study on response to drought stress of MDA content in plants [J]. Forestry Investigation Design, 2019(1): 110−112. [27] 吴建明, 陈荣发, 黄杏, 等. 高等植物赤霉素生物合成关键组分GA20-oxidase氧化酶基因的研究进展[J]. 生物技术通报, 2016, 32(7): 1−12. WU Jianming, CHEN Rongfa, HUANG Xing, et al. Studies on the gene of key component GA20-oxidase for gibberellin biosynthesis in plant [J]. Biotechnology Bulletin, 2016, 32(7): 1−12. [28] CHENG Jun, ZHANG Mengmeng, TAN Bin, et al. A single nucleotide mutation in GID1c disrupts its interaction with DELLA1 and causes a GA-insensitive dwarf phenotype in peach [J]. Plant Biotechnology Journal, 2019, 17(9): 1723−1735. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20240327






 下载:
下载: