-
雷竹Phyllostachys violascens又称早园竹,属禾本科Poaceae刚竹属Phyllostachys,具有出笋早、笋期长、产量高、笋味美等特点,是优良的笋用竹种[1]。自20世纪90年代以来,许多雷竹产区利用砻糠等有机材料进行覆盖栽培,使雷竹出笋提前至春节前,极大提高了雷竹的经济效益[2]。但随着雷竹覆盖栽培年限的增加,雷竹林出现了退化现象,从而限制了雷竹林的可持续发展[3]。因此,为了寻找一种新的方法或优化现有的覆盖增温技术,需要充分了解雷竹笋芽休眠解除的进程。
温度对植物休眠的诱导和解除具有重要的作用,不同植物的休眠解除对温度的需求不同[4]。如百合属Lilium植物休眠解除需要在2 ℃冷藏6~8周[5];李属Prunus植物休眠解除和恢复生长需要6~9 ℃的冷量积累1 000 h[6]。目前,对植物休眠的研究集中在落叶树种。这些植物与竹子不同,它们的芽直接暴露在低温环境下感受低温对其休眠解除带来的影响。雷竹主要通过地下抽鞭发芽,并生长繁殖成林。因此雷竹的侧芽其实是在土壤中完成发育生长,并最终出土成笋,而同时期的土壤和环境温度存在较大差异。因此,雷竹是像落叶树一样由环境低温诱导芽休眠解除,还是由直接接触鞭侧芽的土温诱导尚不清楚。
为了探究笋芽休眠解除过程中雷竹对低温的主要感应部位是地上部分还是地下部分,本研究通过对雷竹盆栽苗不同部位(地下和地上部分)的低温处理,比较各个处理雷竹笋芽休眠进程中相关生理指标的变化规律和差异,探究不同部位低温处理对雷竹笋芽休眠解除的影响,为提高雷竹林经营效率和优化雷竹林覆盖栽培技术提供理论参考。
-
研究区位于浙江省杭州市临安区浙江农林大学翠竹园(30°15′56.965″N,119°44′17.276″E)。该区属中亚热带季风气候,年均降水量为1 613.9 mm,年降水日为158.0 d,年均无霜期为237.0 d。年均气温为16.4 ℃,最低气温为−7.0 ℃,最高气温为41.0 ℃。
-
分别于2021和2022年的10月至翌年3月开展研究。选用2年生雷竹盆栽苗,苗高为1.3 m,苗基径为0.6 cm。育苗盆选用黑色塑料盆(25 cm×21 cm×22 cm)。雷竹盆栽苗地上部分留高约50~100 cm,地下部分留约25 cm的竹鞭。基于许烨荣[7]的前期工作,本研究针对雷竹盆栽苗地上和地下部分设置4个温度处理组:T1 (地上、地下部分18 ℃),T2 (地上部分18 ℃,地下部分自然低温),T3 (地上部分自然低温,地下部分18 ℃),T4 (地上、地下部分自然低温)。每个处理设置15盆雷竹盆栽苗。
温度处理装置主要由人工气候室(WTC)和保温隔热板组成。T1处理是盆栽苗直接放置在WTC中恒温18 ℃;T2处理是在WTC中放置保温隔热箱将盆栽苗隔开,箱体与外界空气互通实现地下部分自然低温,地上部分18 ℃;T3处理在防雨棚下通过加热保温隔热箱实现地下部分18 ℃,地上自然低温;T4处理为直接将盆栽苗放置在防雨棚下。在各处理中随机挑选3盆盆栽苗监测和记录温度变化。研究期间浇灌盆栽苗的水提前放置在相对应的温度环境下,确保浇水时不会影响盆栽苗的温度。
-
于2021年10月15日开始,生理指标每40 d测定1次,各处理每次取5盆盆栽苗。取样时洗净竹鞭后,将箨片剥至笋芽尖完全露出为止,完整切下笋芽并置于−80 ℃冰箱保存,用于各项生理指标测定;于2022年10月20日开始5-乙炔基-2′-脱氧尿苷(EdU)检测和维管束染色试验,每月取1次样,各处理每次取3盆雷竹盆栽苗。
-
雷竹笋芽可溶性糖、蔗糖质量分数采用蒽酮比色法测定,淀粉质量分数采用高氯酸法测定[8];超氧化物歧化酶(SOD)和过氧化物酶(POD)采用苏州科铭生物技术有限公司研制的酶活试剂盒测定;过氧化氢酶(CAT)采用紫外吸收法测定;脱落酸 (ABA)、赤霉素 (GA3)质量分数参考酶联免疫吸附实验法(ELISA)[9]测定。
-
参照相关研究方法[10],本研究将剥除箨片的雷竹笋芽在200 μmol·L−1 EdU中孵12 h,后在1 mL 体积分数为90%的丙酮溶液中冰浴10 min,再用1 mL 1×磷酸盐缓冲液(PBS)洗涤笋芽3次,除去上清液,用1 mL FAA固定液在压力泵中固定笋芽4 h,然后用震动切片将笋芽切成60 μm薄片(尽量取茎尖处的切片),用1 mL 体积分数为0.5% 的Triton X-100 (用1×PBS配制)震动洗涤2次,配制反应混合物对笋芽薄片黑暗避光染色30 min,重复2次,随后用1 mL 1×PBS洗涤笋芽3次,每次20 min。使用荧光显微镜绿光观察拍照。
-
本研究在ESSIAMAH等[11]、XIE等[12]染料吸收和运输速率的方法上稍微改良。每次从各处理随机剪下3段鞭,每根鞭上保留1个芽,尽量保证根须不被剪掉,设3个重复。将其竖着放入50 mL质量分数为0.5%的酸性品红染料中4 h后,在体视显微镜下观察鞭芽对染料的运输情况并拍照记录。
-
将笋芽从竹鞭上完整切下后,在FAA固定液中充分固定。根据石蜡切片的方法将笋芽切成8 μm厚的切片[13],采用高碘酸-锡夫反应法(PAS)染色[14]。
-
采用Excel进行数据统计和处理,用SPSS 26.0进行单因素方差分析(one-way ANVO),Duncan法检验不同处理间的差异显著性(显著性水平为0.05),利用Origin 2021进行非线性曲线拟合及作图。切片使用正置荧光显微镜Olympus BX60观察,用Olympus DP70全自动照相系统拍照。维管观察使用深圳奥斯微体视显微镜观察拍照。
-
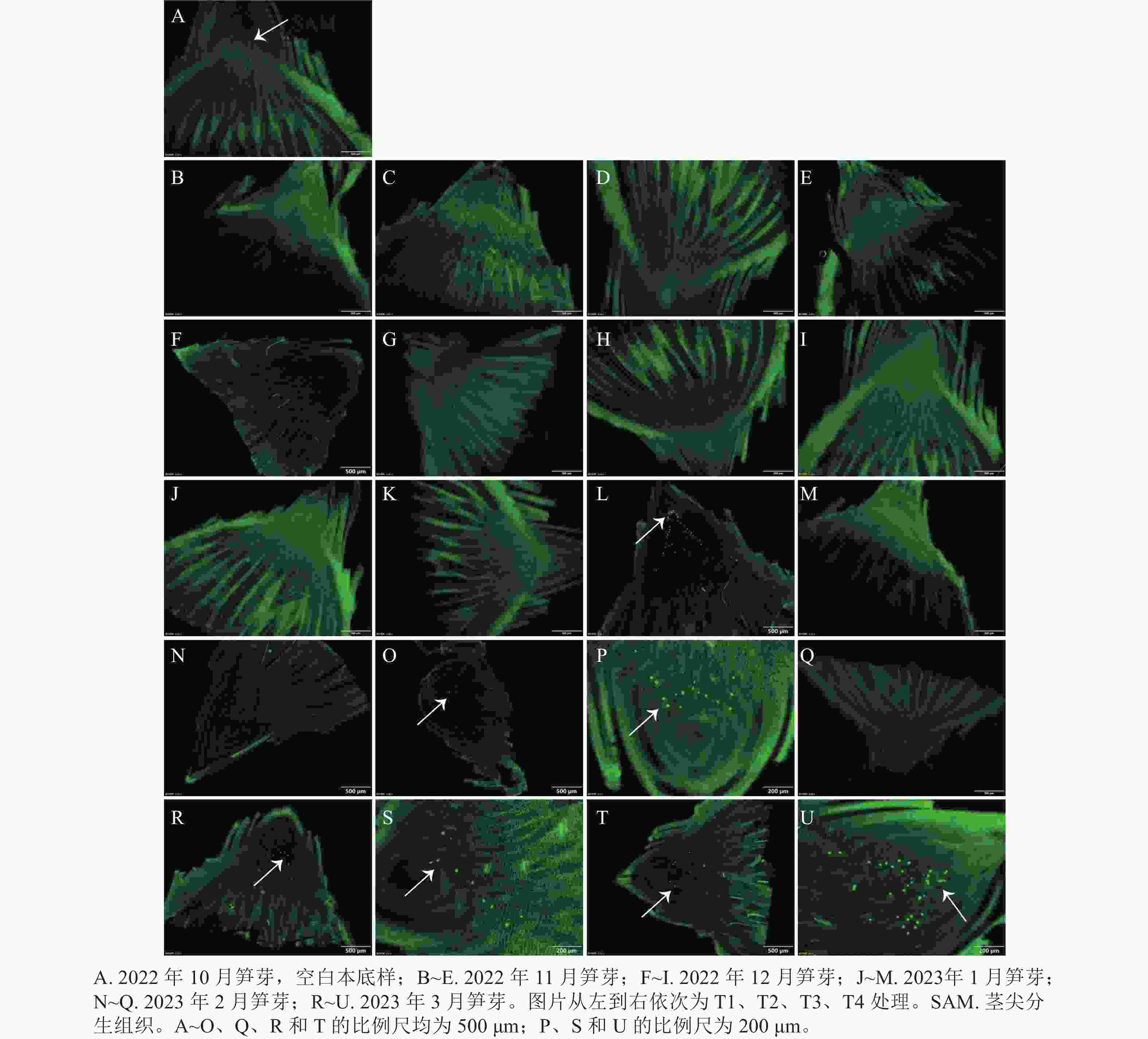
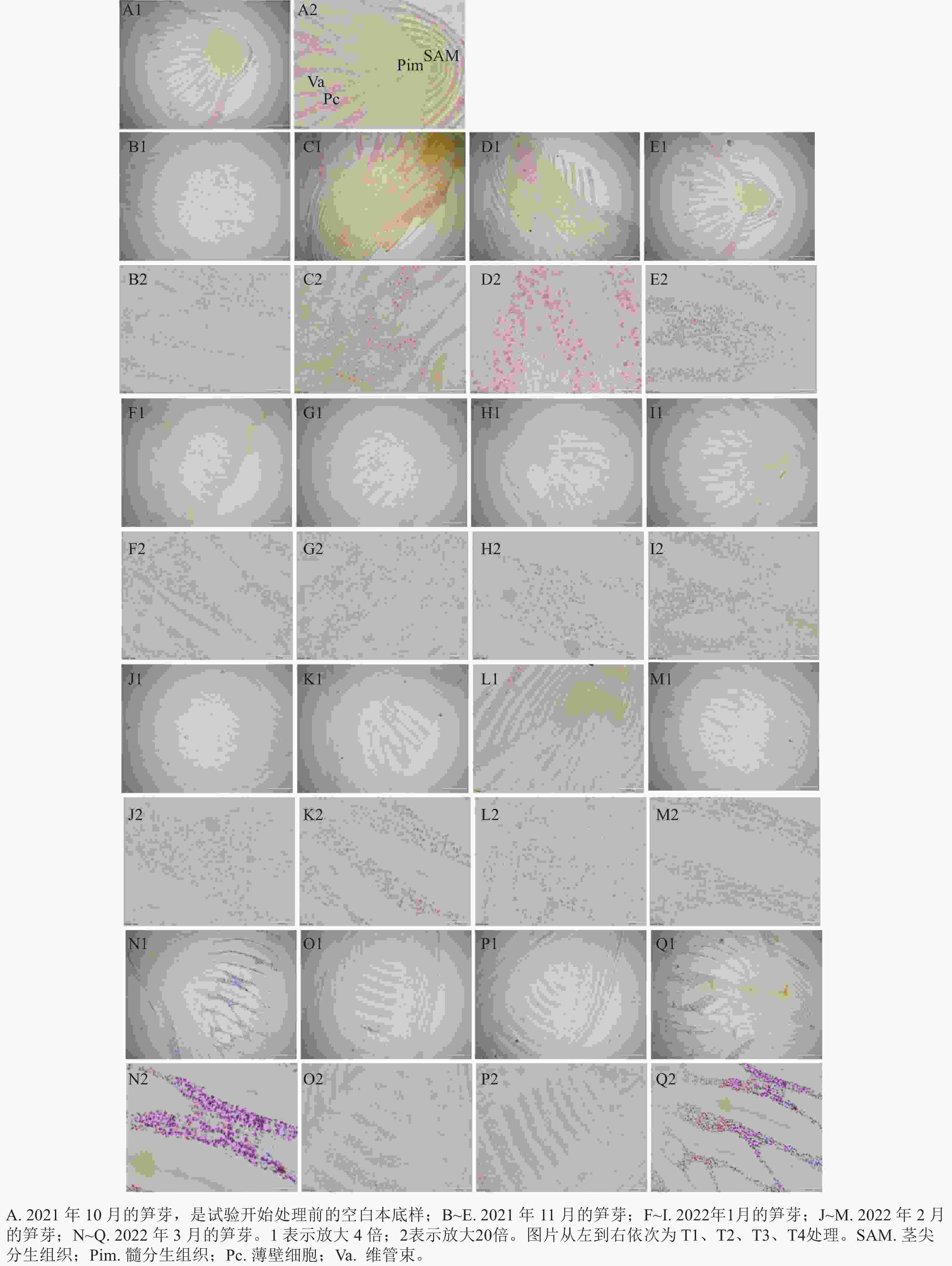
光学显微镜的观察结果(图1)显示:越冬前,雷竹笋芽的淀粉主要储存在薄壁细胞、髓和叶原基中,呈紫粉色。蛋白质主要分布在芽顶端生长锥、腋芽芽尖和维管形成层等分生组织区,呈黄色。芽内淀粉和蛋白质变化基本是同步发生的。在2021年11月,芽内淀粉分布在薄壁细胞、髓和箨原基中,芽顶端生长锥、腋芽芽尖和维管形成层等分生组织呈淡黄色,萘酚黄染色明显。髓部淀粉着色明显,但是淀粉整体颜色比10月淀粉着色浅,整个髓分生组织呈锥形瓶状。芽中部细胞排列规则,纵向压扁,细胞内淀粉等物质聚集在细胞核周围。T2、T4处理的髓部淀粉分布较T3处理少,T4处理的髓部淀粉分布较T2处理少。T1处理变化不明显,髓部淀粉极少,蛋白质着色也不明显。2022年1月,随着温度持续降低,各处理淀粉着色整体变浅,主要在芽顶端生长锥、箨原基、维管形成层及腋芽芽尖。各处理的髓部淀粉着色基本消失,萘酚黄着色逐渐加深。其中T3顶端蛋白质着色较明显,淀粉着色也比12月深。其中T2、T4处理的薄壁细胞中淀粉着色较浅,而T1处理变化不明显。在2月,芽顶端蛋白质着色明显。笋芽中下部淀粉着色较1月稍有变深。T1处理的顶端蛋白质着色明显,淀粉分布基本不变。在随后的3月,T1、T4处理的蛋白质染色明显加深,淀粉被染成深紫色,细胞开始纵向伸长。且T2、T3处理的节间基本分化完毕,已经开始进行高度生长。
-
由图2A可知:可溶性糖质量分数整体随着处理时间的增加呈先升高后下降的趋势。T2和T3处理的可溶性糖质量分数在上升阶段一直都显著高于T1和T4处理(P<0.05),并都在80 d时达到峰值,分别为12.14 和12.37 mg·g−1,此时T2、T3、T4处理的可溶性糖质量分数分别比T1处理显著高20.67%、22.96%和7.91% (P<0.05)。而T1和T4处理的可溶性糖质量分数则在120 d时达到峰值,分别为10.33和10.95 mg·g−1。各处理的可溶性糖质量分数在达到各自峰值后都开始下降,其中T2和T3处理在2月时下降幅度最大,分别下降了24.86%和19.17%,而此时T1和T4处理的可溶性糖质量分数降幅分别为12.83%、15.29%。T2处理的可溶性糖质量分数最大降幅比T1、T3和T4处理分别高12.03%、5.69%和9.75%。
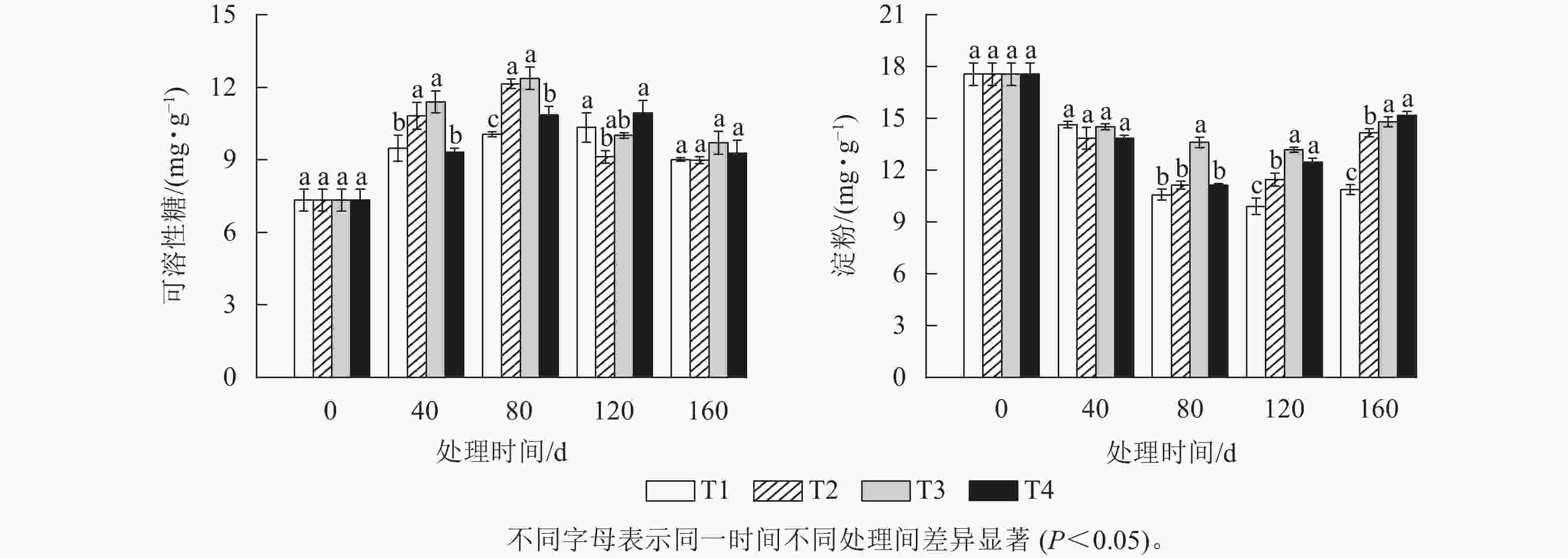
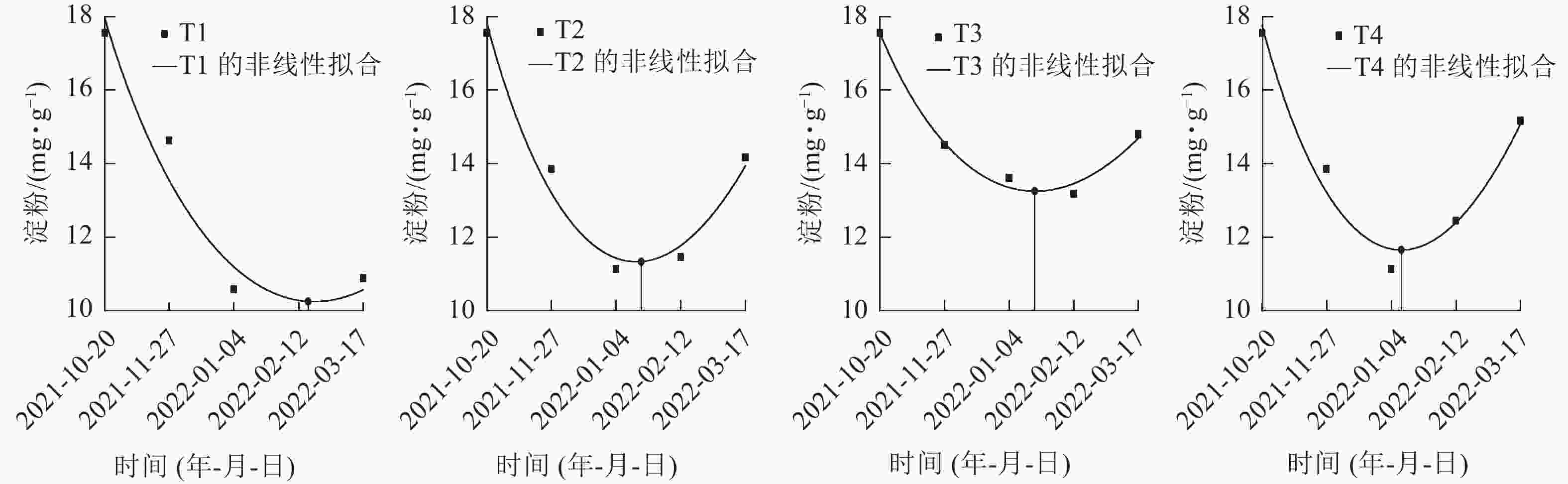
图 2 各处理笋芽中可溶性糖(A)和淀粉(B)质量分数的变化
Figure 2. Changes of soluble sugar (A) and starch (B) content in shoots of different treatments
由图2B可知:各处理组的淀粉质量分数随着处理时间的增加都呈先下降后上升的趋势。T1、T2、T3和T4处理的淀粉质量分数在120 d 时较0 d分别下降了43.59%、36.61%、24.93%和36.61%。各处理间的淀粉质量分数在80 d时首次出现显著差异,此时,T3处理的淀粉质量分数要显著高于T1、T2和T4处理(P<0.05),而T2和T4处理的淀粉质量分数则最先达到其最低值,均为11.13 mg·g−1,比T3处理降低了2.47 mg·g−1。T1和T3处理的淀粉质量分数则在120 d时才出现最低值,淀粉质量分数分别为9.9和13.18 mg·g−1。当各处理的淀粉质量分数降到最低值后又开始逐渐上升。其中最先上升的为T4和T2处理,分别上升了1.33和0.33 mg·g−1。
在对各处理笋芽淀粉切片的动态观察中发现:T2、T3、T4处理的淀粉着色在1月最浅,且2月颜色差异不大(图1),推断出T2、T3、T4处理休眠释放为1—2月。再对T1、T2、T3、T4处理在休眠期间(2021年10月至2022年2月)的淀粉生化指标进行定量分析(图3),并通过非线性拟合找到1—2月淀粉质量分数最低点的横坐标的具体日期,判断淀粉质量分数达到最低的时间分别为:T1处理为2022年2月19日,T2、T3处理为2022年1月20日,T4处理为2022年1月12日。
-
从图4可见:2022年10、11、12月和2023年1月各处理均无红色圆点,只有T3处理在2023年1月出现了少量红色圆点。2023年2月仅T2、T3处理出现了红色圆点,且较为密集。随后的3月,T1、T2处理出现红色圆点较稀疏,并且T3、T4处理均观察到密集的红色圆点。而当笋芽与竹鞭之间的维管束能够被染料标记时,说明笋芽与竹鞭之间建立连接,维管束中导管已经开始进行水分运输,笋芽休眠逐渐解除。
-
当笋芽生态休眠解除后,会进入到快速生长时期。通过EdU的荧光标记来检测笋芽茎尖分生组织中的细胞增殖情况(图5)。在2023年1月的各处理中,T3处理最先检测出EdU荧光信号,整个笋芽切片中的绿色圆点集中在茎尖分生组织区域,原套下方的细胞开始快速生长,进行箨原基、芽原基的分化,说明T3处理的笋芽已经解除生态休眠。2月后,T2处理开始检测出极少的EdU荧光信号,T3处理的荧光信号依然明显,其他处理还未发现荧光信号。在3月,各处理均在茎尖分生组织中检测出EdU信号,此时各处理休眠均已解除。
-
由图6A所示:随着处理时间的增加,各处理的ABA质量分数呈先上升后下降的趋势,T2、T3、T4处理都在80 d时达到最高值,而T1处理在40 d时达到最高值。在120 d时,T2、T3、T4处理的ABA质量分数分别比80 d显著(P<0.05)下降了16.00%、23.13%、31.95%。T1处理的ABA质量分数在整个越冬时期变化不明显。

图 6 各处理笋芽中GA3、ABA质量分数及其比值
Figure 6. Contents and ratios of GA3 and ABA in shoots of different treatments
由图6B所示:GA3质量分数呈先上升后下降随后又上升的趋势,各处理的GA3质量分数都在80 d时达到最低值,且此时T2、T3、T4处理的GA3质量分数较40 d下降显著(P<0.05)。在120 d时,T3处理的GA3质量分数比80 d显著上升了35.07% (P<0.05),T2、T4处理也呈上升趋势。而在整个越冬期T1处理的GA3质量分数在13.88~18.48 ng·g−1内变化。
GA3和ABA的拮抗作用对芽的休眠与萌发有着重要的作用。由图6C可知:T2、T3、T4处理的GA3/ABA均为先上升后下降再上升的变化趋势,80 d时各处理均达到最低值。在120 d时,T3处理的GA3/ABA比80 d增长了约76.1%,T4处理的增长了约62.4%。而在整个越冬期T1处理的GA3/ABA差异不显著。
-
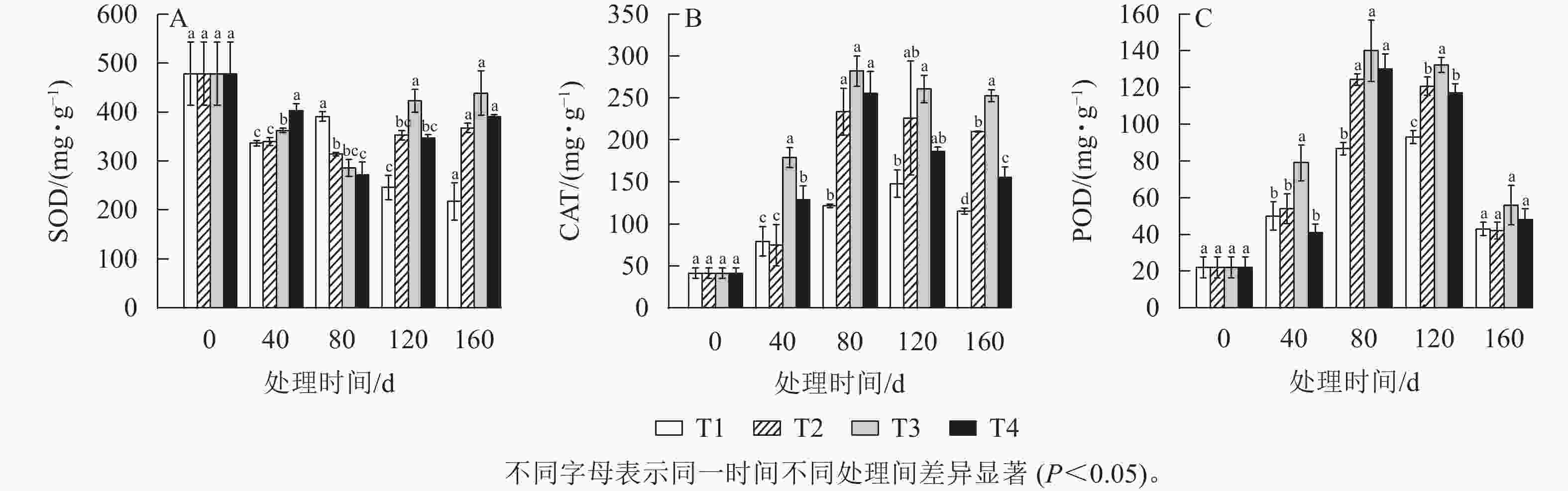
如图7A所示:从40 d开始,各处理的SOD活性开始下降,直到80 d时,T2、T3、T4处理的SOD活性降低到了最低值,且都显著低于T1处理(P<0.05),而此时T1处理的SOD活性反而比40 d时增加了16.27%。到120 d时T2、T3、T4处理的SOD活性均升高,其中T3处理增加了137 mg·g−1,而T1处理继续下降,与其他处理差异显著(P<0.05)。
如图7B所示:各处理的CAT活性呈先上升后下降的趋势。40 d时T1、T2、T3和T4处理的CAT活性分别为0 d的2.27、2.45、3.59、1.86倍。T2、T3、T4处理的CAT活性在80 d时达到最大值,而T1处理在120 d才达到最大值。T3和T4处理的CAT活性与T1处理的CAT活性在40 d时首次出现显著差异(P<0.05)。T1处理的CAT活性整体上变化不显著。
如图7C所示:各处理POD活性在整个处理时间阶段内呈现先上升后下降的趋势。在80 d时各处理的POD活性都达到最大值,其中T2、T3和T4处理的POD活性显著大于T1处理(P<0.05)。在80 d后,除T1处理外,各处理的POD活性均开始下降,直到160 d时POD活性在各处理间差异不显著。在40 d时,T3和T1处理的POD活性差异显著(P<0.05),T3处理比T1处理高53.33 mg·g−1,且显著差异一直持续到160 d。而在80 d时T2、T4处理与T1处理的POD活性差异显著(P<0.05)。在120 d时,T1处理的POD活性显著低于其他3个处理(P<0.05)。
-
碳水化合物是植物休眠解除和芽萌发生过程中各种生理代谢活动的主要能量来源[15]。在休眠解除过程中,淀粉会被酶水解为可溶性糖,不仅为植物提供能量,还可以作为调节休眠的信号物质[16]。在本研究中,T2和T4处理的淀粉质量分数最先达到最低值,说明在低温处理下尤其是地下部分低温处理更加有利于促进淀粉快速水解为可溶性糖,并为雷竹笋芽休眠解除后的生长发育提供能量。在对李属[17]、梨Pyrus pyrifolia[18]、苹果Malus pumila[19]、核桃Juglans regia[20]和白杨Populus tomentosa[21]等的研究中也发现:低温积累处理会使枝条中淀粉质量分数降低,而可溶性糖质量分数增加。因此,本研究推断低温处理尤其是地下部分低温处理可以增强雷竹笋芽对糖的利用能力,从而促进笋芽休眠的解除[22]。
ABA在多种植物中诱导茎停止生长,诱导芽休眠[23]。在本研究中,T2、T3、T4的ABA质量分数均在80 d时达到最高值后开始大幅下降,这与葡萄Vitis vinifera芽中内源ABA质量分数随着休眠的加深而逐步升高,在休眠解除期间又逐渐降低的结果较为一致[24]。说明低温处理可以促进雷竹笋芽ABA质量分数的降低。此外,有研究表明:ABA会抑制α-淀粉酶的活性,从而影响淀粉的降解,说明ABA与糖类之间存在干扰[25]。本研究中,雷竹盆栽苗笋芽ABA与淀粉质量分数都同时增加,正好也验证了这一点。而GA3作为休眠抑制物,在植物芽休眠的解除中发挥着关键作用,可以促进休眠的解除[26]。在芽休眠诱导后GA3质量分数迅速下降,并在休眠期间保持较低的水平,在接近休眠解除和芽萌发开始时升高[27]。本研究中,T2、T3、T4处理的GA3质量分数均在80 d时达到最低后开始急速上升,并且GA3/ABA同样也在80 d达到最低后快速升高。这与淀粉非线性曲线拟合结论是相符的,说明了低温处理可以促进休眠笋芽GA3的合成。同样GA3/ABA对沙棘Hippophae rhamnoides芽的休眠与萌发有控制作用,该比值高可以促进芽的萌发,反之则抑制萌发[28],说明GA3/ABA变化与休眠的解除程度是相吻合的[29]。
BEAUVIEUX等[30]强调了活性氧(ROS)和抗氧化代谢在休眠释放中的关键作用。植物芽中产生的ROS可能是休眠的关键信号[31]。本研究中,T1、T2、T3和T4处理的CAT和POD的活性呈先上升后下降的趋势,T2、T3和T4处理中这2种酶的活性均在80 d时达到最高。在杏树Prunus dulcis花芽休眠解除过程中,CAT和POD活性显著增加的研究结果[32]与本研究结果一致,说明在低温处理下雷竹笋芽休眠解除过程中伴随着氧化应激反应,因为,在低温环境下产生的ROS会对生物大分子造成损害,而植物会使用抗氧化酶以及非酶防御机制来预防或修复氧化损伤[33]。本研究中,T2、T3、T4处理在雷竹笋芽休眠解除过程中SOD酶活性呈先下降后上升的趋势,而在T1处理中SOD活性逐渐降低。根据雷竹笋芽休眠解除时间的确定,说明SOD活性在雷竹笋芽休眠解除后开始增强,并且相比CAT和POD活性,SOD活性一直处于相对较高的水平,说明SOD在雷竹笋芽休眠解除过程中也可能发挥着作用,但其应激反应却不如CAT和POD强烈。
-
一般来说,休眠前期植物器官中物质的化学合成大于水解,主要是低分子量的物质合成高分子量的化合物,而在休眠后期水解作用加强,开始逆向转化[34]。本研究对越冬期笋芽的淀粉及蛋白质分布进行了动态观测,发现在雷竹笋芽感受到低温刺激前,芽髓部有大量的淀粉和蛋白质。雷竹盆栽苗的笋芽随着处理的进行,淀粉着色颜色开始由紫红色变为浅粉色,尤其是地下、地上部分均是自然低温处理的结果变化明显,说明低温处理可以促进淀粉和蛋白质的水解,为笋芽的萌发提供能量和必要的养分[35]。到了120 d时,T2、T3、T4处理的切片中,淀粉颜色较80 d时的深。这是因为休眠解除后,可溶性糖又重新生成了淀粉,为笋芽的后续生长储存了能量[36]。由此可推断出,T2、T3、T4处理的休眠解除为1—2月。
-
在春季,植物汁液的流动也被认为是芽萌发和新陈代谢被激活的重要手段[37]。葡萄芽在休眠过程中与葡萄藤之间的木质部是分离的,而当芽开始膨胀时,它们之间的木质部连续性会重新建立[38]。本研究表明:T3处理的笋芽导管最先在1月恢复运输,说明与其他处理相比,T3处理的笋芽导管最早解除休眠恢复生长。这是因为温度对木质部的形成具有重要作用,当温度达到一定阈值时,将形成植物木质部[39]。并且维管束的重新连接可能与形成层细胞被激活、芽和茎的连接处微观组织重新形成有关[40]。
生长停止和细胞分裂停止是多年生植物芽进入休眠状态的典型特征[41]。本研究发现:T3处理的笋芽茎尖分生组织最先在1月检测出细胞增殖的信号,表明此时笋芽茎尖细胞已开始快速分裂增殖,与其他处理相比,T3处理的雷竹笋芽细胞增殖进程较早。这也与维管束水分运输能力验证实验的结果相符,T1处理没有得到足够的冷积累,其EdU荧光信号检测出的时间最晚且信号最少。虽然T2、T3、T4处理的休眠解除时间相近,但是T3处理的细胞增殖却早于其他处理,这可能是在雷竹笋芽休眠解除后,T3处理地下部分的高温为雷竹笋芽提供适宜的生长温度,使笋芽最早恢复正常的生长发育。
-
本研究发现:越冬前,雷竹笋芽的淀粉主要储存在薄壁细胞、髓和叶原基中,蛋白质主要分布在芽顶端生长锥、腋芽芽尖和维管形成层等分生组织区。随着温度持续的降低,淀粉的分布变为芽顶端生长锥、箨原基、维管形成层及腋芽芽尖,而蛋白质则主要分布在芽顶端。同时,低温处理主要通过增加休眠雷竹笋芽可溶性糖和GA3质量分数、改变维管束的连通状态并促进水分运输、降低淀粉质量分数等促进雷竹笋芽休眠的解除。此外,当笋芽休眠解除后,适宜高温可以促进雷竹笋芽细胞的快速分裂增殖。通过雷竹笋芽切片的淀粉观察和淀粉质量分数的非线性拟合,大致确定了T1处理的雷竹笋芽休眠解除的时间为2022年2月19日,T2、T3处理为2022年1月20日,T4处理为2022年1月12日。在休眠期间雷竹对低温的感知部位主要为地下部分。
Effect of low temperature treatment of different parts on dormancy release of Phyllostachys violascens shoots
-
摘要:
目的 探讨不同部位低温处理对雷竹Phyllostachys violascens笋芽休眠的影响,揭示笋芽休眠解除的生物学机制及感应低温的主要部位。 方法 以2年生雷竹盆栽苗为研究对象,分别于2021和2022年10月至翌年3月设置T1 (地上、地下部分18 ℃),T2 (地上部分18 ℃,地下部分自然低温),T3 (地上部分自然低温,地下部分18 ℃),T4 (地上、地下部分自然低温)共4种温度处理。观测笋芽母竹之间的维管束连接状态、淀粉和蛋白质分布及笋芽细胞的增殖情况,测定笋芽的可溶性糖、淀粉、脱落酸、赤霉素质量分数和抗氧化酶活性,明确不同部位的低温处理对雷竹笋芽休眠解除的影响。 结果 在雷竹笋芽休眠解除过程中,与T1处理相比,T2、T3和T4处理显著增加了笋芽的可溶性糖质量分数(P<0.05),显著降低了笋芽的淀粉质量分数(P<0.05)。根据淀粉切片观察和质量分数拟合,T4处理休眠解除的时间最早,其次为T2和T3处理,2个处理解除时间相同,分别比T1处理提前了37和29 d。T2、T3、T4处理笋芽的脱落酸(ABA)质量分数在120 d时的下降幅度分别是T1处理的1.68、2.18和3.40倍。T2、T3和T4处理笋芽的赤霉素(GA3)质量分数在80 d时分别比T1处理高17.55%、3.27%和10.91%,且T2、T3、T4处理笋芽的GA3/ABA在120 d时的增量分别是T1处理的1.11、3.46、2.67倍;T3处理的笋芽导管和茎尖分生组织均在1月观察到水分运输恢复和细胞增殖的信号,而T1、T2和T4处理则分别在3、2和3月才能观察到此现象。 结论 低温处理尤其是地下部分低温可以促进雷竹休眠笋芽中淀粉水解为可溶性糖,并通过降低ABA质量分数、提高GA3质量分数、增强抗氧化酶(过氧化氢酶和过氧化物酶)活性、促进笋芽维管束的连通和分生组织细胞的快速分裂增殖等来影响雷竹笋芽休眠的解除。综合分析认为:在笋芽休眠期间雷竹对低温的感知部位主要为地下部分。图7参41 Abstract:Objective This study, with an investigation into the effect of low temperature treatment of different parts on dormancy of Phyllostachys violascens shoots, is aimed to reveal the biological mechanism of shoot dormancy relief and the main parts of low temperature induction. Method With 2-year-old Ph. violascens potted plants used as the research subject, 4 treatments were set, namely, T1 (18 ℃ in above-ground parts and underground parts), T2 (18 ℃ in above-ground parts, natural low temperature in underground parts), T3 (natural low temperature in above-ground parts, 18 ℃ in underground parts) and T4 (natural low temperature in above-ground parts and underground parts) lasting from October 2021 to March 2022 and October 2022 to March 2023. Then the content of soluble sugar, starch, ablative acid, gibberellin and antioxidant enzyme activity of bamboo shoots were determined by observing the vascular bundle connection between bamboo shoots and mother bamboo, the distribution of starch and protein, and the proliferation of bamboo shoots. Result Compared with T1 treatment, T2, T3 and T4 treatments could significantly increase the content of soluble sugar (P<0.05) and significantly decrease the content of starch (P<0.05) in bamboo shoots during dormancy release. According to starch slice observation and content fitting, the dormancy release date of shoots treated with T4 was the earliest, followed by T2 and T3 treatments, which were 37 and 29 days earlier than T1 treatment, respectively. The decrease of abscisic acid (ABA) content in shoots treated with T2, T3 and T4 at day 120 was 1.68, 2.18 and 3.40 times of that of T1 in the same period, respectively. The content of GA3 in shoots treated with T2, T3 and T4 at day 80 was 17.55%, 3.27% and 10.91% higher than that treated with T1, respectively, and the increment of GA3/ABA in shoots treated with T2, T3 and T4 at day 120 was 1.11, 3.46 and 2.67 times that of T1, respectively and the signals of water transport recovery and cell proliferation were observed in T3 treated shoot ducts and stem tip meristem in January, while T1, T2 and T4 treated were observed in March, February and March, respectively. Conclusion Low temperature treatment, especially low temperature in the underground part, can promote the hydrolysis of starch to soluble sugar in the dormant shoots of bamboo shoots, and affect the release of dormancy of bamboo shoots by reducing ABA content, increasing GA3 content, enhancing the activities of CAT and POD antioxidant enzymes, promoting the connectivity of vascular bundle and the rapid division and proliferation of meristem cells. Also, with a comprehensive analysis, it was confirmed that the underground part of bamboo was mainly the part that senses low temperature during dormancy. [Ch, 7 fig. 41 ref.] -
-
[1] 胡超宗, 金爱武, 郦章顺. 等. 早竹保护地栽培覆盖材料的研究[J]. 浙江林学院学报, 1996, 13(1): 5−9. HU Chaozong, JIN Aiwu, LI Zhangshun, et al. Mulching materials on Phyllostachys praecox forest of protected cultivation [J]. Journal of Zhejiang Forestry College, 1996, 13(1): 5−9. [2] 方伟, 何钧潮, 卢学可, 等. 雷竹早产高效栽培技术[J]. 浙江林学院学报, 1994, 11(2): 121−128. FANG Wei, HE Junchao, LU Xueke, et al. Cultivation techniques of early shooting and high yielding for lei bamboo sprout [J]. Journal of Zhejiang Forestry College, 1994, 11(2): 121−128. [3] 余树全, 姜春前, 周国模, 等. 雷竹林生态系统健康的研究[J]. 北京林业大学学报, 2003, 25(5): 15−19. YU Shuquan, JIANG Chunqian, ZHOU Guomo, et al. Study on Phyllostachys praecox forest ecosystem health [J]. Journal of Beijing Forestry University, 2003, 25(5): 15−19. [4] HORVATH D. Common mechanisms regulate flowering and dormancy [J]. Plant Science, 2009, 177(6): 523−531. [5] 罗丽兰, 石雷, 张金政. 低温对解除百合鳞茎休眠和促进开花的作用[J]. 园艺学报, 2007, 34(2): 517−524. LUO Lilan, SHI Lei, ZHANG Jinzheng. Advances in studies on the effect of low temperature on releasing domancy and accelerating flower of lily bulbs [J]. Acta Horticulturae Sinica, 2007, 34(2): 517−524. [6] HEIDE O M. Interaction of photoperiod and temperature in the control of growth and dormancy of Prunus species [J]. Scientia Horticulturae, 2008, 115: 309−314. [7] 许烨荣. 温度对雷竹笋芽萌发的影响[D]. 杭州: 浙江农林大学, 2020. XU Yerong. The Effect of Temperature on Phyllostachys violascens Shooting [D]. Hangzhou: Zhejiang A&F University, 2020. [8] HEWITT B R. Spectrophotometric determination of total carbohydrate [J]. Nature, 1958, 182(4630): 246−247 [9] ZHAO Jing, LI Gang, YI Guoxiang, et al. Comparison between conventional indirect competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules [J]. Analytica Chimica Acta, 2006, 571(1): 79−85. [10] TALAREK N, PETIT J, GUEYDON E, et al. EdU incorporation for FACS and microscopy analysis of DNA replication in budding yeast [J]. Methods in Molecular Biology, 2015, 1300: 105−112. [11] ESSIAMAH S, ESCHRICH W. Water uptake in deciduous trees during winter and the role of conducting tissues in spring reactivation [J]. International Association of Wood Anatomists journal, 1986, 7(1): 31−38. [12] XIE Zhaosen, FORNEY C F, BONDADA B. Renewal of vascular connections between grapevine buds and canes during bud break [J]. Scientia Horticulturae, 2018, 233: 331−338. [13] DEL BARRIO R A, ORIOLI G A, BRENDEL A S, et al. Persian walnut (Juglans regia L. ) bud dormancy dynamics in northern Patagonia, Argentina [J/OL]. Frontiers in Plant Science, 2022, 12 : 3341[2024-05-08]. DOI: 10.3389/fpls.2021.803878. [14] 董宁光. 杨树嫩茎生根机理及调控激素的组织细胞原位分析[D].北京: 北京林业大学,2012. DONG Ningguang. Mechanism and Histocytic in situ Analysis of Regulating Hormones during the Rhizogenesis of Poplar Shoot [D]. Beijing: Beijing Forestry University, 2012. [15] LI Xueyan, WANG Chunxia, CHENG Jinyun, et al. Transcriptome analysis of carbohydrate metabolism during bulblet formation and development in Lilium davidii var. unicolor [J/OL]. BMC Plant Biology, 2014, 14 : 358[2024-05-08]. DOI: 10.1186/s12870-014-0358-4. [16] ZHANG Yuxi, YU Dan, LIU Chunying, et al. Dynamic of carbohydrate metabolism and the related genes highlights PPP pathway activation during chilling induced bud dormancy release in tree peony (Paeonia suffruticosa) [J]. Scientia Horticulturae, 2018, 242: 36−43. [17] GONZALEZ-ROSSIA D, REIG C, DOVIS V, et al. Changes on carbohydrates and nitrogencontent in the bark tissues induced by artificial chilling and its relationship with dormancy bud break in Prunus sp. [J]. Scientia Horticulturae, 2008, 118(4): 275−281. [18] HORIKOSHI H M, SEKOZAWA Y, SUGAYA S. Inhibition of carbohydrate metabolism by thermal fluctuations during endodormancy lead to negative impacts on bud burst and incidence of floral necrosis in ‘Housur’ Japanese pear flower buds [J]. Scientia Horticulturae, 2017, 224: 324−331. [19] SIVACI A. Seasonal changes of total carbohydrate contents in three varieties of apple (Malus sylvestris Miller) stem cuttings [J]. Scientia Horticulturae, 2006, 109(3): 234−237. [20] CHARRIER G, POIRIER M, BONHOMME M, et al. Frost hardiness in walnut trees (Juglans regia L. ): how to link physiology and modelling? [J] Tree Physiology, 2013, 33 (11): 1229−1241. [21] ELLE D, SAUTER J J. Seasonal changes of activity of a starch granule bound endoamylase and of a starch phosphorylase in poplar wood (Populus × canadensis Moench robusta) and their possible regulation by temperature and phytohormones [J]. Plant Physiology, 2000, 156(5/6): 731−740. [22] ROHDE A, BHALERAO R P. Plant dormancy in the perennial context [J]. Trends in Plant Science, 2007, 12: 217−223. [23] 李健. 河北杨顶芽休眠解除的miRNA调控机制研究[D]. 北京: 北京林业大学, 2020. LI Jian. Dormancy Release Mechanism of Buds Mediated by miRNAs in Populus hopeiensis [D]. Beijing: Beijing Forestry University, 2020. [24] ZHENG Chuanlin, ACHEAMPONG A K, SHI Zhaowan, et al. Abscisic acid catabolism enhances dormancy release of grapevine buds [J]. Plant Cell and Environment, 2018, 41(10): 2490−2503. [25] PANNEERSELVAM R, JALEEL C A, SOMASUNDARAM R, et al. Carbohydrate metabolism in Dioscorea esculenta (Lour. ) Burk. tubers and Curcuma longa L. rhizomes during two phases of dormancy [J]. Colloids and Surfaces B-Biointerfaces, 2007, 59(1): 59−66. [26] CHEN Zhaoyu, CHEN Yadi, SHI Lanxi, et al. Interaction of phytohormones and external environmental factors in the regulation of the bud dormancy in woody plants [J/OL]. International Journal of Molecular Sciences, 2023, 24 (24): 17200[2024-05-08]. DOI: 10.3390/ijms242417200. [27] ZAWASKI C, KADMIEL M, PICKENS J, et al. Repression of gibberellin biosynthesis or signaling produces striking alterations in poplar growth, morphology, and flowering [J]. Planta, 2011, 234(6): 1285−1298. [28] 李丽霞, 梁宗锁, 魏宇昆, 等. 土壤干旱胁迫下沙棘休眠、萌芽期内源激素变化及外源GA3的调节[J]. 西北林学院学报, 2001, 16(2): 10−14. LI Lixia, LIANG Zongsuo, WEI Yukun, et al. Changes of endogenous hormonr of sea buckthorn in dormancy stage and sprout period under soil water stress and exogenous GA3 regulation [J]. Journal of Northwest Forestry College, 2001, 16(2): 10−14. [29] 郑国生, 盖树鹏, 盖伟玲. 低温解除牡丹芽休眠进程中内源激素的变化[J]. 林业科学, 2009, 45(2): 48−52. ZHENG Guosheng, GAI Shupeng, GAI Weiling. Changes of endogenous hormones during dormancy release by chilling in tree peony [J]. Scientia Silvae Sinicae, 2009, 45(2): 48−52. [30] BEAUVIEUX R, WENDEN B, DIRLEWANGER E. Bud dormancy in perennial fruit tree species: a pivotal role for oxidative cues [J/OL]. Frontiers in Plant Science, 2018, 9 : 657[2024-05-08]. DOI: 10.3389/fpls.2018.00657. [31] HUSSAIN S, NIU Q, YANG F, et al. The possible role of chilling in floral and vegetative bud dormancy release in Pyrus pyrifolia [J]. Biology Plant, 2015, 59: 726−734. [32] PRUDENCIO A S, DÍAZ-VIVANCOS P, DICENTA F, et al. Monitoring the transition from endodormancy to ecodormancy in almond through the analysis and expression of a specific class Ⅲ peroxidase gene [J/OL]. Tree Genetics & Genomes, 2019, 15 (3): 44[2024-05-08]. DOI: 10.1007/s11295-019-1351-8. [33] BHARDWAJ P K, MALA D, KUMAR S. 2-Cys peroxiredoxin responds to low temperature and other cues in Caragana jubata, a plant species of cold desert of Himalaya [J]. Molecular Biology Reports, 2014, 41: 2951−2961. [34] 赵成帅. 枣树营养贮藏蛋白质的细胞学研究[D]. 太原: 山西农业大学, 2014. ZHAO Chengshuai. Cytological Research of Vegetative Storage Proteins in Zizyphus jujuba [D]. Taiyuan: Shanxi Agricultural University, 2014. [35] VITRA A, LENZ A, VITASSE Y. Frost hardening and dehardening potential in temperate trees from winter to budburst [J]. New Phytologist, 2017, 216(1): 113−123. [36] 许曈, 邵灵梅, 王小斌, 等. 多年生单子叶植物的越冬休眠研究进展[J]. 园艺学报, 2022, 49(12): 2703−2721. XU Tong, SHAO Lingmei, WANG Xiaobin, et al. Research progress on winter dormancy of perennial monocots [J]. Acta Horticulturae Sinica, 2022, 49(12): 2703−2721. [37] BAZOT S, BARTHES L, BLANOT D, et al. Distribution of non-structural nitrogen and carbohydrate compounds in mature oak trees in a temperate forest at four key phenological stages [J]. Trees Structure and Function, 2013, 27: 1023−1034. [38] BARTOLINI S, GIORGELLI F. Observations on development of vascular connections in two apricot cultivars [J]. Advances in Horticultural Science, 1994, 8: 97−100. [39] ROSSI S, DESLAURIERS A, ANFODILLO T, et al. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes [J]. Oecologia, 2007, 152(1): 1−12. [40] ALONI R. Role of hormones in controlling vascular differentiation and the mechanism of lateral root initiation [J]. Planta, 2013, 238(5): 819−830. [41] LANG G A, EARLY J D, MARTIN G C, et al. Endodormancy, paradormancy, and ecodormancy-physiological terminology and classification for dormancy research [J]. Hortscience, 1987, 22(3): 371−377. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20240343






 下载:
下载: