-
生物质炭是一类新型环境功能材料,具有发达的孔隙结构、较高的比表面积、丰富的表面官能团和良好的吸附性能[1]。诸多研究证实:生物质炭可改善土壤孔隙结构、持水性、生态功能[2],具有缓释营养物质的作用,在促进植物生长的同时还能减少农业面源污染。因此,生物质炭研究是目前全球研究的热点之一[3]。然而生物质炭制备原料大多是农林业残留物、畜禽粪污等废弃生物质,其中可能含有重金属等污染物[4],其制备过程会将原料中的污染物浓缩,并产生有机酸、焦油、多环芳烃等有机污染物[5],生物质炭对环境存在潜在威胁,在土壤中大规模应用可能会对土壤-植物-微生物系统产生毒害作用[6−7]。因此,在生物质炭应用于土壤前有必要对其安全性进行评估,明确其对作物生长发育的影响,从而科学、合理利用生物质炭。
作物种子萌发、幼苗生长状况决定着作物的产量[8]。无土培养法可评估生物质炭对植物的毒害效应,适用于早期植物毒性检测[9]。萝卜Raphanus sativus在中国各地普遍栽培,具有生长周期短、抗病害能力强等特点。基于此,本研究利用不同生物质炭浸提液培养萝卜种子,探究浸提液对萝卜种子萌发、幼苗生长发育的影响,揭示不同种类生物质炭对萝卜的潜在危害,为典型废弃生物质炭化农业应用潜力筛选及安全利用提供理论依据。
-
供试萝卜购自北京大一韩日国际种苗有限公司。供试生物质炭分别是水稻秸秆炭(RB)、猪粪沼渣炭(PB)和厨余垃圾炭(KB),水稻秸秆采自杭州市富阳区生物质炭农田科学施用研究示范基地,猪粪沼渣采自浙江省一景生态牧业有限公司,厨余垃圾采自浙江科技学院西和公寓食堂。
采用管式热解气氛炉制备生物质炭。制备条件:热解终温为400 ℃,停留2 h,升温速率为10 ℃·min−1。全程通氮气保证无氧环境[10]。
-
3种生物质炭分别与去离子水按m(炭)∶V(水)=1∶20、1∶50、1∶100的比例混合,在室温(约25 ℃)下振荡24 h,抽滤得生物质炭浸提液。浸提液中砷(As)、纳(Na)、钾(K)等典型重金属,钙(Ca)、钾(K)、镁(Mg)、钠(Na)等矿质元素和无机盐总量见表1。浸提液中多环芳烃等有机污染物低于检出限,后续不再分析。
表 1 生物质炭浸提液中总无机盐、重金属和矿质元素
Table 1. Concentrations of the total inorganic salts, the typical toxic metals and mineral elements in de-ionized water elutent from biochars
生物质
炭种类m(炭)∶
V(水)矿质元素质量分数/(mg·g−1) 重金属元素质量分数/(mg·kg−1) 无机盐总量/
(mg·g−1)pH Ca K Mg Na As Cu Zn KB 1∶20 0.47±0.02 c 1.23±0.14 c 0.08±0.04 b 7.85±0.05 c 1.97±0.31 b 1.30±0.14 b 7.08±0.47 b 24.50±1.12 c 9.80 1∶50 1.05±0.11 b 2.82±0.09 b 0.10±0.04 ab 20.01±0.02 b 2.25±0.04 b 2.25±0.37 a 8.25±0.47 b 33.37±0.27 b 8.76 1∶100 1.47±0.12 a 4.80±0.05 a 0.14±0.02 a 36.47±0.02 a 3.95±0.62 a 2.77±0.30 a 10.40±1.99 a 47.46±0.52 a 8.67 PB 1∶20 9.71±0.13 c 2.26±0.09 c 0.23±0.05 b 0.56±0.05 c 0.30±0.62 a 173.35±1.02 b 380.78±6.68 b 30.16±4.68 b 8.56 1∶50 12.67±0.24 b 5.36±0.12 b 0.59±0.02 a 1.31±0.08 b 0.48±0.28 a 194.70±3.60 a 511.28±3.04 b 35.54±3.41 b 7.67 1∶100 13.08±0.36 a 8.87±0.08 a 0.60±0.11 a 2.31±0.07 a 0.68±0.17 a 208.85±1.38 a 547.28±9.04 a 43.75±4.97 a 7.36 RB 1∶20 5.63±0.36 c 3.02±0.13 c 0.30±0.04 c 0.36±0.12 c 3.37±0.17 b 1.18±0.18 b 22.88±1.04 b 19.45±0.69 c 9.26 1∶50 6.73±0.38 b 7.90±0.09 b 0.40±0.02 b 0.86±0.09 b 3.55±0.11 b 2.06±0.35 a 25.05±0.78 a 32.07±4.52 b 8.75 1∶100 8.67±0.15 a 9.49±0.06 a 0.51±0.03 a 1.52±0.07 a 4.17±0.29 a 2.78±0.82 a 21.35±0.71 b 45.67±1.03 a 8.35 说明:KB. 厨余垃圾炭;PB. 猪粪沼渣炭;RB. 水稻秸秆炭。不同小写字母表示同种生物炭同一指标在不同质量体积比浸提液中差异显著(P<0.05)。 -
选取20粒饱满、大小均一的萝卜种子置于培养皿中,分别加入10 mL上述浸提液,对照组添加10 mL去离子水(ck),每个处理重复3次,置于恒温光照培养箱中进行培养。培养温度为25 ℃,光照为3 000 lx,光照时间为12 h·d−1。以芽长超过1 mm为发芽标准,每天9:00统计萝卜种子的发芽数,连续7 d,计算发芽率、发芽势、发芽指数。
-
在育苗桶内放置育苗篮,每桶加入20粒催好芽的、发育状态一致的萝卜种子,分别加入20 mL上述生物质炭浸提液,对照组添加20 mL去离子水,每天观察育苗桶内干湿度,补充浸提液或去离子水3~5 mL,各处理添加量维持相同,持续15 d。
-
种子发芽势为日发芽种子数达到最高峰时的发芽种子占比;种子发芽率为萌发第7天时种子萌发比例;种子发芽指数(GI)计算:GI=Σ(Gt/Dt),其中Gt为在第t天的发芽数,Dt为相应的发芽日数。
随机选取育苗盆中长势较为平均的9株萝卜幼苗,用直尺测量其根长、苗高、叶宽,用游标卡尺测量其茎粗,取平均值,未发芽种子的幼苗不做记录。
生理指标主要包括萝卜幼苗组织内叶绿素质量分数、超氧化物歧化酶(SOD)活性、丙二醛(MDA)质量摩尔浓度、根系活力、过氧化氢酶(CAT)活性。叶绿素质量分数测定采用乙醇提取法,SOD活性测定采用氮蓝四唑法(NBT法),CAT活性测定采用紫外吸收法,MDA质量摩尔浓度测定采用硫代巴比妥酸法,根系活力测定采用TTC法。
-
用Excel 2019整理数据,应用SPSS 26进行统计分析,采用单因素方差分析和Waller Duncan’ s多重比较评价不同处理对萝卜种子的萌发和幼苗生长发育的显著性影响,使用Origin 2021制图。
-
按照GB 8978—1996《污水综合排放标准》最高允许排放质量浓度:As限值为0.5 mg·L−1,Zn限值为5.0 mg·L−1,Cu限值为2.0 mg·L−1。本研究表明:m(炭)∶V(水)=1∶20的厨余垃圾炭中As超标;m(炭)∶V(水)=1∶50的猪粪沼渣炭中Cu、Zn超标,水稻秸秆炭中As、Zn超标(表1)。
由表2可知:浸提液对种子萌发有显著抑制作用(P<0.05),浸提液质量体积比越大,对萝卜种子萌发越不利。m(炭)∶V(水)=1∶100处理下,种子发芽率、发芽势、发芽指数与对照差异不显著。m(炭)∶V(水)=1∶20的厨余垃圾炭、猪粪沼渣炭、水稻秸秆炭浸提液培养的种子发芽率分别比对照降低了56.41%、23.07%、35.90%,不同种类生物质炭之间差异显著(P<0.05),作用从大到小依次为厨余垃圾炭、水稻秸秆炭、猪粪沼渣炭。m(炭)∶V(水)=1∶50的厨余垃圾炭、猪粪沼渣炭、水稻秸秆炭浸提液培养的种子发芽率分别降低了10.26%、10.26%、5.13%。相比于对照,m(炭)∶V(水)=1∶20的浸提液处理下种子发芽势显著降低(P<0.05),但不同种类生物质炭之间无显著差异;m(炭)∶V(水)=1∶50的猪粪沼渣炭发芽势与对照相比无显著差异,其他处理的发芽势均显著低于对照(P<0.05)。
表 2 不同生物质炭浸提液对萝卜种子萌发的影响
Table 2. Effects of different biochar extract solutions on the seed germination of radish
m(炭)∶
V(水)生物质炭
种类发芽率/% 发芽势/% 发芽指数 m(炭)∶
V(水)生物质炭
种类发芽率/% 发芽势/% 发芽指数 0 ck 97.50±3.54 a 47.50±3.54 a 72.50±3.55 a 1∶50 KB 87.50±3.54 a 35.00±3.07 bc 46.55±01.20 b PB 87.50±3.54 a 47.00±0.00 a 49.45±0.65 b RB 92.50±3.54 a 37.50±3.54 b 49.15±1.75 b 1∶20 KB 42.50±3.54 d 25.00±0.00 c 21.45±6.20 d 1∶100 KB 92.50±3.54 a 42.50±3.54 b 69.83±2.41 a PB 75.00±0.00 b 30.50±3.54 bc 34.25±2.45 c PB 87.50±3.54 a 45.00±3.07 ab 70.10±1.45 a RB 62.50±3.54 c 27.50±3.54 c 22.95±1.75 d RB 92.50±3.54 a 38.50±3.54 b 68.00±4.76 a 说明:KB. 厨余垃圾炭;PB. 猪粪沼渣炭;RB. 水稻秸秆炭。不同小写字母表示同一指标不同处理间差异显著(P<0.05)。 种子发芽指数综合反映了堆肥的植物毒性,被认为是最敏感、最可靠的堆肥腐熟度评价指标。一般情况下,发芽指数大于50可认为堆肥对种子基本无毒性。由表2可知:m(炭)∶V(水)=1∶100的浸提液培养的萝卜种子发芽指数均超过50,且与对照相比无显著差异,表明m(炭)∶V(水)=1∶100的浸提液对种子发芽基本无毒副影响。m(炭)∶V(水)=1∶50条件下萝卜种子发芽指数为46.55~49.45,略低于50,显现出轻微的毒害效应。m(炭)∶V(水)=1∶20条件下萝卜种子发芽指数为21.45~34.25,远低于50,毒性显著(P<0.05)。
-
从表3可见:m(炭)∶V(水)=1∶20条件下,水稻秸秆炭浸提液培育的萝卜幼苗存活率为11.67%,猪粪沼渣炭和厨余垃圾炭浸提液培育的萝卜幼苗存活率分别仅为6.67%和3.33%,这导致后续表观指标与生理指标测定无法进行,故育苗实验只探讨m(炭)∶V(水)为1∶50和1∶100条件下生物质炭浸提液对萝卜幼苗的影响。
表 3 不同生物质炭浸提液对萝卜幼苗存活率的影响
Table 3. Effects of different biochar extracts on radish seedling survival rate
生物质炭种类 m(炭)∶V(水) 存活率/% ck 0 100.00±0.00 a KB 1∶20 3.33±5.77 e 1∶50 65.00±0.00 c 1∶100 86.00±0.82 b PB 1∶20 6.67±2.89 de 1∶50 68.33±2.89 c 1∶100 87.33±1.23 b RB 1∶20 11.67±2.89 d 1∶50 90.00±0.00 b 1∶100 88.33±0.47 b 说明:KB. 厨余垃圾炭;PB. 猪粪沼渣炭;RB. 水稻秸秆炭。不同小写字母表示同一指标不同处理间差异显著(P<0.05)。 由表4可知:生物质炭浸提液对萝卜幼苗叶片数无显著影响,说明萝卜幼苗发育程度基本一致。m(炭)∶V(水)=1∶100条件下萝卜幼苗长势与对照接近,m(炭)∶V(水)=1∶50条件下生物质炭浸提液对萝卜幼苗生长发育抑制作用明显,不同生物质炭之间差异显著(P<0.05),作用从大到小依次为厨余垃圾炭、猪粪沼渣炭、水稻秸秆炭。与对照相比,m(炭)∶V(水)=1∶50条件下厨余垃圾炭、猪粪沼渣炭、水稻秸秆炭浸提液处理下的萝卜幼苗的鲜质量分别降低了65.98%、29.55%、51.89%;苗长分别降低了56.01%、37.73%、48.68%;根长分别降低了76.04%、20.77%、53.16%。
表 4 生物质炭浸提液对萝卜幼苗生长的影响
Table 4. Effect of biochar extract solutions on the growth of seedling radish
m(炭)∶V(水) 生物质炭种类 鲜质量/g 苗长/cm 根长/mm 茎粗/mm 叶宽/mm 叶片数 0 ck 2.91±0.01 a 10.23±0.76 a 14.73±0.38 a 1.93±0.26 a 12.60±0.46 b 2.33±0.58 a 1∶50 KB 0.99±0.01 d 4.50±0.20 e 3.53±0.06 e 1.97±0.21 a 7.74±1.48 c 2.33±0.58 a PB 2.05±0.01 b 6.37±0.40 c 11.67±1.00 b 1.85±0.05 a 12.31±1.18 b 2.17±0.29 a RB 1.40±0.01 c 5.25±0.07 d 6.90±0.42 d 1.59±0.14 b 14.25±0.09 a 2.00±0.00 a 1∶100 KB 2.99±0.01 a 8.17±0.46 b 8.1±0.78 c 1.77±0.11 ab 13.94±0.54 a 2.33±0.58 a PB 2.98±0.03 a 9.22±0.92 ab 10.37±1.25 b 1.87±0.18 a 12.1±0.74 b 2.66±0.29 a RB 2.97±0.06 a 9.30±0.75 ab 10.53±0.84 b 1.79±0.29 ab 14.25±0.97 a 2.33±0.58 a 说明:KB. 厨余垃圾炭;PB. 猪粪沼渣炭;RB. 水稻秸秆炭。不同小写字母表示同一指标不同处理间差异显著(P<0.05)。 厨余垃圾炭、猪粪沼渣炭、水稻秸秆炭浸提液处理下均出现死苗现象。观察发现:厨余垃圾炭浸提液培育的萝卜幼苗先是出现烂根,在茎叶交汇处出现茎杆自上而下干枯,后幼苗死亡的现象;类似地,水稻秸秆炭浸提液培育的幼苗也是先在茎叶交汇处出现茎杆失水,短时间内茎杆自上而下干枯,后幼苗死亡的现象;猪粪沼渣炭浸提液培育的幼苗则是先出现叶片茎杆发蔫,后出现萝卜幼苗死亡,且萝卜幼苗叶片颜色偏黄。m(炭)∶V(水)=1∶50条件下猪粪沼渣炭浸提液培育的幼苗表观与对照最接近,但死苗率高达31.65%。结合表1和表2可判断:高质量分数的Cu、Zn对植物幼苗起毒害作用;厨余垃圾炭浸提液中过高的无机盐总量不仅降低植物幼苗存活率,对植物幼苗的生长发育也有明显的抑制效应;水稻秸秆炭浸提液对萝卜幼苗的毒害机理与厨余垃圾炭相似,但无机盐总量低于厨余垃圾炭,对萝卜幼苗生长发育的抑制效果减轻,幼苗存活率也提高。
-
与对照相比,m(炭)∶V(水)=1∶50条件下生物质炭浸提液对萝卜种子萌发、幼苗生长的影响显著(P<0.05),m(炭)∶V(水)=1∶100的生物质炭浸提液影响不显著(表2和表4),故只探究m(炭)∶V(水)=1∶50条件下生物质炭浸提液对萝卜幼苗生理指标的影响。
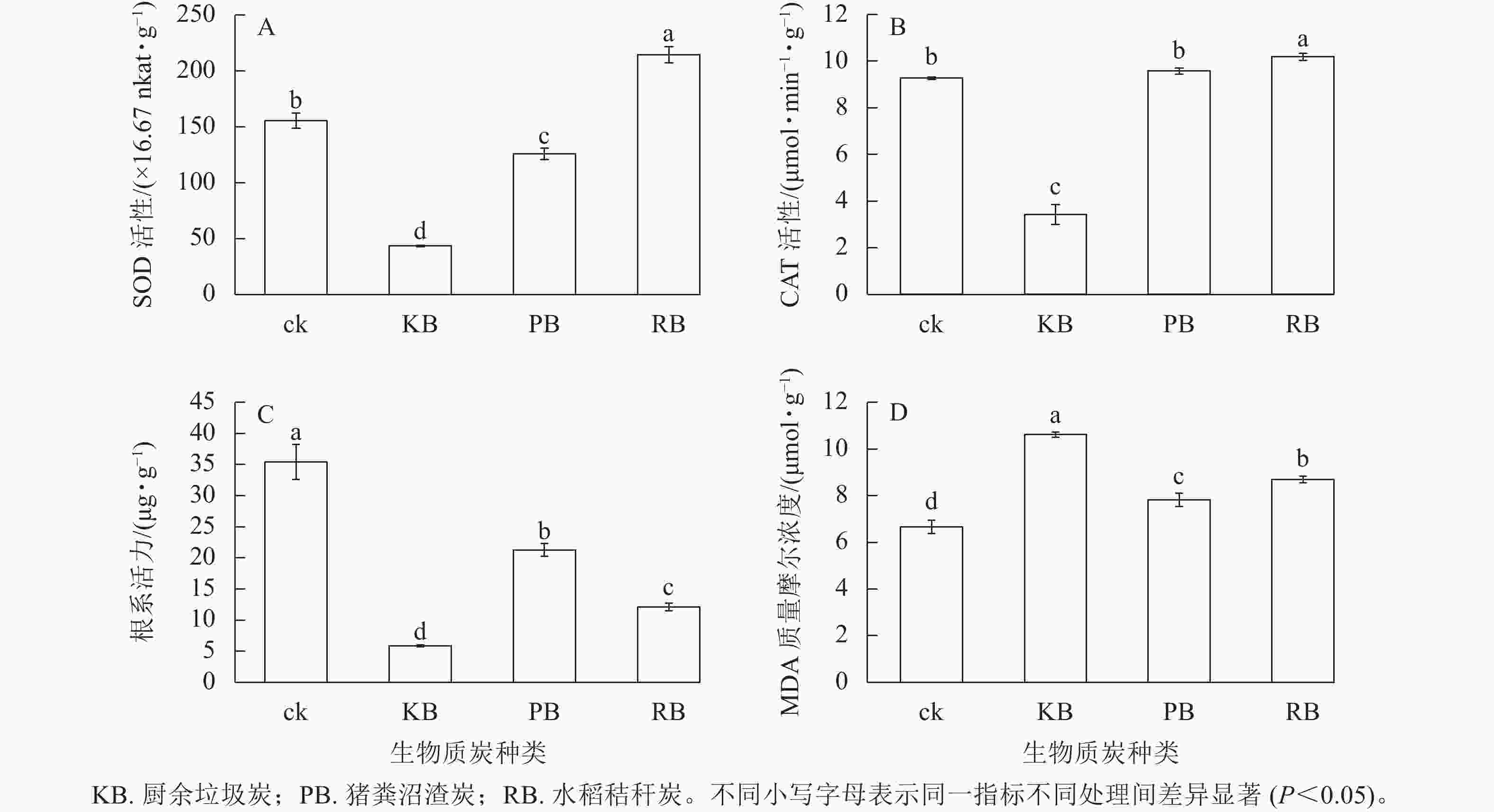
m(炭)∶V(水)=1∶50的厨余垃圾炭浸提液、猪粪沼渣炭浸提液、水稻秸秆炭浸提液培育的萝卜幼苗叶绿素总量较对照相比分别降低了21.85%、12.00%、24.32%;叶绿素a分别降低了21.55%、11.44%、24.51%;叶绿素b分别降低了21.85%、13.27%、24.32%,变化趋势一致。厨余垃圾炭和水稻秸秆炭浸提液处理无显著差异(图1),说明两者对幼苗生长发育影响机理接近。浸提液培养对萝卜幼苗叶绿素的影响与对萝卜幼苗表观(鲜质量、苗长、根长)的影响趋势基本一致。厨余垃圾炭和猪粪沼渣炭浸提液培育的萝卜幼苗SOD活性相比于对照分别降低了71.95%、19.04%,水稻秸秆炭浸提液培育下提高了37.85% (图2A)。厨余垃圾炭浸提液培育的萝卜幼苗CAT活性相比于对照降低了63.09%,水稻秸秆炭浸提液培育下提高了9.87%,而猪粪沼渣炭浸提液培育下CAT活性相比于对照差异不显著(图2B),变化趋势与叶宽一致。厨余垃圾炭、猪粪沼渣炭、水稻秸秆炭浸提液培育的萝卜幼苗根系活力相比于对照分别降低了83.57%、39.95%、65.93% (图2C),降低趋势与根长接近。厨余垃圾炭、猪粪沼渣炭浸提液培育的萝卜幼苗MDA质量摩尔浓度相比于对照分别增加了59.46%、17.49%、30.58% (图2D),变化趋势与根系活力相反。
-
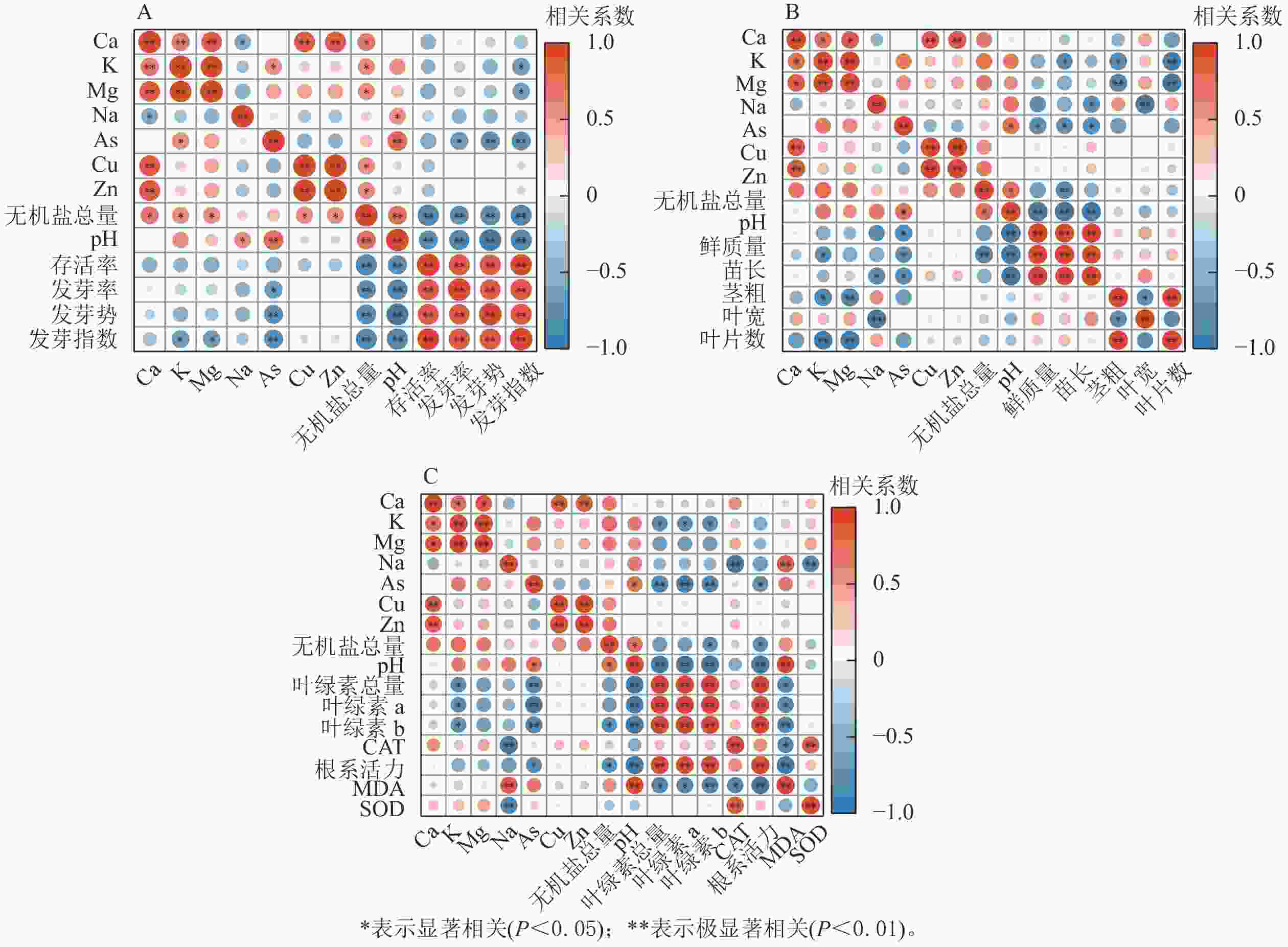
生物质炭浸提液pH、无机盐总量、As与萝卜种子萌发和幼苗生长发育均呈显著负相关(P<0.05)(图3),是抑制萝卜生长发育的重要环境因子,也是影响萝卜幼苗存活率的主要因素。除生物质炭浸提液的pH、无机盐总量外,萝卜幼苗苗长、叶绿素与K呈显著负相关(P<0.05);根长、叶宽、CAT和SOD活性与Na呈显著负相关(P<0.05);MDA与Na呈显著正相关(P<0.05)。
-
pH对植物的营养代谢有较大的影响,生物质炭pH和无机盐总量,生物质炭制备原料、温度、升温速率有关[11]。本研究所制备的生物质炭pH为碱性,高质量体积比条件下浸提液为碱性高盐环境,会对种胚细胞结构造成破坏,从而抑制种子萌发[12−13],因此萝卜种子萌发与生物质炭浸提液的pH、无机盐总量呈极显著负相关(P<0.01)。萝卜种子发芽率、发芽势、发芽指数均与As呈显著负相关(P<0.05),这与符佳豪等[14]的研究结果一致。As对种子萌发有明显的抑制作用,且As质量分数越大抑制作用越强。K、Mg是植物生长发育所必需的营养元素,但本研究中,萝卜种子发芽指数与K、Mg呈显著负相关(P<0.05),说明浸提液中K、Mg质量分数过高,已对萝卜种子的萌发造成胁迫。
本研究设置的3个处理中,随着质量体积比的降低,浸提液pH降低,对萝卜种子萌发的抑制作用减轻,浸提液对萝卜种子的毒害作用减轻。生物质炭种类不同,浸提液的pH、无机盐总量和种类存在差异,发芽过程中种子从浸提液吸收的水分和有害物质存在差异,故发芽率存在差异。生物质炭种类对萝卜种子毒害作用从大到小依次为厨余垃圾炭、水稻秸秆炭、猪粪沼渣炭。
-
萝卜种子萌发期主要靠种子胚提供营养,而幼苗期生长发育则需从外界吸收营养,因此生物质炭浸提液对幼苗的影响强于对种子的影响。m(炭)∶V(水)=1∶20条件下,较高的pH不仅破坏种胚细胞结构,抑制萝卜种子萌发,还严重破坏萝卜幼苗输导组织与分生组织,导致幼苗死亡[15−16]。
As在环境中易累积、难降解,是强毒性环境污染的重金属元素,对植物生长发育有明显的抑制作用[17−18]。K、Na、Ca离子在植物中具有复杂的信号功能,是植物生长发育所必需的营养元素,但当浸提液中K、Na、Ca过高时会改变渗透势,因此影响植物生长发育。本研究生物质炭浸提液中的K、Na、Ca等元素已对萝卜幼苗生长发育造成胁迫。
萝卜幼苗的根部比冠部对有害物质更敏感,受抑制作用影响也更明显[19]。厨余垃圾炭、水稻秸秆炭浸提液处理下,有害物质通过抑制幼苗根部细胞有丝分裂过程进而影响幼苗根部生长[20]。当幼苗根部受抑制程度达一定阈值时,幼苗组织内的水分、养分运输就会受阻,影响幼苗冠部生长发育[21]。厨余垃圾炭浸提液较高的pH和无机盐导致幼苗的根部无法进行正常的养分吸收[22];水稻秸秆炭浸提液中无机盐稍低于厨余垃圾炭,萝卜幼苗根部可以吸收营养物质供幼苗生长发育,但水稻秸秆炭浸提液中较高的K+、Na+,会影响幼苗信号传递功能,阻碍幼苗生长发育[23−25]。猪粪沼渣炭浸提液中有害物质并未抑制萝卜幼苗根部的有丝分裂,但较高的重金属元素抑制了萝卜幼苗的生长发育[26]。
-
叶绿素减少会导致幼苗光合作用效率降低[27]。生物质炭浸提液降低了萝卜幼苗叶绿素质量分数,因此减弱了幼苗光合作用效率,从而抑制了萝卜冠部生长发育。
与对照相比,m(炭)∶V(水)=1∶50条件下,生物质炭浸提液增加了萝卜幼苗MDA质量摩尔浓度,植物处于受胁迫环境时会启动抗氧化保护酶系统,减轻外界对植物的损伤[28]。表明萝卜幼苗正处于逆境胁迫中。幼苗MDA质量摩尔浓度增长趋势与根系活力降低趋势一致,进一步证明浸提液对幼苗根部细胞有丝分裂过程有抑制作用。
m(炭)∶V(水)=1∶50条件下,厨余垃圾炭浸提液培育的萝卜幼苗MDA质量摩尔浓度增加最显著(P<0.05),同时SOD、CAT活性最低,表明萝卜幼苗在厨余垃圾炭浸提液形成的高盐胁迫环境下抗氧化保护酶系统受到了严重损伤,无法自主调节抗氧化酶活性。水稻秸秆炭浸提液培育的幼苗MDA质量摩尔浓度增加量低于厨余垃圾炭,但SOD、CAT活性均高于对照,表明水稻秸秆炭浸提液虽对幼苗造成盐胁迫,但幼苗可通过自主调节抗氧化酶活性,增强幼苗清除活性氧的能力,减轻环境对幼苗造成的损伤[29]。猪粪沼渣炭浸提液培育的幼苗MDA质量摩尔浓度增加量最少,CAT活性与对照差异不显著,SOD活性低于对照,表明猪粪沼渣炭浸提液对萝卜幼苗几乎不造成盐胁迫,但依然对萝卜幼苗有毒害作用。
-
本研究表明:厨余垃圾炭、猪粪沼渣炭、水稻秸秆炭浸提液在m(炭)∶V(水)>1∶50条件下,对萝卜种子萌发及幼苗生长发育均存在显著抑制作用,抑制作用从大到小依次为厨余垃圾炭、水稻秸秆炭、猪粪沼渣炭,且在萝卜幼苗生长过程中均存在氧化应激。这表明以上3种生物质炭对萝卜种子萌发和幼苗生长存在潜在危害。因此,在每种生物质炭广泛应用于农田改良之前,应对其进行相应的植物响应分析研究,以确保其科学合理使用。
Toxic effects of the elution from the different feedstock biochars on seeds germination and seedling growth of radish
-
摘要:
目的 探究生物质炭对植物种子萌发及幼苗生长的毒副作用,以期为典型有机废弃物炭化农业应用潜力筛选及安全利用剂量提供理论依据。 方法 以厨余垃圾炭、猪粪沼渣炭、水稻秸秆炭等3种典型生物质炭为材料,设置m(炭)∶V(水)分别为1∶20、1∶50、1∶100等3个浸提处理,以去离子水为对照,研究生物质炭浸提液对萝卜Raphanus sativus种子萌发、幼苗生长发育的毒害效应。 结果 与对照相比,m(炭)∶V(水)为1∶100的生物质炭浸提液对萝卜种子萌发与幼苗生长影响不显著,m(炭)∶V(水)为1∶20、1∶50的浸提液对萝卜种子萌发有显著抑制作用(P<0.05)。m(炭)∶V(水)为1∶20时,萝卜幼苗存活率<12%。m(炭)∶V(水)为1∶50时,幼苗表观指标(鲜质量、根长、苗长)相比于对照组分别降低了29.55%~51.89%、20.77%~76.04%、37.73%~56.01%,呈显著的生长抑制效应(P<0.05),抑制作用从大到小依次为厨余垃圾炭、水稻秸秆炭、猪粪沼渣炭。浸提液的pH、无机盐总量、砷(As)、纳(Na)、钾(K)均与萝卜种子萌发、幼苗生长发育呈显著负相关(P<0.05),是抑制萝卜生长发育的主要因素。m(炭)∶V(水)为1∶50浸提液培养的萝卜幼苗根系活力降低、丙二醛提高,幼苗根系细胞膜完整性被破坏,发生脂质过氧化,表现出明显的毒害效应。 结论 厨余垃圾炭、猪粪沼渣炭和水稻秸秆炭的m(炭)∶V(水)>1∶50时,浸提液对萝卜种子萌发和幼苗期生长存在危害。图3表4参29 Abstract:Objective This study, with an investigation of the toxic effects of biochar on seed germination and seedling growth, is aimed to provide a theoretical basis for the potential screening of typical organic waste carbonization in agriculture and the safe use of dosage, which is conducive to the safe application of biochar in agriculture. Method With kitchen waste biochar (KB), pig manure biogas digestion residues derived biochar (PB), and rice straw biochar (RB), chosen as the raw materials and the control group treated with deionized water, an assessment was conducted of the influence of biochar extracts with different solid-liquid ratios (1∶20, 1∶50, 1∶100, m/V) on seed germination and seedling growth of radish (Raphanus sativus). Result Compared with the control without biochar extracts, the biochar extracts with the solid-liquid ratio of 1∶100 have no significant effect on radish seed germination and seedling growth but those with the solid-liquid ratio of 1∶20 and 1∶50 inhibit the radish seed germination significantly (P<0.05). The seedling fresh weight, root length, and seedling length cultured by biochar extracts with a 1∶50 solid-liquid ratio were reduced by 29.55%−51.89%, 20.77%−76.04%, and 37.73%−56.01%, respectively, indicating a significant growth inhibition effect of biochar extracts (P<0.05). When cultured with 1∶20 biochar extract, the survival rate of radish seedlings was lower than 12%. The inhibitory effects of different kinds of biochar reduced in the following order: kitchen waste biochar>rice straw biochar>pig manure biogas digestion residues derived biochar. The pH, total concentration of inorganic salts, As, Na, and K concentration of the extract solution were negatively correlated with radish seed germination and seedling growth (P<0.05) and served as the main factors inhibiting radish growth. The root activity of radish seedlings cultured with the 1∶50 solid-to-liquid ratio decreased whereas the content of malondialdehyde (MDA) increased, indicating the destruction of seedling root cell membranes and the occurrence of resultant lipid peroxidation. Conclusion The biochar extracts with a high solid-liquid ratio (>1∶50, m/V) had toxic effects on radish seed germination and seedling growth. [Ch, 3 fig. 4 tab. 29 ref.] -
Key words:
- biochar elution solution /
- seeds germination /
- seedling growth /
- chlorophyll /
- antioxidant enzyme
-
表 1 生物质炭浸提液中总无机盐、重金属和矿质元素
Table 1. Concentrations of the total inorganic salts, the typical toxic metals and mineral elements in de-ionized water elutent from biochars
生物质
炭种类m(炭)∶
V(水)矿质元素质量分数/(mg·g−1) 重金属元素质量分数/(mg·kg−1) 无机盐总量/
(mg·g−1)pH Ca K Mg Na As Cu Zn KB 1∶20 0.47±0.02 c 1.23±0.14 c 0.08±0.04 b 7.85±0.05 c 1.97±0.31 b 1.30±0.14 b 7.08±0.47 b 24.50±1.12 c 9.80 1∶50 1.05±0.11 b 2.82±0.09 b 0.10±0.04 ab 20.01±0.02 b 2.25±0.04 b 2.25±0.37 a 8.25±0.47 b 33.37±0.27 b 8.76 1∶100 1.47±0.12 a 4.80±0.05 a 0.14±0.02 a 36.47±0.02 a 3.95±0.62 a 2.77±0.30 a 10.40±1.99 a 47.46±0.52 a 8.67 PB 1∶20 9.71±0.13 c 2.26±0.09 c 0.23±0.05 b 0.56±0.05 c 0.30±0.62 a 173.35±1.02 b 380.78±6.68 b 30.16±4.68 b 8.56 1∶50 12.67±0.24 b 5.36±0.12 b 0.59±0.02 a 1.31±0.08 b 0.48±0.28 a 194.70±3.60 a 511.28±3.04 b 35.54±3.41 b 7.67 1∶100 13.08±0.36 a 8.87±0.08 a 0.60±0.11 a 2.31±0.07 a 0.68±0.17 a 208.85±1.38 a 547.28±9.04 a 43.75±4.97 a 7.36 RB 1∶20 5.63±0.36 c 3.02±0.13 c 0.30±0.04 c 0.36±0.12 c 3.37±0.17 b 1.18±0.18 b 22.88±1.04 b 19.45±0.69 c 9.26 1∶50 6.73±0.38 b 7.90±0.09 b 0.40±0.02 b 0.86±0.09 b 3.55±0.11 b 2.06±0.35 a 25.05±0.78 a 32.07±4.52 b 8.75 1∶100 8.67±0.15 a 9.49±0.06 a 0.51±0.03 a 1.52±0.07 a 4.17±0.29 a 2.78±0.82 a 21.35±0.71 b 45.67±1.03 a 8.35 说明:KB. 厨余垃圾炭;PB. 猪粪沼渣炭;RB. 水稻秸秆炭。不同小写字母表示同种生物炭同一指标在不同质量体积比浸提液中差异显著(P<0.05)。 表 2 不同生物质炭浸提液对萝卜种子萌发的影响
Table 2. Effects of different biochar extract solutions on the seed germination of radish
m(炭)∶
V(水)生物质炭
种类发芽率/% 发芽势/% 发芽指数 m(炭)∶
V(水)生物质炭
种类发芽率/% 发芽势/% 发芽指数 0 ck 97.50±3.54 a 47.50±3.54 a 72.50±3.55 a 1∶50 KB 87.50±3.54 a 35.00±3.07 bc 46.55±01.20 b PB 87.50±3.54 a 47.00±0.00 a 49.45±0.65 b RB 92.50±3.54 a 37.50±3.54 b 49.15±1.75 b 1∶20 KB 42.50±3.54 d 25.00±0.00 c 21.45±6.20 d 1∶100 KB 92.50±3.54 a 42.50±3.54 b 69.83±2.41 a PB 75.00±0.00 b 30.50±3.54 bc 34.25±2.45 c PB 87.50±3.54 a 45.00±3.07 ab 70.10±1.45 a RB 62.50±3.54 c 27.50±3.54 c 22.95±1.75 d RB 92.50±3.54 a 38.50±3.54 b 68.00±4.76 a 说明:KB. 厨余垃圾炭;PB. 猪粪沼渣炭;RB. 水稻秸秆炭。不同小写字母表示同一指标不同处理间差异显著(P<0.05)。 表 3 不同生物质炭浸提液对萝卜幼苗存活率的影响
Table 3. Effects of different biochar extracts on radish seedling survival rate
生物质炭种类 m(炭)∶V(水) 存活率/% ck 0 100.00±0.00 a KB 1∶20 3.33±5.77 e 1∶50 65.00±0.00 c 1∶100 86.00±0.82 b PB 1∶20 6.67±2.89 de 1∶50 68.33±2.89 c 1∶100 87.33±1.23 b RB 1∶20 11.67±2.89 d 1∶50 90.00±0.00 b 1∶100 88.33±0.47 b 说明:KB. 厨余垃圾炭;PB. 猪粪沼渣炭;RB. 水稻秸秆炭。不同小写字母表示同一指标不同处理间差异显著(P<0.05)。 表 4 生物质炭浸提液对萝卜幼苗生长的影响
Table 4. Effect of biochar extract solutions on the growth of seedling radish
m(炭)∶V(水) 生物质炭种类 鲜质量/g 苗长/cm 根长/mm 茎粗/mm 叶宽/mm 叶片数 0 ck 2.91±0.01 a 10.23±0.76 a 14.73±0.38 a 1.93±0.26 a 12.60±0.46 b 2.33±0.58 a 1∶50 KB 0.99±0.01 d 4.50±0.20 e 3.53±0.06 e 1.97±0.21 a 7.74±1.48 c 2.33±0.58 a PB 2.05±0.01 b 6.37±0.40 c 11.67±1.00 b 1.85±0.05 a 12.31±1.18 b 2.17±0.29 a RB 1.40±0.01 c 5.25±0.07 d 6.90±0.42 d 1.59±0.14 b 14.25±0.09 a 2.00±0.00 a 1∶100 KB 2.99±0.01 a 8.17±0.46 b 8.1±0.78 c 1.77±0.11 ab 13.94±0.54 a 2.33±0.58 a PB 2.98±0.03 a 9.22±0.92 ab 10.37±1.25 b 1.87±0.18 a 12.1±0.74 b 2.66±0.29 a RB 2.97±0.06 a 9.30±0.75 ab 10.53±0.84 b 1.79±0.29 ab 14.25±0.97 a 2.33±0.58 a 说明:KB. 厨余垃圾炭;PB. 猪粪沼渣炭;RB. 水稻秸秆炭。不同小写字母表示同一指标不同处理间差异显著(P<0.05)。 -
[1] HAO Shilai, ZHU Xiangdong, LIU Yuchen, et al. Production temperature effects on the structure of hydrochar-derived dissolved organic matter and associated toxicity [J]. Environmental Science and Technology, 2018, 52(13): 7486 − 7495. [2] LEHMANN J, COWIE A, MASIELLO C A, et al. Biochar in climate change mitigation [J]. Nature Geoscience, 2021, 14(12): 883 − 892. [3] HAGEMANN N, JOSEPH S, SCHMIDT H P, et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility [J/OL]. Nature Communications, 2017, 8(1): 1089[2023-04-01]. doi:10.1038/s41467-017-01123-0. [4] WOOLF D, AMONETTE J E, STREET-PERROTT F A, et al. Sustainable biochar to mitigate global climate change [J]. Nature, 2010, 1: 1 − 9. [5] OLESZCZUK P, JOSKO I, KUSMIERZ M. Biochar properties regarding to contaminants content and ecotoxicological assessment [J]. Journal of Hazardous Materials, 2013, 260: 375 − 382. [6] JANUS A, WATERLOT C, DOUAY F, et al. Ex situ evaluation of the effects of biochars on environmental and toxicological availabilities of metals and polycyclic aromatic hydrocarbons [J]. Environmental Science and Pollution Research, 2020, 27(2): 1852 − 1869. [7] JIA Huiting, ZHAO Yuanyuan, DENG Hui, et al. Significant contributions of biochar-derived dissolved matters to ecotoxicity to earthworms (Eisenia fetida) in soil with biochar amendment [J/OL]. Environmental Technology & Innovation, 2023, 29: 102988[2023-04-01]. doi:10.1016/j.eti.2022.102988. [8] CELLETTI S, BERGAMO A, BENEDETTI V, et al. Phytotoxicity of hydrochars obtained by hydrothermal carbonization of manure-based digestate [J/OL]. Journal of Environmental Management, 2021, 280: 111635[2023-04-01]. doi:10.1016/j.jenvman.2020.111635. [9] 李阳, 黄梅, 沈飞, 等. 生物炭对小麦种子萌发与幼苗生长的植物毒理效应[J]. 生态毒理学报, 2017, 12(1): 234 − 242. LI Yang, HUANG Mei, SHEN Fei, et al. Phytotoxic effects of biochar on seed germination and early growth of wheat [J]. Asian Journal of Ecotoxicology, 2017, 12(1): 234 − 242. [10] 张进, 刁韩杰, 王敏艳, 等. 稻壳与污泥共热解对污泥炭特性及其重金属生态风险的影响[J]. 环境科学学报, 2019, 39(4): 1250 − 1256. ZHANG Jin, DIAO Hanjie, WANG Minyan, et al. Effects of rice husk and sewage sludge co-pyrolysis on characteristics of the sludge biochar and its ecological risk of heavy metals [J]. Acta Scientiae Circumstantiae, 2019, 39(4): 1250 − 1256. [11] YANG Fan, XU Zibo, YU Lu, et al. Kaolinite enhances the stability of the dissolvable and undissolvable fractions of biochar via different mechanisms [J]. Environmental Science and Technology, 2018, 52(15): 8321 − 8329. [12] 胡选萍, 秦公伟, 曹小勇, 等. 不同pH值溶液对蓝莓种子萌发特性的影响[J]. 种子, 2019, 38(4): 24 − 30. HU Xuanping, QIN Gongwei, CAO Xiaoyong, et al. Effect of different gradient of pH on seed germination characteristics of blueberry [J]. Seed, 2019, 38(4): 24 − 30. [13] 彭钼植, 吉小敏, 姜黎. 温度与盐分对荒漠植物骆驼蓬种子萌发和幼苗生长的影响[J]. 北方园艺, 2023(2): 50 − 56. PENG Muzhi, JI Xiaoming, JIANG Li. Effects of temperature and salt stress on seed germination and seedling growth of Peganum harmala [J]. Northern Horticulture, 2023(2): 50 − 56. [14] 符佳豪, 范少茹, 庄秋荣, 等. 砷胁迫下纳米硫对油菜幼苗生长、砷积累及土壤砷形态的影响[J]. 江苏农业科学, 2022, 50(12): 51 − 59. FU Jiahao, FAN Shaoru, ZHUANG Qiurong, et al. Effects of nano-sulfur on growth of rapeseed seedlings, As accumulation, and soil As speciation under arsenic stress [J]. Jiangsu Agricultural Sciences, 2022, 50(12): 51 − 59. [15] MRIDHA D, PAUL I, DE A, et al. Rice seed (IR64) priming with potassium humate for improvement of seed germination, seedling growth and antioxidant defense system under arsenic stress [J/OL]. Ecotoxicology and Environmental Safety, 2021, 219: 112313[2023-04-01]. doi:10.1016/j.ecoenv.2021.112313. [16] ZHANG Tao, SUN Kaile, CHANG Xiaoke, et al. Comparative physiological and transcriptomic analyses of two contrasting pepper genotypes under salt stress reveal complex salt tolerance mechanisms in seedlings [J/OL]. International Journal of Molecular Sciences, 2022, 23(17): 9701[2023-04-01]. doi:10.3390/ijms23179701. [17] 俞佳, 黄颖, 张晋龙, 等. 砷胁迫下外源磷对香蒲根表铁膜形成及吸附砷的影响[J]. 生态环境学报, 2021, 30(4): 866 − 873. YU Jia, HUANG Ying, ZHANG Jinlong, et al. Effect of phosphorus on the formation and adsorption of arsenic in the iron film of the fennel root table under arsenic stress [J]. Ecology and Environmental Sciences, 2021, 30(4): 866 − 873. [18] SHUKLA J, MOHD S, KUSHWAHA A S, et al. Endophytic fungus Serendipita indica reduces arsenic mobilization from root to fruit in colonized tomato plant [J/OL]. Environmental Pollution, 2022, 298: 118830[2023-04-01]. doi: 10.1016/j.envpol.2022.118830. [19] RAGURAJ S, KASIM S, JAAFAR N M, et al. Influence of chicken feather waste derived protein hydrolysate on the growth of tea plants under different application methods and fertilizer rates [J]. Environmental Science and Pollution Research, 2023, 30(13): 37017 − 37028. [20] SPANÒ C, MUCCIFORA S, CASTIGLIONE M R, et al. Polystyrene nanoplastics affect seed germination, cell biology and physiology of rice seedlings in-short term treatments: evidence of their internalization and translocation [J]. Plant Physiology and Biochemistry, 2022, 172: 158 − 166. [21] 荆蓉, 彭祚登, 李云, 等. 刺槐林腐殖质浸提液对自身种子萌发和幼苗生长的影响[J]. 西北农林科技大学学报(自然科学版), 2023, 51(7): 1 − 15. JING Rong, PENG Zuodeng, LI Yun, et al. Effects of humus extracts from Robinia pseudoacacia forests on seed germination and seedling growth [J]. Journal of Northwest A&F University, 2023, 51(7): 1 − 15. [22] MIKULIC P M, VEBERIC R, HUDINA M, et al. HPLC-DAD-MS dentification and quantification of phenolic components in Japanese Knotweed and American Pokeweed extracts and their phytotoxic effect on seed germination [J/OL]. Plants Basel, 2022, 11(22): 3053[2023-04-01]. doi:10.3390/plants11223053. [23] MUNSON R D, HUBER D M, ARNY D C. Potassium in Agriculture [M]. Wisconsin: Amer Society of Agronomy, 1985. [24] LERMA M R, LOPEZ G J A, SUAREZ E F, et al. Mitigation of phytotoxic effect of compost by application of optimized aqueous extraction protocols [J/OL]. Science of the Total Environment, 2023, 873: 162288[2023-04-01]. doi: 10.1016/j.scitotenv.2023.162288. [25] 刘泽勋, 刘超, 郑康, 等. 不同钠盐胁迫对刺槐种子萌发和幼苗生长的影响[J]. 种子, 2022, 41(10): 65 − 72. LIU Zexun, LIU Chao, ZHENG Kang, et al. Effects of different sodium salt stress on seed germination and seedling growth of Robinia pseudoacacia [J]. Seed, 2022, 41(10): 65 − 72. [26] SENEVIRATNE M, RAJAKARUNA N, RIZWAN M, et al. Heavy metal-induced oxidative stress on seed germination and seedling development: a critical review [J]. Environmental Geochemistry and Health, 2019, 41: 1813 − 1831. [27] KUMAR A, SINGH S, MUKHERJEE A, et al. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress [J/OL]. Microbiological Research, 2021, 242: 126616[2023-04-01]. doi:10.1016/j.micres.2020.126616. [28] JIANG Dan, LU Bin, LIU Liantao, et al. Exogenous melatonin improves salt stress adaptation of cotton seedlings by regulating active oxygen metabolism [J/OL]. PeerJ, 2020, 8: e10486[2023-04-01]. doi:10.7717/peerj.10486. [29] FAIZAN M, BHAT J A, CHEN Chen, et al. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato [J]. Plant Physiology and Biochemistry, 2021, 161: 122 − 130. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20230227







 下载:
下载: