-
蛹虫草Cordyceps militaris,又称为北虫草,北冬虫夏草,隶属于真菌界的子囊菌门Ascomycota肉座菌目Hypocreales麦角菌科Clavicipitaceae,是世界性广布种[1]。其所含的虫草素比天然冬虫夏草Cordyceps sinensis还要高,药效与天然冬虫夏草相似[2],具有明显的抗疲劳、抗肿瘤、抗衰老等作用[3-5]。人工栽培蛹虫草子实体生产周期长,产量有限,国内外学者一直致力于蛹虫草液体发酵培养的研究[6-7]。研究表明,采用液体培养的虫草菌丝体,其所含的主要成分与天然子实体十分接近[8],且具有培养条件易控制、产量高、生长周期短且活性物质易于提取等特点,有望成为野生虫草的替代品[9-11]。因此,液体发酵生产蛹虫草菌丝体不仅具有重要的应用价值,而且对保护有限的野生虫草资源也具有重要的意义。近年来,蛹虫草液体培养的研究取得了很大进展,但由于蛹虫草菌株及培养基配方等原因,使培养产物有诸多差异[12-13]。目前,对蛹虫草液体培养基配方的优化主要从单因素试验和正交试验两方面[14-19],对配方的优化方法的研究还不够深入,不能确定各因素之间的协同关系。本研究分别采用单因素试验、正交试验、Plackett-Burman(BP)设计、中心组合试验及响应面法系统全面地对蛹虫草液体培养的培养基配方进行优化,以菌丝体干质量浓度为响应值,采用多元二次回归拟合方程,通过求偏导获得主要影响因子的最佳浓度配比,筛选出最佳的培养基配方。
HTML
-
蛹虫草菌株DY-1由四川省农业科学院蚕业研究所养蚕法研究室分离并保存。 所用试剂硫酸镁、磷酸二氢钾、磷酸氢二钾、硫酸亚铁、硝酸钾、氯化钠等均为分析纯,购自国药集团药业股份有限公司,试验用水为自制双蒸水和去离子水。
BP1211S 1/10J电子天平(德国赛多利斯有限公司),ZK-82A型真空干燥箱(上虞市宏兴机械仪器制造有限公司),KQ-600型超声波清洗器(苏州江东精密仪器有限公司),DHZ-DA恒温振荡培养箱(江苏太仓仪器设备有限公司),TG16-WS台式离心机(湖南沪康离心机有限公司);YX400高压蒸汽灭菌锅(上海安锐自动化仪表有限公司)。
-
土豆200.00 g,葡萄糖20.00 g,蒸馏水1 000.00 mL,自然pH值。121 ℃灭菌30 min,备用。
-
M1:土豆200.00 g,牛肉膏5.00 g,硫酸镁1.00 g,磷酸二氢钾0.60 g,硫酸铵1.00 g,碳酸钙3.00 g,蒸馏水1 000.00 mL;M2:酵母粉5.00 g,蛋白胨10.00 g,氯化钠10.00 g,蒸馏水1 000.00 mL;M3:葡萄糖20.00 g,蛋白胨10.00 g,硫酸镁1.50 g,磷酸二氢钾1.50 g,蒸馏水1 000.00 mL;M4:牛肉膏3.00 g,蛋白胨5.00 g,蔗糖10.00 g,酵母膏1.00 g,蒸馏水1 000.00 mL;M5:葡萄糖16.00 g,可溶性淀粉2.50 g,蛋白胨10.00 g,豆饼粉26.00 g,玉米浆1.00 g,氯化钠7.50 g,硫酸铵4.00 g,蒸馏水1 000.00 mL。自然pH值。培养基用聚乙烯膜封口,121 ℃灭菌30 min,备用。
-
从固体斜面培养基上取1.0 cm2的菌丝块2块,接种到已灭菌的装有400.00 mL种子培养基的1 000.00 mL三角瓶中,22 ℃,180 r·min-1,振荡培养72 h,备用。
-
将10.00 mL活化的菌株孢子悬液接种于已灭菌的装有100.00 mL基础培养基的500.00 mL三角瓶中(孢子浓度用血球计数板测定为2.0×106个·mL-1),22 ℃,180 r·min-1,振荡培养5 d,重复2次·处理-1。
-
采用菌丝体干质量法,取发酵液100.00 mL,转速3 000 r·min-1离心15 min,倾去上清液,菌丝体于80 ℃烘干至恒量,用分析天平称量,取2次重复试验的平均值为菌丝体质量。
-
分别采用1.2.2节中的5种基础培养基对菌株DY-1进行液体发酵,通过测定菌丝体干质量浓度,筛选出干质量浓度最高的培养基配方,在此基础上进行培养基配方的优化。
-
所筛选出的基础培养基中的碳源采用葡萄糖、蔗糖、可溶性淀粉、土豆、玉米粉,以20.00 g·L-1添加,以不含碳源的培养基为对照,重复2次·处理-1。测定菌丝体干质量浓度,比较不同碳源对菌丝体干质量浓度的影响。有机氮源分别选取蛋白胨、牛肉膏、黄豆饼粉、酵母浸膏粉进行单因素分析,基础培养基以10.00 g·L-1添加;无机氮源分别选取硝酸钾、硫酸铵、氯化铵、硝酸铵进行单因素分析,基础培养基以1.00 g·L-1添加;其他方法同1.4.2节。
-
将筛选到的最佳碳源、有机氮源和无机氮源参照正交表L16(45)进行正交试验设计,其因素与水平的设置见表 1,重复2次·处理-1。测定菌丝体干质量浓度,比较不同C/N比对菌丝体干质量浓度的影响。
处理 因素水平/( g▪L-1) 葡萄糖 酵母浸膏粉 硝酸钾 1 2.00 1.00 0.50 2 5.00 2.00 1.00 3 10.00 5.00 2.00 4 20.00 10.00 5.00 Table 1. Arrangement of factors and levels
-
本研究首先进行了不同无机盐对DY-1菌株菌丝干质量浓度的单因素影响试验,筛选出了对菌丝干质量浓度影响较大的3种无机盐硫酸镁、磷酸二氢钾、硫酸亚铁。本研究对这3种无机盐,根据正交表L9(34)进行正交试验设计,其因素与水平的设置见表 2,重复2次·处理-1。测定菌丝体干质量浓度,比较不同C/N比对菌丝干质量浓度的影响。
处理 因素水平/(g▪L-1) 硫酸镁 磷酸二氢钾 硫酸亚铁 1 0.20 1.00 0.01 2 0.50 1.50 0.02 3 1.00 3.00 0.05 Table 2. Arrangement of factors and levels
-
根据以上试验结果,选取6个影响显著的因子进行试验,其因素水平及编码见表 3。
因素 因素水平/( g.L-1) -1 0 1 葡萄糖 5.00 10.00 15.00 酵母浸膏粉 2.00 5.00 7.00 硝酸钾 0.50 1.00 1.50 硫酸镁 0.10 0.20 0.40 磷酸二氢钾 0.50 1.50 2.00 硫酸亚铁 0.10 0.20 0.30 Table 3. Factors and coded val es of design
-
利用Minitab 14软件对试验数据进行一元线性回归分析,根据试验拟合的一次多项式方程找出主要影响因子,并确定最陡爬坡的方向及步长,由此接近最大响应区域[20]。
-
在部分因子试验和最陡爬坡试验的基础上,进行中心组合试验设计。本阶段试验设计、数据分析及模型的建立是借助于Design Expert 7.0和SAS 9.0辅助完成[21]。
-
试验数据采用SAS 9.0数据分析软件进行统计分析。
1.1. 材料及主要试剂和仪器
1.2. 培养基配方
1.2.1. 种子液培养基配方
1.2.2. 基础培养基配方
1.3. 试验方法
1.3.1. 菌株的活化方法
1.3.2. 接种及培养方法
1.3.3. 菌丝体生物量测定方法
1.4. 培养基配方优化的方法
1.4.1. 基础培养基的筛选
1.4.2. 单因素试验
1.4.3. 不同碳氮肥比(C/N)对菌株DY-1菌丝体干质量浓度的影响
1.4.4. 不同无机盐对菌株DY-1菌丝体干质量浓度的影响
1.4.5. Plackett-Burman(BP)试验设计
1.4.6. 最陡爬坡试验
1.4.7. 中心组合试验
1.5. 数据处理
-
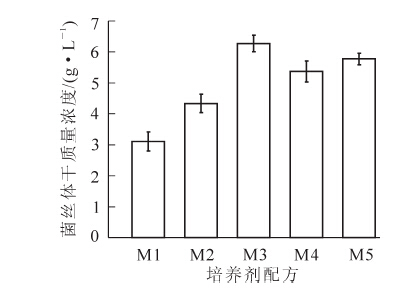
由图 1可知:M3培养基中菌丝体干质量浓度最高,经SAS 9.0分析,选择M3培养基作为基础培养基进行其他因素的筛选。筛选出的最佳碳源、最佳有机氮源和最佳无机氮源分别为葡萄糖、酵母浸膏粉和硝酸钾。
-
以葡萄糖为最佳碳源,酵母浸膏粉、硝酸钾为最佳有机氮源、无机氮源,进行正交试验。采用SAS 9.0数据分析软件对正交试验结果进行方差分析,分析结果见表 4。葡萄糖、酵母浸膏粉、硝酸钾对菌丝体干质量浓度均有极显著影响。分别分析3种因素结果表明:葡萄糖10.00 g·L-1,酵母浸膏粉5.00 g·L-1,硝酸钾 1.00 g·L-1为最佳的C/N比组合。
因素 均方 F值 P>F 葡萄糖 10.773 11.67 0.006 5 酵母浸膏粉 5.377 5.83 0.003 3 硝酸钾 8.954 9.7 0.010 2 Table 4. Effects of different C/N on mycelium dry weight of strain DY-1
-
以4种不同无机盐对菌株DY-1菌丝体干质量浓度的影响进行正交试验。经SAS 9.0数据分析软件对其结果进行方差分析,结果见表 5。分析结果表明:硫酸镁、磷酸二氢钾和硫酸亚铁对菌丝体干质量浓度的影响均达到显著水平。分析可知影响菌丝体干质量浓度最高的无机盐的组合为:硫酸镁0.20 g·L-1,磷酸二氢钾 1.50 g·L-1,硫酸亚铁0.02 g·L-1。
因素 均方 F值 P>F 硫酸镁 12.568 35.64 0.027 3 磷酸二氢钾 26.348 74.72 0.013 2 硫酸亚铁 11.013 31.23 0.031 0 Table 5. Effect of different inorganic salts on mycelium dry weigh
-
根据上述试验可知,对蛹虫草菌丝体具有显著影响作用的共有以下6个因素:葡萄糖、酵母浸膏粉、硝酸钾、硫酸镁、磷酸二氢钾和硫酸亚铁。采用Minitab软件进行PB试验设计,并对试验结果进行方差分析,得到各因素对菌丝体干质量浓度的影响效果(表 6)。由表 6可以看出:影响菌丝体干质量浓度的极显著因素有葡萄糖(P=0.000)和磷酸二氢钾(P=0.006)。其他因素如酵母浸膏粉(P=0.099),硝酸钾(P=0.219),硫酸镁(P=0.141)和硫酸亚铁(P=0.632)等对菌丝体干质量浓度的影响均不显著。对试验数据进行分析,并拟合一次回归方程,其模型为:C7 =26.3 + 2.41C1 - 0.439C2 + 0.312C3 - 0.384C4 + 0.907C5 - 0.116 C6。(其中:C1代表葡萄糖;C2代表酵母浸膏粉;C3代表硝酸钾;C4代表硫酸镁;C5代表磷酸二氢钾;C6代表硫酸亚铁;C7代表菌丝体干质量浓度)。该方程的R2(adj)为90.7%,表明该回归方程拟合良好[21]。
自变量 系数 T P 常量 26.3 122.7 0.000 葡萄糖 2.41 10.41 0.000 酵母浸膏粉 -0.439 -1.9 0.099 硝酸钾 0.312 1.35 0.219 硫酸镁 -0.384 -1.66 0.141 磷酸二氢钾 0.907 3.92 0.006 硫酸亚铁 -0.116 -0.5 0.632 Table 6. The main effects of factors
-
根据PB试验结果,进一步选择葡萄糖,磷酸二氢钾这2个显著因素进行最陡爬坡试验,当葡萄糖为15.00 g·L-1,磷酸二氢钾为1.70 g·L-1时,菌丝体干质量浓度最高,为32.00 g·L-1,试验结果见表 7。
组合 规范变量 自然变量/( g▪L-1) 菌丝体干质量浓度/(g▪L-1) 葡萄糖 磷酸二氢钾 葡萄糖 磷酸二氢钾 1 0.38 5.00 0.20 原点-△ -1 -0.38 5.00 1.30 24.34 原点 0 0 10.00 1.50 26.45 原点%△ 1 0.38 15 1.7 32 原点+2△ 2 0.76 20 1.90 30.65 原点+3△ 3 1.14 25.00 2.10 27.67 原 点 + 4△ 4 1.52 30.00 2.30 24.32 原 点 + 5△ 5 1.90 35.00 2.5 22.32 Table 7. Experimental designs and results of the steepest accent experiment
-
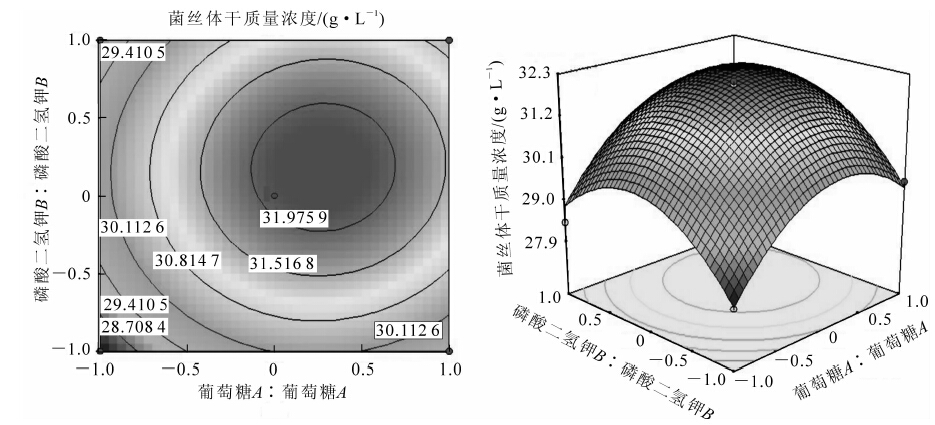
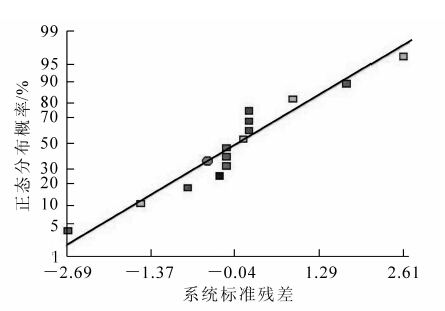
根据最陡爬坡试验结果可知:处理3的菌丝体干质量浓度已接近最大,故选用处理3的培养基作为中心组合试验的中心点,进行中心组合试验设计。其因素水平见表 8。利用SAS 9.0中的响应面回归命令对试验结果数据进行分析,建立以菌丝体干质量浓度为响应面的多元线性回归模型,并且对模型系数和概率进行统计分析,结果见表 9。响应面图及等高线见图 2。从等高线图可以直观的反映出两变量相互作用的显著程度,圆形表示2因素交互作用不显著,而椭圆形与之相反。图 2中等高线呈近似圆形,表明两因素的交互作用不显著。中心组合试验的失拟性分析结果见图 3。从图 3中可以看出试验数据的拟合度较好,试验数据的可信度高。由表 9拟合的全变量编码水平的二次回归方程(y为菌丝体干质量浓度):
因素 水平/(g.L-1) -1.414 -1 0 1 -1.414 葡萄糖 7.9 10 15 20 22.1 磷酸二氢钾 1.00 1.20 1.7 2.20 2.40 Table 8. Factors and coded values of central composite design
来源 平方和 自由度 均方 F值 P>F 模型 35.114 5 7.023 109.793 <0.000 1 A-A 4.779 1 4.779 74.715 <0.000 1 B-B 2.085 1 2.085 32.598 0.000 7 A# 0.035 1 0.035 0.541 0.486 0 A2 14.989 1 14.989 234.331 <0.000 1 B2 15.397 1 15.397 240.71 <0.000 1 残差 0.448 7 0.064 失拟值 0.438 3 0.146 58.821 0.000 9 系统误差 0.010 4 0.003 总误差 35.677 13 说明:A代表葡萄糖,B代表磷酸二氢钾,AB代表葡萄糖与磷酸二氢钾的交互作用项。 Table 9. ANOVA for the regression equation of central composition design
模型的可靠性可由方差分析及决定系数来检验,P=0.001 4(P<0.01)说明模型是极显著的。决定系数R2(adj)=97.44,这表明97.44%的试验数据可用此模型解释,因此,回归方程拟合程度良好。由方程可知:由于二次项系数均为负值,方程代表的抛物面开口向下,因而该方程有极大值点。对方程利用SAS 9.0进行分析,得到模型的极值点(A=0.277;B=0.186),即葡萄糖为16.40 g·L-1,磷酸二氢钾为1.80 g·L-1时,菌丝体干质量浓度达到最高,预测值达到32.22 g·L-1,实测值(32.11 g·L-1)与预测值基本相符。
2.1. 单因素试验结果
2.2. 不同C/N比对菌株DY-1菌丝体干质量浓度的影响结果
2.3. 不同无机盐对菌株DY-1菌丝体干质量浓度的影响结果
2.4. Plackett-Burman(PB)试验结果
2.5. 最陡爬坡试验结果
2.6. 中心组合试验结果
-
通常优化培养基的方法主要包括单次单因子法和正交试验设计法。单次单因子试验是讨论一种因素的影响,由于考察因素间经常存在交互作用,所以该方法并非总能获得最佳的优化条件[22];而正交试验不能给出整个区域上因素和响应值之间的一个明确的函数表达式即回归方程,从而无法找到整个区域上因素的最佳组合和响应值的最佳组合。因此,国内外不少学者[23-24]通过响应面分析法在培养基优化方面取得了良好效果。
通过响应面分析法建立各因素与响应值之间的数学模型,可以较直观看出不同因素之间的交互作用,进行有针对性的调整,减少试验次数,提高效率,在生产实际中有广泛的应用价值。在本研究中,通过响应面分析法建立了葡萄糖和磷酸二氢钾等2个影响显著的因素和响应值菌丝体生物量之间的数学模型,并通过直观的等高线图证实2个影响显著因素的交互作用不显著。通过试验验证,在响应面优化后所获得实际值与预测的最大响应值间拟合程度良好,模型的回归效果显著,能很好地预测蛹虫草菌丝的生长状况,表明中心组合设计和响应面分析法在培养基优化方面的应用具有实际指导作用。优化得到蛹虫草液体培养的最佳培养基配方为:葡萄糖16.40 g·L-1,酵母浸膏粉5.00 g·L-1,硝酸钾1.00 g·L-1,硫酸镁0.20 g·L-1,磷酸二氢钾1.80 g·L-1,硫酸亚铁0.02 g·L-1。在此条件下,通过验证菌丝体生物量达到32.22 g·L-1,比单因素优化提高了1.01倍,较基础培养基提高了4.12倍;而蔡友华等[25]、周广麒等[26]及李珺等[27]对虫草液体发酵培养基优化得到的菌丝体生物量均低于此值。
在所试验的6种碳源中,葡萄糖最有利于虫草菌的生长;这与蔡友华等[25]和刘苗苗等[28]的研究一致,而周广麒等[26]筛选出的蛹虫草的最佳碳源是蔗糖,这可能与蛹虫草菌株的性状不同有关系;在所选的氮源中,酵母浸膏粉和硝酸钾能很好地促进菌丝体的生长,碳氮源是微生物生长所必须的营养物质,碳氮源的种类不仅影响微生物的生长,还会影响多糖等代谢产物的合成。液体培养的周期比固体培养时间大大缩短,而生物量达到30.00 g·L-1以上,且发酵产生的菌丝体呈白色,味清香,可以加入到面食糕点中,提高营养价值。
利用最陡爬坡试验及响应面分析法,对解决优化发酵工艺,获得高产量的蛹虫草菌丝体的问题,具有十分重要的意义和广泛的应用前景,同时这种工艺可在其他微生物发酵过程中得到广泛应用。












 DownLoad:
DownLoad: