-
瓯柑Citrus suavissima是浙江南部地区的一个古老的地方栽培种,具有祛热生津、清凉解毒等特殊的医药功效,素有“端午瓯柑似羚羊”之说。‘无籽’瓯柑Citrus suavissima ‘Seedless’是瓯柑的无核突变体,2004年2月通过浙江省林木良种审定委员会的良种认定并命名[1]。前期的研究[2-4]表明:花粉败育是‘无籽’瓯柑无核的原因之一。植物雄性不育是指植物有性繁殖过程中不能产生正常的花药、花粉或雄配子的遗传现象。雄性不育可以分为细胞核雄性不育(NMS)及质核互作雄性不育(CMS)。关于植物CMS分子机制,普遍认为与呼吸链和三磷酸腺苷(ATP)合成基因的嵌合基因以及核糖核酸(RNA)的编辑等有关[5-7]。胡志勇等[8]成功地从温州蜜柑Citrus unshui克隆到了3个线粒体基因atp6,cob和coxⅡ的片段,它们分别编码F1F0-ATP合成酶的第6亚基、细胞色素b的亚基和细胞色素C氧化酶复合体的第2亚基,并通过实时荧光定量聚合酶链式反应(real-time PCR)技术证明了这3个基因在小孢子发育后期一直到形成成熟的花粉粒的过程中线粒体基因的表达减弱,即供给小孢子发育的能量可能在减少,进而导致了花粉的败育,由此推测温州蜜柑的雄性不育与呼吸作用及能量代谢的异常相关。Qiu等[9]在对‘无籽’椪柑Citrus reticulata ‘Seedless’差异表达基因的分析研究中,筛选到一些参与雄配子体发育的基因。本课题组从‘无籽’瓯柑和普通瓯柑的数字基因表达谱(digital gene expression,DGE)中筛选出2个脂类代谢相关的基因片段LTP(lipid transfer proteins)和LOX(lipoxygenase)(另文发表)。植物转脂蛋白(lipid transfer proteins,LTPs)是一类广泛存在于高等植物中的碱性小分子量蛋白质,生理功能包括参与角质的形成、脂代谢、生物膜的形成、体细胞发育、花粉与雌蕊的相互作用等,特别是在抗性信号转导和植物防御功能中的作用,成为近年来关注的焦点[10-11]。脂氧合酶(LOX)是一类广泛存在于动植物中的非血红素铁蛋白,参与脂肪酸氧化途径中的LOX途径,专一催化含有顺, 顺-1, 4-戊二烯结构的多元不饱和脂肪酸的加氧反应[12]。为进一步明确‘无籽’瓯柑花粉败育与其脂质代谢的相关性,本研究采用同源序列法,结合cDNA末端快速扩增(rapid amplification of cDNA ends,RACE)技术,克隆获得‘无籽’瓯柑脂转移酶基因(CsLTP)和脂氧合酶基因(CsLOX)的全长cDNA序列。通过实时荧光定量PCR(realtime PCR)技术,对2个目标基因mRNA表达量在花粉发育的不同时期进行实时定量分析,期望能够进一步解析‘无籽’瓯柑花粉败育的分子基础,为揭示‘无籽’瓯柑无核机制奠定基础。
HTML
-
供试材料采自浙江省丽水市林业科学研究院百果园内的普通瓯柑和‘无籽’瓯柑。
-
取‘无籽’瓯柑的花蕾,采用Trizol Kits(Invitrogen)进行总RNA抽提。经DNaseⅠ(1×16.67 nkat·L-1)(Promega)处理纯化后,按照SMARTTM cDNA Library Construction Kit(TaKaRa)操作,分别反转录获得3′-RACE和5′-RACE的模板cDNA。根据数字基因表达谱(DGE)测序分析的BLASTn结果,用Primer Premier 5.0软件分别在2个基因内部设计基因特异引物(GSP),并使用3′-RACE或5′-RACE的模板进行3′-RACE和5′-RACE。PCR扩增产物经分离、回收后,连接pMD18-T测序载体,转化大肠埃希菌Escherishia coli TG1,调取阳性克隆,由上海生工测序。
-
利用DNAMAN软件进行序列分析,预测目标cDNA编码的蛋白质,将氨基酸序列提交到美国国家生物技术信息中心(NCBI)进行BLAST比对,并用DNAMAN和ORF Finder进行ORF分析序列的开放阅读框和保守域,用ClustalX和MEGA4[13]软件对蛋白序列进行多重比对和系统进化分析。利用在线工具分析氨基酸序列的二级结构,物理性质及功能域,并对亚细胞定位进行预测。
-
2013年4月9日瓯柑花蕾可见时开始采集样品,采样1次·d-1,根据花蕾大小差异将‘无籽’瓯柑和普通瓯柑各分为5个时期:Ⅰ(花粉母细胞形成期)、Ⅱ(四分体时期)、Ⅲ(花粉粒形成期)、Ⅳ(花粉粒成熟期)和Ⅴ(花朵开放期)等5个时期的花蕾。其中,‘无籽’瓯柑(N):NⅠ,NⅡ,NⅢ,NⅣ,NⅤ;普通瓯柑(Y):YⅠ,YⅡ,YⅢ,YⅣ,YⅤ。液氮冷冻,于-80 ℃保存备用。
分别提取‘无籽’瓯柑和普通瓯柑各自不同时期花蕾的总RNA,采用PrimerScriptTM RT regent kit with gDNA Eraser(Perfect Real Time,TaKaRa)试剂盒反转录成cDNA,采用CFX96 Real-Time System实时荧光定量PCR仪(Bio-Rad),反应程序为95 ℃,30 s;95 ℃,5 s;57 ℃,30 s,39个循环;65~95 ℃,隔5 s上升0.5 ℃做融解曲线。重复3次·样品-1,取平均值。实时定量PCR引物分别为:CsLTP_F(3′-CACCATGCATCGGTTTCTT-5′)和CsLTP_R(3′-AGTTTGGCGGTCAGGTGT-5′);CsLOX_F(3′-CTGAAATTCGAACTGTGGGA -5′)和CsLOX_R(3′-CAATGTTGCATCTTGCAGTG-5′)。
1.1. 试验材料
1.2. ‘无籽’瓯柑CsLTP和CsLOX全长cDNA的克隆
1.3. 生物信息学分析
1.4. 实时荧光定量PCR分析
-
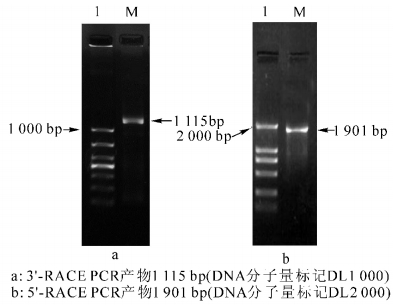
利用LTP+98(3′-ATGTGGGCAGGTGAGTGGCT-5′)和LTP-118(3′-GAGCCACTCACCTGCCCACAT-5′)作为特异引物,3′-RACE和5′-RACE完成后,PCR扩增产物在质量浓度为1%的琼脂糖胶上电泳检测,结果3′-RACE产物约为500 bp(图 1a),5′-RACE产物约为150 bp(图 1b);测序结果表明:它们分别为527 bp和154 bp。根据重叠序列将3′-RACE和5′-RACE的结果拼接,经测序,CsLTP cDNA全长667 bp,包含1个开放阅读框),编码215个氨基酸,分子量为1.26 kD,等电点(pI)为9.42,带负电荷的氨基酸残基总数(Asp+Glu)为3,总的正电荷氨基酸残基(Arg+Lys)总数为12,推测基因所编码的蛋白位置在细胞外。BLASTn结果显示:与脐橙Citrus sinensis(AF369931)和粗柠檬C. jambhiri(AB437259)的脂转移蛋白LTP相似性分别为95%和60%,序列提交GenBank,登录号为KF772872,命名为CsLTP。利用LOX-GSP3(3′-ATAGCAACCAATCGGCAACTCAGCGTG-5′)和LOX-GSP5(3′-GCTTGTCGAGCCAAAC CATTGA-5′)为特异引物,3′-RACE和5′-RACE完成后,PCR扩增产物在质量浓度为1%的琼脂糖胶上电泳检测。结果显示:3′-RACE产物约为1 000 bp(图 2a),5′-RACE产物约为2 000 bp(图 2b)。测序结果表明:它们分别为1 115 bp和1 901 bp。根据重叠序列将3′-RACE和5′-RACE的结果拼接,经测序,CsLOX cDNA全长2 915 bp,包含1个开放阅读框,编码895个氨基酸(图 3b),分子量为10.176 6 kD,等电点(pI)为6.09,带负电荷的氨基酸残基总数(Asp+Glu)为120个,总的正电荷氨基酸残基(Arg+Lys)总数为108个,推测该基因所编码的蛋白位置在细胞核内(76.0%),线粒体基质(50.2%),过氧化物酶体(35.8%),线粒体内膜上(22.2%)。BLASTn结果显示:与粗柠檬(AB039745),美洲黑杨Populus deltoids(DQ131179)的脂氧合酶相似性分别为99%和51%,序列提交GenBank,登录号为KF772871,命名为CsLOX。
-
根据NCBI CDS(http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)分析氨基酸序列,CsLTP包含nsLTP1保守功能域,属于非特异性脂转运蛋白1型(nsLTP1)亚科,属于AAI_LTSS超基因家族(图 4a),即α-淀粉酶抑制子(AAI)-脂转移(LT)-种子储藏(SS)蛋白家族。CsLOX包含植物脂氧合酶相关蛋白结构域,属于PLAT超基因家族(图 4b),即多囊蛋白1-脂氧合酶-α-毒素蛋白家族。
利用在线分析软件,选择Hphob./Kyte & Doolittle模式,对‘无籽’瓯柑CsLTP和CsLOX基因的蛋白疏水性进行分析预测。CsLTP基因蛋白的疏水指数为-2.00~2.82,约在第18~21位出现较强的疏水性,在第74~75位出现较强的亲水性;CsLOX基因蛋白的疏水指数从-2.57到2.46,约在第150,743,817位出现较强的疏水性,在第225,250,300,398,638,726,778位出现较强亲水性。
利用在线同源建模分析软件http://swissmodel.expasy.org/对蛋白质三级结构预测,得到了CsLTP和CsLOX的三级结构[14-16]。CsLTP蛋白经过几次折叠后主要以α短螺旋为主;CsLOX蛋白主要以α螺旋为主,包括长螺旋和短螺旋,并伴有一些β折叠,其间由一些转角等相互连接。
-
利用BLASTP对CsLTP和CsLOX预测蛋白的氨基酸序列进行同源性检索,并比对同源性较高的物种氨基酸序列进行系统进化树分析(图 5)。结果表明:CsLTP与龙眼Dimocarpus longan的亲缘关系较近(图 5a);而CsLOX与蓖麻Ricinus communis的亲缘关系较近(图 5b)。
-
根据花粉的发育过程,分别采集Ⅰ(花粉母细胞形成期),Ⅱ(四分体时期),Ⅲ(花粉粒形成期),Ⅳ(花粉粒成熟期),Ⅴ(花朵开放期)等5个时期的花蕾,通过荧光定量PCR技术分别检测‘无籽’瓯柑和普通瓯柑各自花粉发育的5个时期中CsLTP和CsLOX的表达水平,以CsLTP和CsLOX的实时表达量与各自在普通瓯柑的第Ⅰ时期的表达量的比值计算,以ACTIN(GU911361)为内参。以SPSS 14.0.软件进行0.05和0.01水平的统计分析。结果显示:CsLTP(图 6a)和CsLOX(图 6b)在‘无籽’瓯柑和普通瓯柑中均有表达,其中CsLTP的表达趋势在2个品种中相似,即花粉母细胞形成期表达量最高,而后随发育时期的进行显著下降,但‘无籽’瓯柑中CsLTP表达量的下降比普通瓯柑延后1个时期,并且从Ⅲ时期开始,‘无籽’瓯柑中CsLTP的表达量显著低于普通瓯柑;CsLOX在‘无籽’瓯柑中的表达趋势与普通瓯柑相反,即表达量从Ⅱ时期(四分体时期)开始显著下降,Ⅲ时期(花粉粒形成期)达到最低值,并极显著低于普通瓯柑,其余4个时期均显著高于普通瓯柑(特别是花粉母细胞形成期)。
2.1. ‘无籽’瓯柑CsLTP和CsLOX基因全长cDNA的克隆及序列分析
2.2. CsLTP,CsLOX基因的推导氨基酸序列分析
2.3. ‘无籽’瓯柑CsLTP和CsLOX的系统进化树分析
2.4. CsLTP和CsLOX的荧光定量表达
-
脂类物质对于动植物细胞结构和功能调节具有非常重要作用[17]。本研究结合‘无籽’瓯柑与普通瓯柑的差异表达基因筛选,克隆了与脂类代谢相关的脂转移酶基因CsLTP和脂氧合酶基因CsLOX。甜橙Citrus sinensis基因组测序[18]已经完成,对现有数据库()进行Blastn检索,发现CsLTP在甜橙的6号染色体可能存在同源序列,CsLOX则在3,7,9号染色体可能存在拷贝。研究发现:植物转脂蛋白(LTP)与动物非特异性脂转移蛋白(nonspecific lipid-transfer protein,nsLTP)非常相似,能与磷脂和脂肪酸结合,进行膜间的脂类转运[19]。LTP在植物的生长发育以及抗逆等生命活动中具有广泛的生物学功能,近年来LTP在生殖发育中的重要作用也开始引起人们的广泛关注[20-21],特别是在小孢子的各发育时期中油脂等营养物质的传递与积累,以及花粉壁等结构物质的形成等方面发挥着重要的作用[22-28]。本研究中,CsLTP从花粉粒形成期开始表达量显著下降,降低了生殖细胞对周围营养物质的吸收效率,无法为小孢子发育提供营养,因而花粉粒不能正常发育。
脂氧合酶是动植物体内不饱和脂肪酸代谢的关键酶[29-30]之一,参与茉莉酸(JA)的生物合成,影响雄蕊花丝的伸长、花药开裂以及花粉生活力,甚至是雄蕊的形态发育[31]。LOX在小麦Triticum aestivum光敏雄性不育系BS366中的表达较低[32],引起花药开裂异常。何永明等[33]发现水稻Oryza sativa OsLOX-RCI1的表达水平在颖花开放时明显上调,开放后又下降。本研究中,CsLOX的表达在‘无籽’瓯柑花粉粒形成期急剧下降,则可能引起花粉粒成熟进程异常,进而导致花粉粒不能正常的发育。
前期研究[3]表明:‘无籽’瓯柑的花药自然散粉率极低,在花粉粒成熟期,‘无籽’瓯柑花粉粒内大多中空,其绒毡层附近有大量脂类物质残存。本试验中,‘无籽’瓯柑CsLTP和CsLOX的表达水平都在花粉粒形成期开始锐减,绒毡层细胞内的营养物质无法被花粉粒充分利用,严重影响了花粉粒的成熟进程,最终导致花粉败育。
-
从‘无籽’瓯柑中克隆到的脂转移酶基因CsLTP和脂氧合酶基因CsLOX序列分析表明:它们具有植物转脂蛋白(LTPs)和脂氧合酶(LOXs)的特征结构域。两者在花粉发育不同时期转录本的丰度变化表明:‘无籽’瓯柑花粉粒发育初期脂类物质转运效率的下降,以及花粉粒成熟进程的中止,可能成为绒毡层脂类营养物质滞留,花粉发育关键时期营养不良,并最终成为‘无籽’瓯柑花粉败育的原因之一。
















 DownLoad:
DownLoad: