-
放牧是天然草地最主要的利用与管理方式,影响草地生态系统植被结构和功能[1],并可能引起草地养分状况的变化,导致草地荒漠化和贫瘠化。适度放牧能够增加草地生态系统的生物多样性和生产力[2],过度放牧是威胁草地生态系统生物多样性和生产力的主导因素,特别是在干旱和半干旱地区[3-4]。放牧对草地植物最大的影响在于牲畜采食和践踏造成的机械损伤。诸多研究表明:机械损伤可导致植物体内活性氧类(reactive oxygen species,ROS)水平显著升高,抗氧化系统由于ROS的影响表现出不同的响应机制[5-6]。ROS是植物正常生长过程进行有氧代谢的副产物,受到生物胁迫或非生物胁迫时,打破植物体内ROS生成与清除之间的动态平衡引起ROS积累,导致植物受到伤害[7-8]。过氧化氢(hydrogen peroxide,H2O2)和超氧阴离子自由基(superoxide radical,O2·-)是最重要的ROS,其中O2·-是形成其他ROS的前体,作为前体比其本身对植物的伤害更具有毒性[9]。ROS的积累可加速细胞膜质过氧化,使细胞内丙二醛(malondialdehyde,MDA)水平升高,抑制抗氧化酶活性和抗氧化剂的水平,破坏生物膜的结构和功能[10]。同时,ROS作为信号分子,介导植物体内抗氧化酶基因的表达,增强超氧化物歧化酶(superoxide dismutase,SOD),过氧化氢酶(catalase,CAT)和过氧化物酶(peroxidase,POD)活性消除ROS的影响[11]。除此之外,适度的胁迫环境可以启动植物体内抗坏血酸-谷胱甘肽循环(ascorbate-glutathione cycle,AsA-GSH cycle)。该循环中的抗坏血酸(ascorbate,AsA)和谷胱甘肽(glutathione,GSH)相耦联起作用,有效清除由于逆境胁迫而过多产生的ROS[12-14]。以羊草Leymus chinensis和大针茅Stipa grandis为主的内蒙古草原典型草原,在连续多年过度放牧压力下均可退化演替为以冷蒿Artemisia frigida为主的单一优势种群[15-17]。近年来,对冷蒿的研究集中在冷蒿挥发性有机化合物[18]、群落结构特征[19-20]以及对土壤低磷环境适应性[21]等方面,关于冷蒿的耐牧抗损伤机制鲜见报道。本研究从生理生化的角度出发,拟解决以下问题:①揭示机械损伤后冷蒿体内ROS产生状况;②阐明机械损伤对冷蒿体内抗氧化酶活性和AsA-GSH循环的影响;③探讨冷蒿叶片和根系对机械损伤响应的差异及相关性。上述研究旨在揭示冷蒿在不同牧压下的耐损伤机制,探讨冷蒿在不同机械损伤下的适应对策;同时为退化草地恢复、草地生物多样性保护以及可持续发展利用提供理论依据。
HTML
-
供试材料冷蒿采自内蒙古自治区锡林浩特毛登牧场内蒙古大学草地生态学研究基地,地理位置为44°10′2″N,116°28′56″E,海拔1 160 m。全年平均气温为-0.4 ℃,最冷月(1月)平均温度-22.3 ℃,最热月(7月)平均气温18.8 ℃,≥0 ℃年积温为2 410.0 ℃,≥10 ℃积温为1 597.9 ℃,无霜期91 d,植物生长期为150 d左右。年降水量为365.6 mm,降水多集中在6-8月,占年降水量的80%左右。本研究区域主要草原植物为羊草,糙隐子草Cleistogenes squarrosa,克氏针茅Stipa krylovii,大针茅,防风Saposhnikovia divaricata,冷蒿,瓣蕊唐松草Thalictrum petaloideum和阿尔泰狗哇花Heteropappus altaicus等。土壤为栗钙土。
-
2014年6月中旬在采样地选取自然条件下生长健壮的返青期冷蒿植株,栽植于盛有采样地原土的花盆中(直径18 cm,高20 cm),1丛·盆-1。盆栽苗置于浙江农林大学实验室温室中,自然光照,相对湿度为30% ± 2%,白天温度为(25 ± 2) ℃,夜晚温度为(20 ± 2) ℃。缓苗生长20 d后进行实验处理。选取株高一致、生长良好、无病虫害冷蒿12盆,随机分为4组,为模拟动物采食对冷蒿的伤害,试验以剪刀损伤冷蒿地上枝叶的方式处理冷蒿植株,损伤冷蒿地上枝叶1/4为轻度、损伤1/3为中度、损伤1/2为重度、不作处理为对照。3盆·处理-1,独立重复1个·盆-1。在处理后6 h,对冷蒿叶片和根系分别取样,地上部分剩余枝叶全部剪碎混匀后取样,根系用清水洗去附着土壤,再用蒸馏水冲洗擦干后将整个根系剪碎混匀后取样。样品用液氮速冻后放置于-80 ℃低温冰箱内保存。
-
O2·-测定参照SHAH等[14]的氮蓝四唑法;过氧化氢测定参照RAI等[22]的方法;MDA测定参照HODGES等[23]的方法。
-
酶液提取方法:取0.2 g冷冻样品液氮研磨,加5 mL磷酸缓冲溶液(50 mmol·L-1,pH 7.8)匀浆,10 000 g离心10 min(4 ℃)。上清液用于SOD,POD和CAT活性的测定。SOD活性测定参照GIANNOPOLITIS等[24]的方法; CAT和POD活性测定参照KUMARI等[25]的方法。
-
AsA和GSH测定参照RAI等[26]的方法。
-
抗坏血酸过氧化物酶(ascorbate peroxidase,APX)活性测定参照NAKANO等[27]的方法;谷胱甘肽还原酶(glutathione reductase,GR)活性测定参照SCHAEDLE等[28]的方法;单脱氢抗坏血酸还原酶(monodehydroascobate reductase,MDHAR)活性测定参照HOSSAIN等[29]的方法;脱氢抗坏血酸还原酶(dehydroascorbate reductase,DHAR)活性测定参照DOULIS等[30]的方法。
-
所有的数据均为3次重复的平均值±标准误差,利用Origin 8软件(美国OriginLab公司)进行统计分析和作图。统计方法采用One-Way ANOVA进行检验,并进行Tukey多重比较(P<0.05)。采用Two-Way ANOVA分析冷蒿组织×机械损伤处理之间相互作用的影响。
1.1. 采样地概况
1.2. 试验材料处理
1.3. 方法
1.3.1. ROS和MDA质量摩尔浓度测定
1.3.2. 抗氧化酶活性测定
1.3.3. AsA和GSH质量摩尔浓度测定
1.3.4. AsA-GSH循环相关酶活性测定
1.4. 数据处理
-
随着损伤程度的增加,冷蒿叶片中O2·-水平逐渐升高,重度损伤下达到对照2.1倍(表 1);在中度和重度损伤下,冷蒿根系O2·-水平分别为对照的2.4倍和2.8倍。冷蒿过氧化氢水平随着处理强度的增强而增加,在轻度、中度和重度处理下,冷蒿叶片过氧化氢水平与对照相比显著升高21.0%,27.8%和41.7%;冷蒿根系过氧化氢水平在中度和重度处理下分别比对照显著增加27.0%和56.0%。在轻度、中度和重度处理下,冷蒿叶片的MDA摩尔质量浓度分别比对照显著增加28.7%,82.8%和107.7%,冷蒿根系MDA摩尔质量浓度分别比对照增加30.5%,48.1%和50.1%。从各处理间差异显著性分析结果来看,轻度处理后,冷蒿叶片H2O2和MDA摩尔质量浓度显著高于对照,说明冷蒿在机械损伤影响下遭受了ROS的伤害;冷蒿叶片的O2·-水平与对照相比无显著差异,可能是由于O2·-非常活泼,作为其他ROS的前体被转化。虽然冷蒿叶片和根系的ROS和MDA水平都随损伤程度的增加而升高,但冷蒿叶片中ROS和MDA摩尔质量浓度在中度和重度损伤之间的差异不显著,说明冷蒿的ROS产生被抑制。冷蒿根系的ROS和MDA摩尔质量浓度显著低于叶片。这可能是由于机械损伤直接作用于冷蒿叶片,对根系的影响较小。
植物组织 机械损伤 O2·-(A540)/(min•-1g-1) 过氧化氢(μmol•g-1) MDA/(μmol•g-1) 叶片 对照(未处理) 24.72±3.94B 22.86±0.47C 10.05±0.46C 轻度 29,17±0,93B 27.67±1.01B 12.93±0.53B 中度 43.89±3.94A 29.23±0.67AB 18.37±0.22A 重度 51.67±3.63A 32.39±0.94A 20.88±1.53A 平方和 组间自由度 1 422.45 141.79 220.23 组内自由度 88.31 4.97 4.54 根系 对照(未处理) 11.67±1.48b 16,37±0,56 c 8.06±1.2b 轻度 19.17±0.44b 18.39±0.17bc 10.74±1.01a 中度 28,06±2,28a 20.79±0.94b 11.94±0.37a 重度 33.06±1.73 a 25.52±0.10a 12.13±0.49a 平方和 组间自由度 809.43 139.94 31.69 组内自由度 118.17 37.25 9.01 机械损伤的影响 ** ** ** 植物组织的影响 ** ** ** 植物组织与损伤的交互作用 不显著 不显著 ** 说明:同列不同大写字母表示冷蒿叶片的差异显著性,同列的不同小写字母表示冷蒿根系的差异显著性。 Table 1. Effect of mechanical damage on O2·-, H2O2 and MDA content in Artemisia frigida
-
冷蒿的抗氧化酶活性随处理强度的增加而增强(图 1)。在轻度、中度和重度处理下,冷蒿叶片SOD活性与对照相比差异显著,分别为对照的1.4,2.3和3.4倍;轻度处理下,冷蒿根系的SOD活性与对照相比无显著差异,中度处理下根系SOD活性比对照显著增加80.1%,但与轻度相比差异不显著,重度处理下根系SOD活性比对照显著增加140.0%,但与中度相比差异不显著(图 1A);且在各个处理下,冷蒿叶片的SOD活性显著高于根系,这可能由于机械损伤直接作用于冷蒿叶片,对叶片和根系的抗氧化系统影响有所差异造成的。
冷蒿的CAT活性变化总趋势是随处理强度的增大逐渐增强(图 1B)。但冷蒿叶片的CAT活性在轻度处理与对照之间差异不显著,中度处理与轻度处理之间差异不显著,重度处理与中度处理之间差异不显著;冷蒿根系的CAT活性3个处理之间的差异均不显著;叶片的CAT活性显著高于根系,说明冷蒿叶片和根系的抗氧化系统对机械损伤的响应是不同的。
在轻度、中度和重度处理下,冷蒿叶片的POD活性分别比对照增加30.6%,87.3%和132.8%;冷蒿根系POD活性分别比对照增加59.7%,87.8%和116.7%(图 1C);冷蒿叶片和根系在各个处理间差异显著,增幅明显,说明POD因机械损伤的刺激活性增强,在消除过氧化氢中起重要的作用。
-
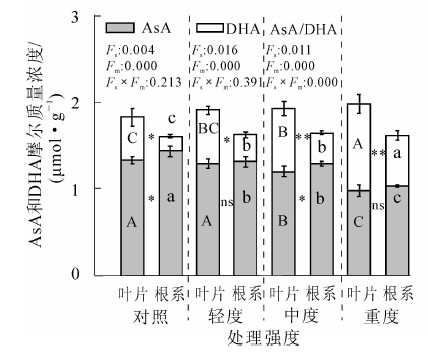
冷蒿叶片和根系的AsA摩尔质量浓度随损伤程度的增加逐渐降低,而脱氢抗坏血酸(dehydroascorbate,DHA)水平随机械损伤程度的增加逐渐增加,AsA/DHA持续下降(图 2)。在重度处理下,DHA摩尔质量浓度高于AsA,ρAsA/ρDHA比对照降低64.3%。在不同损伤程度下,叶片的DHA摩尔质量浓度显著高于根系。
-
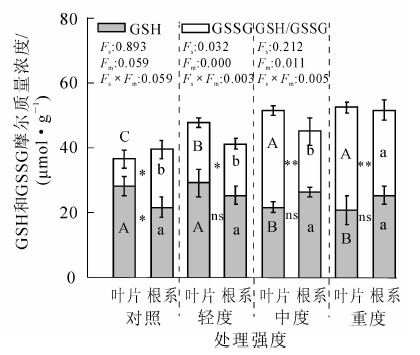
冷蒿叶片和根系的总谷胱甘肽水平随损伤程度的增加而缓慢增加(图 3)。轻度处理下,冷蒿叶片GSH摩尔质量浓度与对照相比差异不显著,中度处理下,叶片GSH摩尔质量浓度与对照相比显著下降23.3%;与叶片不同,冷蒿根系GSH水平在不同处理间的差异不显著。除对照外,在轻度、中度和重度损伤强度下,冷蒿叶片和根系之间的GSH水平差异不显著。冷蒿叶片的还原型谷胱甘肽(GSSG)摩尔质量浓度随机械损伤强度的增加而显著增加,在轻度、中度和重度处理下分别为对照的2.2,3.6和3.7倍,差异显著;冷蒿根系GSSG摩尔质量浓度重度处理下比对照显著增加46.6%。随机械损伤强度的增加,在中度和重度处理下,冷蒿叶片与根系之间的GSSG摩尔质量浓度差异极显著。冷蒿叶片的ρGSH/ρGSSG随机械损伤强度的增加持续下降,在轻度、中度和重度处理下分别比对照降低56.8%,80.2%和82.2%,与轻度相比,中度和重度处理下的叶片ρGSH/ρGSSG增幅明显减缓;冷蒿根系的ρGSH/ρGSSG随机械损伤强度的增加小幅增加后降低,重度处理下根系ρGSH/ρGSSG比对照减少21.6%。根系的ρGSH/ρGSSG在不同机械损伤强度之间差异不显著。
-
随着损伤处理程度增加,冷蒿叶片和根系的APX活性逐渐增加(图 4A),在重度处理下与对照相比的增幅分别达到94.0%和132.8%;冷蒿叶片的APX活性显著高于根系。
冷蒿根系的GR活性随损伤程度的增加而增强(图 4B);在中度处理下,冷蒿叶片的GR活性高于其他处理,与对照相比的增幅为97.4%。在轻度和中度处理下,叶片与根系之间的GR活性差异极显著;重度处理下,冷蒿叶片和根系之间的GR活性无显著差异。
在中度处理下,冷蒿叶片和根系DHAR活性高于其他处理且差异极显著,分别为对照的1.3倍和1.9倍(图 4C);在重度处理下,冷蒿叶片和根系的DHAR活性与对照相比增幅分别为88.5%和106.7%,且叶片与根系之间差异显著。
随损伤程度的增加,冷蒿叶片和根系的MDHAR活性逐渐升高(图 4D)。在重度处理下与对照相比的增幅分别为88.5%和106.7%,冷蒿叶片的MDHAR活性显著高于根系。
2.1. 机械损伤对冷蒿O2·-,过氧化氢和MDA质量摩尔浓度影响
2.2. 机械损伤对冷蒿抗氧化酶活性的影响
2.3. 机械损伤对冷蒿AsA水平的影响
2.4. 机械损伤对冷蒿GSH水平的影响
2.5. 机械损伤对冷蒿AsA-GSH循环相关酶活性的影响
-
诸多研究表明:机械损伤可导致植物体内ROS摩尔质量浓度显著升高,抗氧化系统由于ROS的影响表现出不同的响应机制[5-6]。胁迫往往使ROS和MDA摩尔质量浓度升高[31],抗氧化酶活性增强[32]。冷蒿试验结果表明:随机械损伤强度的增加,冷蒿体内O2·-和MDA摩尔质量浓度升高,冷蒿SOD酶活性逐渐增强,由于SOD的作用,O2·-增幅明显减缓;冷蒿体内过氧化氢水平随机械损伤强度的增加逐渐升高,过氧化氢的积累刺激冷蒿体内CAT和POD应激反应使活性增强,由于酶系统的作用,过氧化氢的增幅逐渐减缓。总之,冷蒿在抗氧化酶防御系统作用下,有效消除因机械损伤过量积累的ROS,使重度处理下的ROS水平与中度相比无显著差异。由于机械损伤处理直接作用于冷蒿的地上部分,对叶片的抗氧化系统影响更大,因此冷蒿叶片的ROS水平和酶活性明显高于根系。
维持细胞内较高的ρGSH/ρGSSG和ρAsA/ρDHA比值有利于维护植物体内的氧化还原环境,减少胁迫所造成的ROS伤害[33]。MAHALINGAM等[34]认为AsA再生系统受到胁迫产生ROS的不利影响,导致AsA摩尔质量浓度下降。本试验表明:随着机械损伤强度的增加,冷蒿叶片中AsA和GSH摩尔质量浓度下降,DHA和GSSG摩尔质量浓度上升,与罗娅等[35]研究结果一致;在轻度和中度处理下,冷蒿体内ρAsA/ρDHA与对照相比增幅不显著,可能是因为冷蒿体内的AsA再生酶(MDHAR和DHAR)活性增加,维持了AsA的摩尔质量浓度;与李晓云等[36]的研究结果相吻合。AsA-GSH循环清除过氧化氢的能力依赖于DHAR和GR对还原型AsA和GSH库的维护作用[37-38]。重度处理下,冷蒿叶片AsA和GSH因清除过氧化氢而被消耗[39];且冷蒿叶片GR和DHAR活性与中度处理相比有所下降,不能提供充足GSH来减少DHA,使ρAsA/ρDHA降低。单长卷等[40]研究认为,严重胁迫下,冰草叶片内AsA-GSH循环相关酶活性呈降低趋势。冷蒿根系ρAsA/ρDHA和ρGSH/ρGSSG在重度处理下显著高于叶片,可能是由于ROS为了传递应激反应的信号而进行的精密调制并引导冷蒿根系AsA-GSH系统新平衡[41-42],因此在损伤胁迫下冷蒿根系GSH库在避免机械损伤中发挥更重要的作用。总之,冷蒿体内的AsA-GSH循环通过增强相关酶活性维持抗氧化剂的再生,在轻度和中度损伤胁迫下有效消除过氧化氢的同时保证细胞内氧化还原的平衡,重度胁迫下由于根系GSH库的维护作用,引导了冷蒿体内AsA-GSH系统新平衡。
综上所述,随着机械损伤强度的增加,冷蒿叶片中的O2·-和过氧化氢摩尔质量浓度升高,MDA摩尔质量浓度升高;因机械损伤的刺激,冷蒿叶片内的抗氧化防御酶活性逐渐增强;AsA-GSH 循环相关酶活性随机械损伤强度的增加而增强,通过维持非酶抗氧化剂AsA和GSH的再生,保障冷蒿体内过氧化氢产生与消除之间的相对平衡;冷蒿根系的抗氧化各项指标在机械损伤影响下的变化趋势与叶片相似,但是变化幅度低于叶片。说明冷蒿可以在酶系统与非酶系统的共同作用下,使O2·-和过氧化氢摩尔质量浓度的增幅明显减缓,有效消除机械损伤产生的ROS伤害,具有较强的抗氧化能力。














 DownLoad:
DownLoad: