-
松材线虫Bursaphelenchus xylophilus是公认的检疫性有害生物,破坏性地危害欧亚地区的松林资源[1]。松材线虫的生活史分为2个阶段:繁殖阶段和扩散阶段[2]。在温度适宜的夏季和秋季,松材线虫进入繁殖阶段,在树体内经过卵、繁殖型幼虫(J1~J4),再发育为成虫。在不良环境条件下,松材线虫进入扩散阶段,即滞育型周期,繁殖型J2会转型发育为扩散型J3幼虫和扩散型J4幼虫,在此过程中体内脂滴数量增加,并发生脂滴融合现象,形成超大脂滴或者块状脂肪[3]。扩散型J4幼虫口针退化不取食,依靠消耗体内超大脂滴作为能量侵染新的寄主植物。因此,脂滴对于松材线虫的生存和传播都具有重要作用。中性脂肪(甘油三酯和胆固醇)在线虫中主要以脂滴的形式存在,是松材线虫体内脂肪的主要储存形式[4-6]。松材线虫的肠道是脂类物质沉积的主要场所。由于线虫虫体是透明的,可在显微镜下清楚地看到从头部到尾部的整个肠道,是研究脂肪沉积的一种理想模型[7]。模式线虫秀丽隐杆线虫Caenorhabditis elegans可采用染料苏丹黑B、尼罗红和油红O等方法检测脂肪储存和代谢,不同染色方法下脂肪含量的表征呈现一定程度的差异。利用脂溶性的染料苏丹黑B来着色脂滴,脂滴在肠和皮下组织中可见。荧光染料尼罗红和油红O可将脂滴染成红色[8]。目前在松材线虫中尚无相关脂滴染色研究报道,因此,本研究使用不同的染料标记松材线虫体内的脂滴分布,通过使用ImageJ软件测量线虫脂滴的平均像素强度,来表征脂肪含量,确定适合松材线虫脂滴染色的方法[9]。
-
松材线虫NXY61虫株为中国林业科学院森林保护研究所从浙江省宁波市罹病马尾松Pinus massoniana中分离获得。使用马铃薯葡萄糖琼脂培养基(PDA)培养的灰葡萄孢Botrytis cinerea来培养繁殖型松材线虫[2]。混合龄期的繁殖型线虫置于PDA培养基上生长3 d,在加入灭菌双蒸水(ddH2O)的贝尔曼漏斗中将线虫从培养基上洗下来,3 000 r·min−1离心3 min,清除上清液。扩散型3龄幼虫从当年采集的疫木中分离,扩散型4龄幼虫从媒介昆虫松墨天牛Monochamus alternatus体内分离。
-
将松材线虫用M9缓冲液(3.00 g KH2PO4, 6.00 g Na2HPO4, 5.00 g NaCl, 1 mL 1 mol·L−1 MgSO4,加入蒸馏水定容至1 L)洗涤离心3次(3 000 r·min−1,1 min);将线虫置于含体积分数为1%多聚甲醛(paraformaldehyde,PFA)的M9缓冲液中,4 ℃过夜;−80 ℃冰箱冻融3次;将M9洗涤固定的线虫经梯度体积分数乙醇(25%,50%和70%)分级脱水,每级5 min;随后,在溶于体积分数为70%乙醇的苏丹黑B溶液中进行染色[10]。显微镜观察和拍摄染色后的线虫,并对皮下和肠道内染色的脂滴像素统计。
-
将线虫用体积分数为1%多聚甲醛溶液固定,使线虫细胞和亚细胞结构保持稳定状态[9]。线虫和多聚甲醛溶液混合,并于室温摇动2~3 h后,静置使线虫沉降;在−80 ℃冰箱冻融3次,固定后的线虫逐级脱水;在M9缓冲液的线虫沉淀中加入1 mL尼罗红(1 mg·L−1),在避光的环境下混匀并染色25~30 min,染色期间轻轻翻转离心管,使得虫体均匀接触染料;用1×PBS(8.00 g NaCl,0.20 g KCl,1.44 g Na2HPO4和0.24 g KH2PO4,溶于800 mL蒸馏水,用HCl调节溶液pH至7.4,最后加蒸馏水定容至1 L)清洗1次,去除线虫表面的染料,然后使线虫自然沉降。最后用显微镜观察拍照。
-
用M9缓冲液将松材线虫洗至离心管中,在体积分数为1%多聚甲醛室温或4 ℃固定15~30 min;然后置于−80 ℃冰箱中冷冻15 min,在43 ℃水浴中迅速解冻;低速离心1 min,弃上清;用1×PBS冲洗3次,弃去PBS;用体积分数为1%Triton X-100、异丙醇油红O溶液浸泡30 min;用1×PBS冲洗3次。用异丙醇置于载玻片上,显微镜下观察拍照[11]。
-
使用改良的后置油红O染色方案[12]。将油红O溶于异丙醇,制成质量浓度为5.00 g·L−1原液。采用混合法制备油红O工作液(60%的原液和40%的水)。油红O工作液静置后用0.22 mm自旋过滤器过滤。将线虫用体积分数为1%多聚甲醛/PBS在室温下固定摇动30 min;然后立即将样品在冰箱中冷冻干燥15 min,并在自来水中溶解,共3次;低速离心1 min,弃上清;用1×PBS洗涤样品3次,用体积分数为60%异丙醇脱水2 min;用1 mL油红O工作液摇动1 h进行染色。染色后的样品用1×PBS洗涤3次,再水合1×PBS,并安装在琼脂填充的载玻片上,显微镜观察拍照。
-
用徕卡荧光正置显微镜(DM4B)使用40×物镜观察固定的尼罗红、油红O和苏丹黑B染色的线虫,用CCD相机(DFC7000T)拍摄16位图像,并用ImageJ(V2.1.4.7)进行图像处理分析。
-
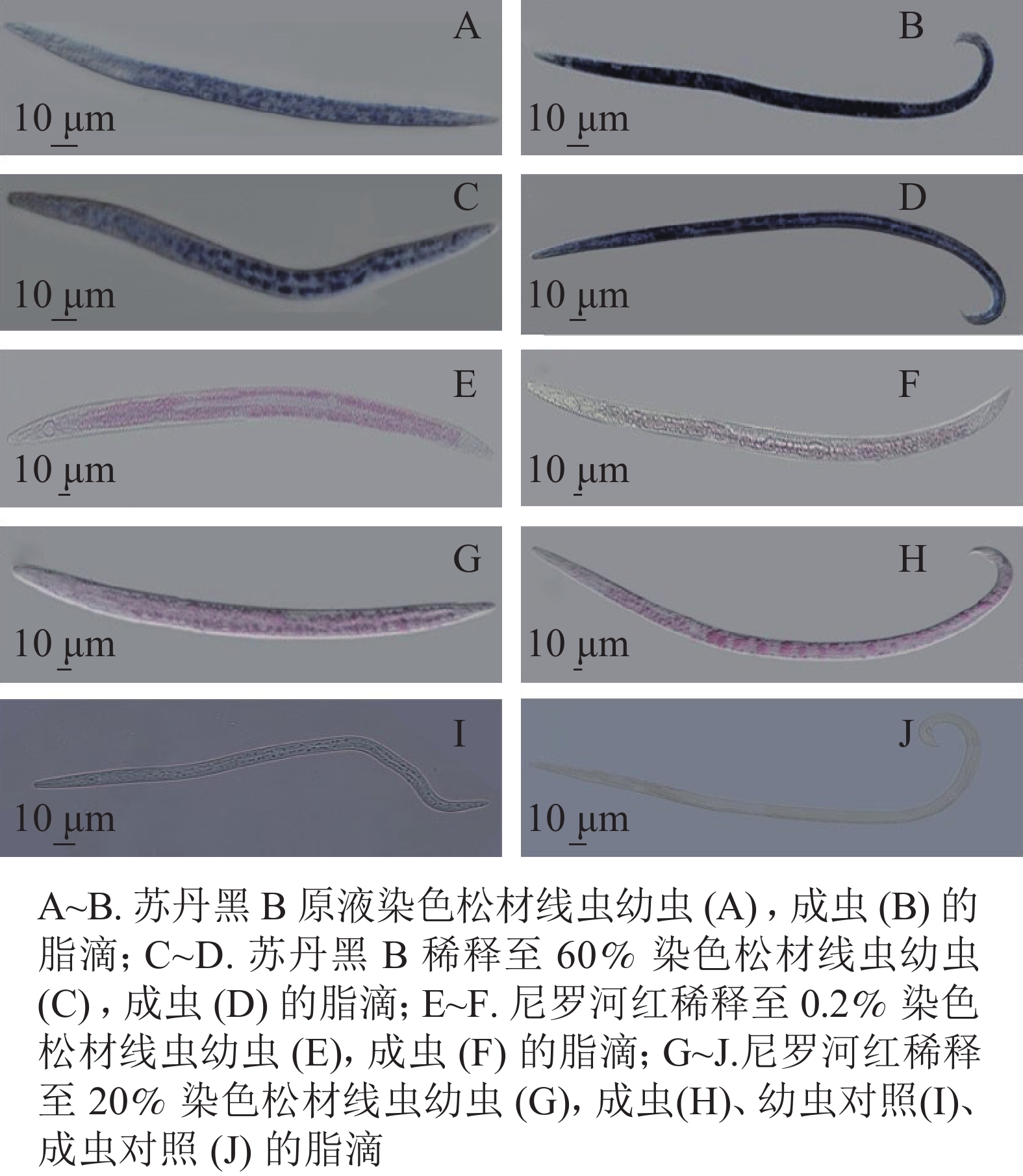
将固定后的虫体置于显微镜下观察,与未染色线虫相比,苏丹黑B染色线虫的肠道普遍被染成蓝色(图1)。在实验过程中,用苏丹黑B原溶液染色线虫如图1A~B,显微镜下观察到的脂滴边界不清晰,整条成虫呈现深蓝色;将苏丹黑B稀释60%,染色观察如图1C~D,幼虫和成虫脂滴边界尚清楚,但仍会影响观察松材线虫的脂滴动态变化。
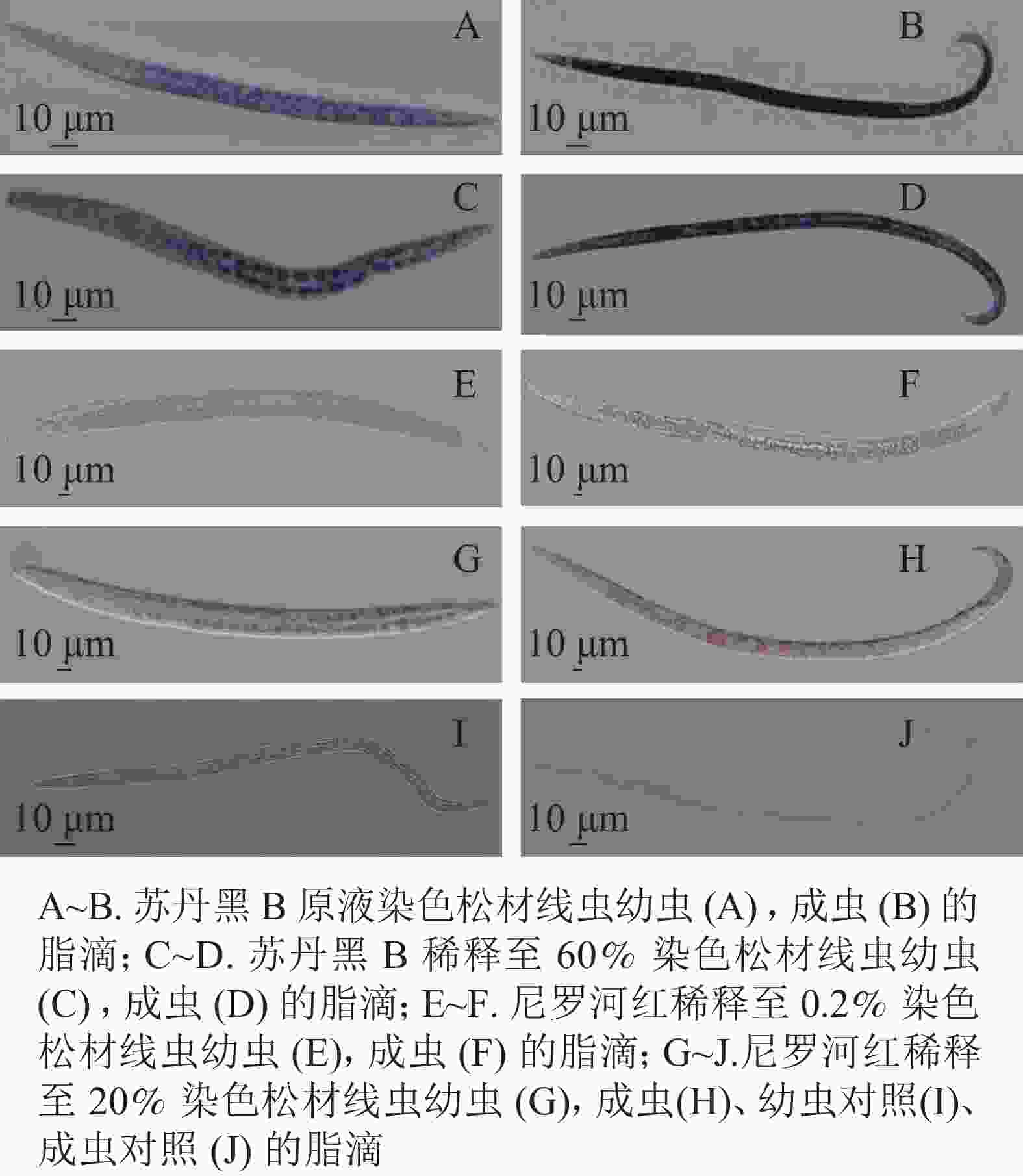
图 1 苏丹黑B、尼罗河红染色线虫中相同脂滴
Figure 1. Sudan black B, fixed Nile red stained nematode in same lipid droplets
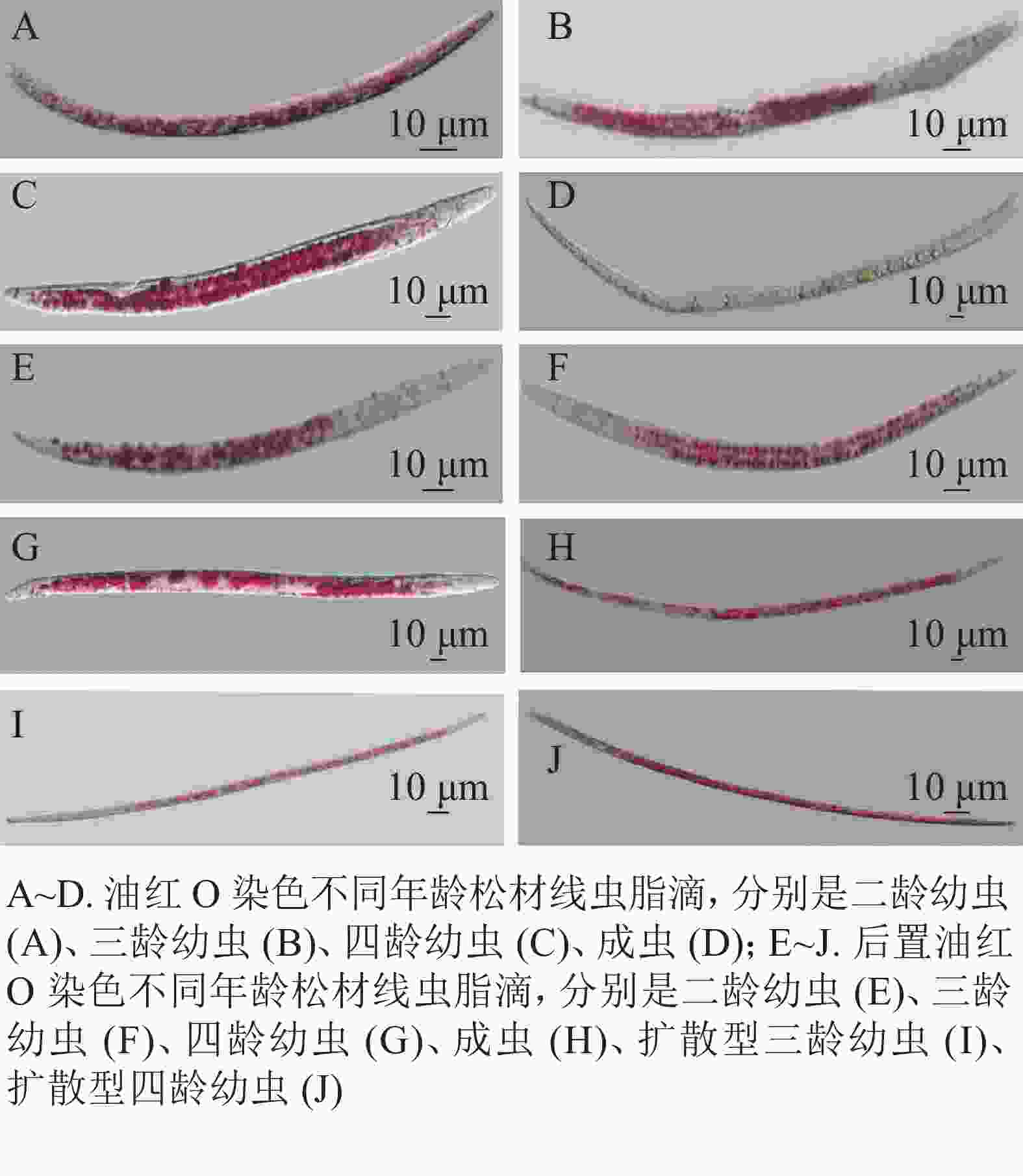
尼罗红与苏丹黑B共同标记了肠道周围的脂滴。尼罗红染色线虫在显微镜下观察到部分脂滴呈浅红色(图1E~H)。将配置的母液染料稀释500倍,染色时间30 min,显微成像效果不佳(图1E~F),大部分虫体脂滴染色不均,其中成虫的色泽较浅。当提高工作液浓度,仅将母液稀释了100倍,为5 mg·L−1, 并将染色时间增加为1 h (图1G~J),可以分辨分布在成虫肠道周围的圆形大脂滴,而幼虫的脂滴边界相对比较模糊,不便于测量松材线虫脂滴的大小和脂肪存储量。
-
在用油红O与后置油红O染色线虫时,培养的同步发育繁殖型线虫和野外采集的扩散型线虫都呈现明显的红色脂滴(图2)。油红O染色如图2A~D,可以观察到,当虫体内出现较多脂滴时,单个脂滴未能良好分离,会影响测量松材线虫脂肪储存量化及繁殖型与扩散型松材线虫的脂滴大小的变化。用后置油红O方法染色线虫结果如图2E~J,在显微镜下观察到脂滴边界清晰,其中J2和J3幼虫大部分较小的脂滴分布在肠道和表皮,并能观察到从幼虫J4到成虫出现小脂滴聚集成大脂滴的现象。扩散型J3较大的脂肪块与扩散型J4脂质小滴之间的脂滴大小变化十分明显。
-
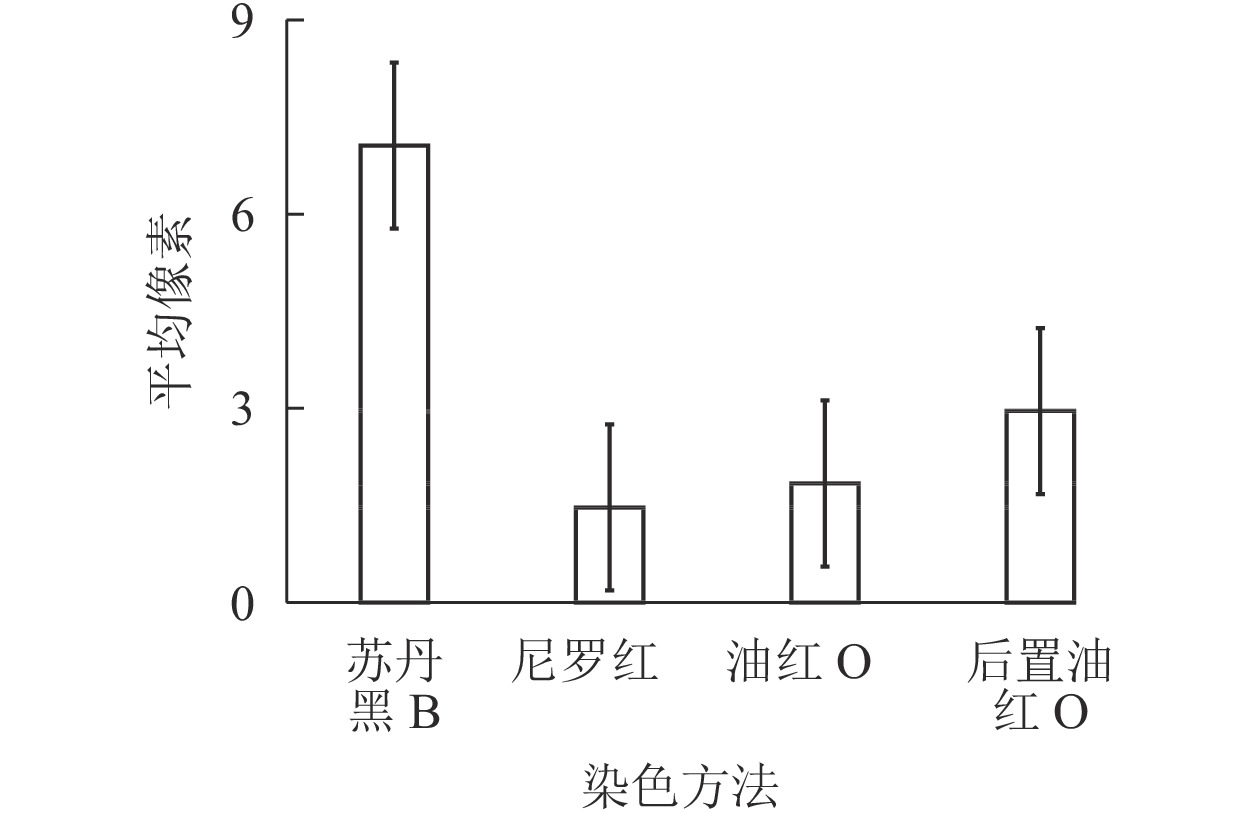
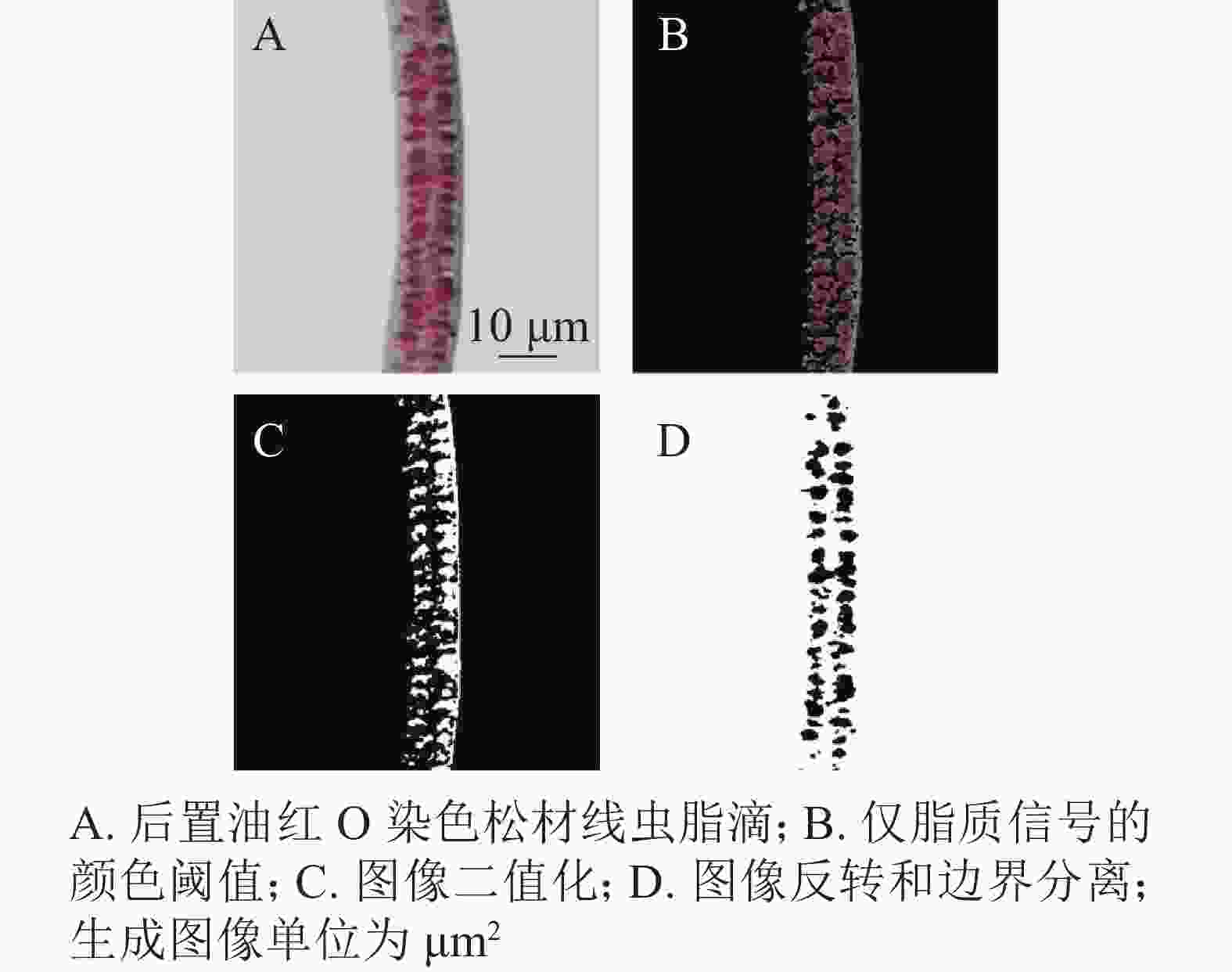
通过光学显微镜捕获的苏丹黑B、尼罗红、油红O染色松材线虫脂滴图片,用ImageJ进行图像处理,计算线虫脂滴图片的平均像素强度。图3结果显示:苏丹黑B和后置油红O染色松材线虫脂滴的平均像素强度明显高于尼罗红和油红O染色,其中苏丹黑B染色后整条虫呈现蓝色,所以脂滴平均像素强度最高,而尼罗红的最低,因为颜色较浅。这就说明脂滴平均像素强度偏高或偏低都会影响表征脂肪。经ImageJ图像处理比较分析,后置油红O染色脂滴经计算转换与原始图像的一致,具有单个脂滴的良好分离(图4)。
-
如表1所示:综合考虑染色方法的简易性、染色时间的长短[11]、染色的观察效果,后置油红O染色方法在4种染色方法中的染色时间相对较短,脂滴呈现的效果比较清晰,能实现脂滴的单个分离,方法步骤比较简单,认为后置油红O染色方法是松材线虫脂滴的最佳染色方法。
表 1 不同染料标记和方法的比较
Table 1. Comparison of different dyestuff markers and methods
染料 储备溶液 脱水步骤 染色时间/min 输出颜色 实现单个脂滴分离 油红O 5 g·L−1异丙醇 30 红色 否 苏丹黑B 体积分数为70%乙醇 乙醇系列 360 深蓝色 否 尼罗红 丙酮 乙醇系列 30 浅红色 否 后置油红O 5 g·L−1异丙醇 体积分数为60%异丙醇 60 红色 是 -
本研究通过使用染料标记的不同测定法来探索松材线虫的最佳脂滴染色方法。在染色前,需要先对虫体进行有效固定,多聚甲醛可很好地保存生物体组织的细微结构特征。固定以后,组织中的各种物质会沉淀和凝固,产生不同的折射率,促使产生光学差异,方便染色后观察。
油红O、苏丹黑B、尼罗红染色一般都能观察到线虫体内脂肪储存,但在本研究中呈现不同显色效果。苏丹黑B作为一种经典的染料,在固定过程中经乙醇的洗涤,无法标记线虫中脂肪储存[9]。本研究也发现:苏丹黑B染色松材线虫脂滴观察效果不佳,染色时间也比较长。尼罗红是一种由尼古蓝衍生的脂类染料,在疏水环境时发荧光,既可染色脂滴,又可检查完整活体动物的脂肪含量[13-15],经常被作为秀丽隐杆线虫的脂肪酸代谢研究的染料标记。最近研究还发现,鲜活的线虫经尼罗红染色没有显示染色模式的差异[12]。当线虫被喂食尼罗红时,染料会在与溶酶体有关的细胞器中积聚[16],固定尼罗红比喂食尼罗红更能显示脂肪储存情况。本研究初期观察到的脂滴色泽非常浅,甚至整条线虫未被染色,呈透明状态,经过多步实验探索,线虫中脂滴有部分染色,但仍有染色不完整的脂滴。而且由于尼罗红是亲水性染料,可能会染色脂褐素,导致固定染色不能将中性脂肪存储区与脂褐素区分开,影响结果[17]。油红O是一种油溶性偶氮染料,溶于乙醇和丙酮,在脂肪内能高度溶解,从而染色,颜色比较鲜红,染色效果相对于前面2种染料要好。后置油红O染色用体积分数为1%多聚甲醛的固定液在分子杂交炉中室温条件摇动30 min,通过摇动,使线虫充分接触固定液。将样品放在−80 ℃冰箱中冻融3次,并在自来水中溶解,相比于油红O染色(在43 ℃水浴中迅速解冻),可增加线虫的缓冲性。在后置油红O染色过程中,异丙醇脱水使线虫显微成像时观察到的组织结构更清晰,用自旋过滤器过滤油红O工作液,可以减少沉淀杂质的产生。用琼脂填充幻灯片成像,可以防止线虫干死,也可以更好地固定已染色的线虫。经过多次实验探究,油红O染色之后,观察到的大小脂滴聚集,脂滴边界未能体现,红色会成片区域出现,当测量脂滴大小变化、脂滴数量时,会加大实验数据误差;后置油红O染色方法经过改良,线虫在室温下用体积分数为1%多聚甲醛摇动,并经多次冻融以后用体积分数为60%的异丙醇脱水,染色效果比较理想,可直接观察脂滴大小变化。综上所述,后置油红O染色方法是最佳松材线虫脂滴染色方法。
Comparison of four lipid droplets staining methods in Bursaphelenchus xylophilus
-
摘要:
目的 从4种脂滴染色方法中筛选最适合松材线虫Bursaphelenchus xylophilus的脂滴染色方法。 方法 采用苏丹黑B染色、尼罗红染色、油红O染色和后置油红O染色等4种染色方法来固定和表征松材线虫体内脂滴的分布,对染色后的线虫进行显微观察和拍摄,并用ImageJ软件对皮下和肠道内染色的脂滴像素进行统计。 结果 4种脂滴染色方法对松材线虫的脂滴都有一定的染色效果。显微拍照后观察脂滴与ImageJ软件图像处理后像素强度比较结果显示:苏丹黑B染色脂滴像素为200.00×1017 m2,尼罗红染色脂滴像素41.64×1012 m2,油红O染色脂滴像素52.12×1017 m2,后置油红O染色脂滴像素83.85×1017 m2。苏丹黑B染色脂滴平均像素强度最高,尼罗红染色脂滴平均像素强度最低,而后置油红O法染色脂滴经计算转换产生了与原始图像一致的表征,实现单个脂滴的良好分离。 结论 综合染色方法的简易、染色时间的长短和染色后的效果,改良后的后置油红O法是最佳的松材线虫脂滴染色方法,能清晰显示脂滴大小及分布。图4表1参17 Abstract:Objective With a comparison of the four commonly used methods for staining Caenorhabditis elegans lipid droplets, this study is aimed to figure out the most suitable dyeing method for Bursaphelenchus xylophilus lipid droplets. Method Four were used to fix and characterize After the distribution of lipid droplets in B. xylophilus was defined with its features revealed employ four dyeing methods including Sudan black B staining, Nile red staining, oil red O staining, and post-fix oil red O staining, the stained nematodes were observed and photographed microscopically, and then ImageJ software was used to count the subcutaneous and intestinal lipid droplet pixels. Result 1) The four dyeing methods have a certain dyeing effect on the lipid droplets of B. xylophilus. 2) Upon the observation of the lipid droplets after microphotographing and the comparison of the pixel intensity after image processing by ImageJ software, the stained lipid droplets pixels with Sudan black B, Nile red, oil red O and post-fix oil red O are 200×1017 m2, 41.64×1012 m2, 52.12×1017 m2, and 83.85×1017 m2 respectively. 3) Lipid droplets stained by Sudan black have the highest average pixel intensity, and lipid droplets stained by Nile red have the lowest average pixel intensity, whereas the lipid droplets stained by post-fix oil red O, after calculation and conversion, produced a consistent characterization with the original image, with a favorable separation of individual lipid droplet. Conclusion In conclusion, in light of the simplicity of the dyeing method, the length of dyeing time and the effect of dyeing, the improved post-fix oil red O method is the optimal method for dyeing lipid droplets in B. xylophilus, with the size and distribution of lipid droplets clearly shown. [Ch, 4 fig. 1 tab. 17 ref.] -
Key words:
- Bursaphelenchus xylophilus /
- lipid droplets staining /
- Nile red /
- oil red O /
- Sudan black /
- post-fix oil red O
-
表 1 不同染料标记和方法的比较
Table 1. Comparison of different dyestuff markers and methods
染料 储备溶液 脱水步骤 染色时间/min 输出颜色 实现单个脂滴分离 油红O 5 g·L−1异丙醇 30 红色 否 苏丹黑B 体积分数为70%乙醇 乙醇系列 360 深蓝色 否 尼罗红 丙酮 乙醇系列 30 浅红色 否 后置油红O 5 g·L−1异丙醇 体积分数为60%异丙醇 60 红色 是 -
[1] 于浩, 吴海燕. 植物寄生线虫滞育机制研究进展[J]. 植物保护, 2009, 35(4): 20 − 23. YU Hao, WU Haiyan. Research progresses in the mechanism of dormancy and diapause in plant parasitic nematodes [J]. Plant Prot, 2009, 35(4): 20 − 23. [2] 田浩楷, 张帅, 柳小龙, 等. 扩散型与繁殖型松材线虫数字基因表达谱对比分析[J]. 生物安全学报, 2017, 26(2): 111 − 121. TIAN Haokai, ZHANG Shuai, LIU Xiaolong, et al. Comparative analysis of dispersal and propagative larval stages in the pinewood nematode (Bursaphelenchus xylophilus) based on a digital gene expression library[J]. J Biosafety, 2017, 26(2): 111−121. [3] MAMIYA Y. The life history of the pine wood nematode, Bursaphelenchus lignicolus [J]. Jap J Nematol, 1975, 5: 16 − 25. [4] MARTIN S, PARTON R G. Lipid droplets: a unified view of a dynamic organelle [J]. Nat Rev Mol Cell Biol, 2006, 7(5): 373 − 378. [5] DUCHARME N A, BICKEL P E. Lipid droplets in lipogenesis and lipolysis [J]. Endocrinology, 2008, 149(3): 942 − 949. [6] GOODMAN J M. The gregarious lipid droplet [J]. J Biol Chem, 2008, 283(42): 28005 − 28009. [7] JONES K T, ASHRAFI K. Caenorhabditis elegans as an emerging model for studying the basic biology of obesity [J]. Dis Models Mech, 2009, 2: 224 − 229. [8] SOUKAS A A, KANE E A, CARR C E, et al. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans [J]. Genes Dev, 2009, 23(4): 496 − 511. [9] YEN K, LE T T, BANSAL A, et al. A comparative study of fat storage quantitation in nematode Caenorhabditis elegans using label and label-free methods [J]. PLoS One, 2010, 5(9): e12810. doi: 10.1371/journal.pone.0012810. [10] KIMURA K D, TISSENBAUM H A, LIU Y, et al. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans [J]. Science, 1997, 277(5328): 942 − 946. [11] 徐林丽, 高学娟, 屈长青. 秀丽隐杆线虫体内脂肪染色方法的探究[J]. 中国组织化学与细胞化学杂志, 2010, 19(6): 615 − 616. XU Linli, GAO Xuejun, QU Changqing. Study on the method of fat staining in Caenorhabditis elegans [J]. Chin J Histochem Cytochem, 2010, 19(6): 615 − 616. [12] BROOKS K K, LIANG B, WATTS J L. The influence of bacterial diet on fat storage in C. elegans [J]. PLoS One, 2009, 4: e7545. doi: 10.1371/journal.pone.0007545. [13] O’ROURKE E J, SOUKAS A A, CARR C E, et al. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles [J]. Cell Metab, 2009, 10(5): 430 − 435. [14] KLAPPER M, EHMKE M, PALGUNOW D, et al. Fluorescence based fixative and vital staining of lipid droplets in Caenorhabditis elegans reveal fat stores using microscopy and flow cytometry approaches [J]. J Lipid Res, 2011, 52(6): 1281 − 1293. [15] FOWLER S D, GREENSPAN P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O [J]. Histochem J Cytochem, 1985, 33: 833 − 836. [16] SCHROOEDER L K, KREMER S, KRAMER M J, et al. Function of the Caenorhabditis elegans ABC transpor the biogenesis of a lysosome-related fat storage organelle [J]. Mol Biol Cell, 2007, 18: 995 − 1008. [17] VIEGAS M S, MARTINS T C, SECO F, et al. An improved and cost-effective methodology for the reduction of autofluorescence in direct immunofluorescence studies on formalin-fixed paraffin-embedded tissues [J]. Eur J Histochem, 2007, 51(1): 59 − 66. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20200674






 下载:
下载: