-
氮元素是植物生长所需要的重要的大量元素元素之一,在植物生长过程中起到关键作用。菌根真菌氮元素的吸收利用研究被人们所关注[1-2]。通过接种,菌根真菌能有效地促进植株对氮素的吸收利用[3-5]。特殊内生菌根杜鹃花类菌根(ericoid mycorrhiza,ERM),对杜鹃花类植物克服恶劣环境、加强养分吸收和提高生长量起着重要作用[6-8]。ERM菌根共生体能够帮助杜鹃花科Ericaceae植物缓解环境压力,改善营养获取方式,吸收复杂有机态的氮[9-10]。张春英等[11]报道了云锦杜鹃Rhododendron fortunei幼苗接种形成菌根苗后,能提高对各种氮源营养的吸收,直接表现为增加植株的干物质积累。桃叶杜鹃Rhododendron annae为常绿灌木,成年树冠为圆球形,花色丰富,花期为晚春,主要分布于贵州海拔1 800~1 830 m高山地区,在城市园林建设中具有较高的开发应用潜力。野生桃叶杜鹃菌根结构复杂且侵染率较高[12],通过接种,菌根真菌提高了菌根苗叶片的叶绿素含量,增强光合性能,促进了碳同化的高效运转和有机物的积累,提高了菌根苗同源激素含量,最终表现为菌根苗生物量的增加[13-14]。笔者研究ERM菌株接种后对桃叶杜鹃菌根苗硝酸还原酶(NR)活性和氮元素积累的影响,为桃叶杜鹃等高山常绿杜鹃菌根化园林栽培应用提供理论依据与技术支持。
-
12株供试菌株从野生桃叶杜鹃根部分离得到,编号为TY02,TY07,TY12,TY14,TY18,TY19,TY21,TY24,TY29,TY34,TY35和TY41[14]。分离菌株培养液为PDA培养基,置于28 ℃摇床上黑暗振荡160 r·min-1,培养15 d打碎并制成液体菌剂备用。
-
试验苗为实验室通过种子和土壤灭菌后培养的2年生实生桃叶杜鹃苗。育苗基质采自百里杜鹃风景区桃叶杜鹃林下腐殖质土,土样带回实验室进行土壤理化性质试验。供试土壤理化性质如下:pH 4.8,有机质44.1 g·kg-1,全氮1.5 g·kg-1,全磷0.2 g·kg-1,碱解氮269.0 mg·kg-1,速效磷7 mg·kg-1,速效钾206. mg·kg-1。
-
育苗基质经121 ℃高温蒸汽灭菌2 h,自然冷却后80 ℃烘2 h,然后放置室温后装入花盆(规格24 cm × 16 cm × 20 cm)。花盆装基质3 kg·盆-1,移植无菌桃叶杜鹃幼苗1株·盆-1。接种处理采用单因素完全随机设计,试验设13个处理(含对照ck),5盆·处理-1,重复3次。移栽3 d后每株苗根部各施入真菌液体菌剂10 mL,以浇不含菌的PDA培养液为对照,以后隔7 d浇1次菌液,连续浇3次结束,试验处理严格保证土壤微生物区系一致。接种后随机放置贵州大学林学院苗圃温室进行培养,按照常规育苗方法进行管理。
-
接种培养180 d后,随机取出幼苗10株·盆-1,流水洗净后吸干水分,在105 ℃杀青20 min,置于80 ℃烘箱48 h烘干至恒量,取出后分别称量地下部分(根)和地上部分(叶、茎和芽)的干质量,计算总生物量。采用Phillips等[15]的改进法统计侵染率。测定苗分地下根系及地上部分全氮采用H2SO4-H2O2消煮-半微量蒸馏法。用分光光度计测定法测定硝酸还原酶(NR)活性。以上测试重复3次。
-
运用Excel 2003记录及绘图;使用SPSS 11.5软件进行统计分析,采用单因素方差分析(one-way ANOVA),应用Duncan多重分析法进行方差检验(P=0.01)。
-
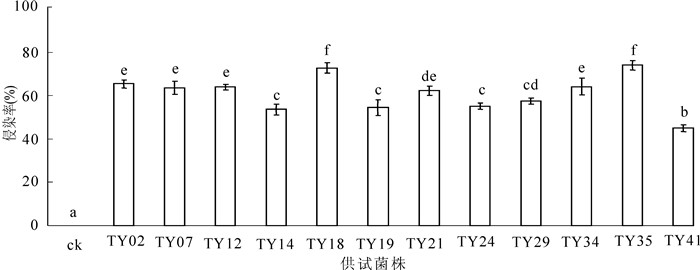
从图 1可以看出:接种苗菌根侵染率达到45.0%~74.1%。不同菌株对幼苗的侵染率表现出差异性,说明不同菌株与幼苗之间有相互选择性。
-
从表 1可以看出:不同菌株侵染对桃叶杜鹃地上部分和地下根系生物量的影响存在显著差异,接种苗地上部分干质量比对照增加2.5%~99.7%,地下部分干质量增加6.0%~27.3%,接种苗总生物量比对照增加3.9%~61.2%。从植株外观上也明显看出接种对宿主生物量的影响较大(图 2)。
表 1 不同ERM菌株接种对桃叶杜鹃幼苗干质量的影响(平均值±标准差)
Table 1. Effects of inoculation with different ericoid mycorrhizal strains on growth of Rhododendron annae seedling (mean±SD)
处理 地上部分生物量 地下部分生物量 总生物量 干质量/g 增幅/% 干质量/g 增幅/% 干质量/g 增幅/% ck 0.213±0.003 A 0.0 0.183±0.027 A 0.0 0.396±0.030 A 0.0 TY02 0.335±0.001 D 57.3 0.210±0.011 ABCDE 14.4 0.544±0.010 E 37.4 TY07 0.267±0.004 C 25.3 0.212±0.008 DCDE 15.8 0.479±0.004 D 21.0 TY12 0.365±0.001 E 71.4 0.223±0.004 CDE 21.6 0.588±0.004 E 48.5 TY14 0.219±0.007 AB 2.7 0.203±0.006 ABCD 10.7 0.411±0.010 AB 3.8 TY18 0.425±0.009 G 99.7 0.214±0.012 BCDE 16.5 0.639±0.021 F 61.4 TY19 0.275±0.006 C 29.3 0.197±0.002 ABC 7.6 0.473±0.005CD 19.4 TY21 0.260±0.002C 21.9 0.206±0.006 ABCDE 12.5 0.466±0.004 CD 17.7 TY24 0.235±0.005 B 10.3 0.201±0.010 ABCD 9.8 0.436±0.008 BC 10.1 TY29 0.397±0.001 F 86.4 0.233±0.015 CD 27.3 0.630±0.014 F 59.1 TY34 0.259±0.030 C 21.6 0.204±0.006 ABCD 11.3 0.463±0.033 CD 16.9 TY35 0.377±0.002 EF 76.8 0.227±0.006 DE 23.8 0.604±0.008 EF 52.5 TY41 0.218±0.002 AB 2.5 0.194±0.002 AB 6.0 0.413±0.004 AB 4.3 说明:同列不同大写字母表示差异达到极显著水平(P < 0.01)。 -
由图 3可见:接种苗地上部分叶的氮质量分数均高于对照,除TY19,其他菌根差异极显著(P < 0.01),较未接种处理,接种幼苗叶部氮质量分数提高2.8%~50.6%。地下根系氮质量分数除TY19低于对照外(差异不显著),其他处理均高于对照,且差异极显著(P < 0.01),接种苗根部氮质量分数提高2.0%~40.3%。这表明通过接种处理后,增强了幼苗对氮的吸收,但不同菌株之间的影响有差异。

图 3 不同ERM菌株接种对桃叶杜鹃幼苗氮的影响
Figure 3. Effect on nitrogen content in Rhododendron annae seedling inoculationed with different strains of ericoid mycorrhizae
从图 4可以看出:不同菌株接种提高了幼苗硝酸还原酶活性,且各处理差异极显著(P < 0.01)。接种苗根部硝酸还原酶活性除TY41外,高于对照0.9%~29.3%,叶部硝酸还原酶活性除TY41和TY24外,高于对照6.5%~43.9%。不同器官硝酸还原酶活性表现为根系 > 叶片。
-
从表 2相关性分析可知:侵染率与地下部分干质量呈极显著正相关,与总生物量和叶部硝酸还原酶活性呈显著相关关系。这表明侵染率的高低直接影响桃叶杜鹃幼苗的生物量积累、硝酸还原酶活性。根部及叶部硝酸还原酶活性与地上干质量、总生物量有相关性,但叶部的相关性大于根部;叶部硝酸还原酶活性与叶部氮质量分数呈极显著正相关关系。
表 2 菌根侵染率、生物量、氮吸收量及硝酸还原酶活性Pearson相关系数
Table 2. Pearson correlation coefficient of mycorrhizal colonize, biomass, nitrogen content and nitrate reductase activity
项目 侵染率 地下部分干质量 地下部分干质量 总生物量 根部氮量分数 叶部氮质量分数 根部硝酸还原酶活性 叶部硝酸还原酶活性 侵染率 1 地下部分干质量 0.706** 1 地上部分干质量 0.584 0.831** 1 总生物量 0.613* 0.872** 0.996** 1 根部氮质量分数 0.595 0.308 0.119 0.144 1 叶部氮质量分数 0.565 0.451 0.490 0.496 0.360 1 根部硝酸还原酶活性 0.515 0.505 0.647* 0.642* 0.207 0.423 1 叶部硝酸还原酶活性 0.579* 0.653 0.815** 0.806** 0.194 0.272** 0.763** 1 说明:*差异达到显著水平(P < 0.05);**差异达到极显著水平(P < 0.01)。 -
研究桃叶杜鹃菌根氮效应极有意义,因为对于杜鹃花科植物来说,土壤中能供给此类植物的氮养分多数以有机态形式存在,因此在杜鹃花科植物氮养分吸收中菌根共生体起到了重要的作用。对于杜鹃花类菌根吸收利用氮素的原因可能有:①由于杜鹃花生长土壤为酸性土,pH值较低,土壤中铵盐比硝酸盐多,氮素矿物化过程缓慢,氨离子在土壤中的流动性不大,其扩散速度一般会小于根的吸收速度,所以,杜鹃花类植物的吸收根周围会形成一个缺氮区。根外菌丝越过缺氮区能够把远处的铵盐吸收到菌根中来。②ERM真菌的吸收系统对氮有很高的亲和力,能在含氮量少的低浓度溶液中吸收氨离子。③利用植物所不能利用的或很少利用的有机氮源[16]。
本研究得出桃叶杜鹃幼苗接种后,地上部分与地下部分的氮含量均显著增加,说明菌根真菌促进了幼苗对氮的吸收与利用。这与云锦杜鹃菌根苗试验结果相似[11, 17]。宋福强等[18]认为菌根改善宿主植物的氮营养状况的作用主要表现在2个方面,一是根外菌丝直接吸收土壤氮的作用;二是菌根首先改善植物的磷营养状况,进而促进植物对土壤氮的吸收作用。菌根真菌提高植物对氮、磷元素的吸收报道很多,桃叶杜鹃接种后根部与叶部的磷含量均比对照增加且大于氮的吸收强度(文章待发),有些宿主吸收氮的强度大于磷,这可能与不同宿主及不同菌根有关,因此,菌根是否先通过改善植物的磷营养状况,进而促进植物对土壤氮的吸收有待试验和验证。施氮情况下,菌根宿主云锦杜鹃[11]、刺槐Robinia pseudoacacia[19]生物量增加,吸氮量及硝酸还原酶活性发生变化。由于本研究所有基质均一致,未额外添加不同氮源,所以菌根真菌吸收和利用氮源情况及与宿主的共生机理关系还需进一步深入研究。
Foissner等[20]采用激光共聚焦的方法发现真菌激发子诱导烟草表皮细胞浆和叶绿素的一氧化氮迸发。一氧化氮影响植物的生长发育等生理代谢过程,而硝酸过原酶硝酸还原酶普遍具有合成一氧化氮的功能。Kaiser等[21]发现,硝酸还原酶催化NO2-产生的一氧化氮在脱落酸诱导气孔关闭的过程中有重要的作用,通过对脱落酸钝感型等突变体的研究发现,由硝酸还原酶催化生成的一氧化氮是脱落酸诱导气孔关闭所必需。在相关性分析数据中,桃叶杜鹃菌根苗根部硝酸还原酶与地下根部生物量相关,与地上部和总生物量显著相关,叶部硝酸还原酶与地上根部生物量相关,与地上部和总生物量极显著相关。接种后菌根苗体内的脱落酸含量增加[15],这说明生物量的增加与接种真菌具有很大关系。硝酸还原酶是一种诱导酶,同时是一种调节酶和限速酶,存在于植物的根部质体中和叶绿体中,菌根真菌是如何激活和诱导硝酸还原酶,菌根植物硝酸还原酶是如何合成一氧化氮等分子机制有待深入研究。
Nitrate reductase activity and N absorption of Rhododendron annae seedlings with ericoid mycorrhiza inoculation
-
摘要: 研究12株杜鹃花类菌根(ericoid mycorrhiza, ERM)菌株对2年生桃叶杜鹃Rhododendron annae幼苗生长及矿质元素氮积累的影响。结果表明:接种ERM真菌的植株根系均被有效地感染, 不同菌株促生效应显著, 接种ERM真菌显著增加幼苗地上、地下部分及总生物量。与对照相比, ERM菌株接种后, 显著提高了接种幼苗氮质量分数和硝酸还原酶(NR)活性, 且各处理差异极显著(P < 0.01)。接种苗根部氮质量分数提高了2.0%~40.3%, 叶部提高了2.8%~50.6%。接种苗根部硝酸还原酶活性除菌株TY41外, 其余处理高于对照0.9%~29.3%, 叶部除菌株TY41和菌株TY24外, 高于对照6.5%~43.9%。不同器官硝酸还原酶活性表现为根系大于叶片。Abstract: To measure growth and N accumulation in two-year-old Rhododendron annae seedlings, twelve ericoid mycorrhiza (ERM) fungal strain isolates were used for inoculation. The test design set 13 treatments (including ck), each disposed 5 trees, 3 times repeated. The inoculated method was soil inoculation. Results showed that ERM fungi colonized the seedling roots improving biomass of seedling aboveground and belowground parts. Compared to uninoculated controls, inoculation with different ERM strains showed a highly significant increase (P < 0.01) for N content in the leaves and roots of seedlings as well as nitrate reductase (NR) activity. Root N content increased 2.0%-40.3%, and foliar N increased 2.8%-50.6%. Except for TY14, root NR activity was higher than the control (0.9%-29.3%), and except for TY41 and TY24, foliar NR activity was higher than the control 6.5%-43.9%. NR activity in different organs showed root>leaf. ERM fungal strain promoted the growth, NR activity and N content of R. annae seedlings, which meant to promote seedling nitrogen absorption and utilization. Effects of mycorrhiza varied by different ERM fungi strain.
-
Key words:
- botany /
- ericoid mycorrhiza(ERM) /
- nitrogen /
- nitrate reductase /
- Rhododendron annae
-
表 1 不同ERM菌株接种对桃叶杜鹃幼苗干质量的影响(平均值±标准差)
Table 1. Effects of inoculation with different ericoid mycorrhizal strains on growth of Rhododendron annae seedling (mean±SD)
处理 地上部分生物量 地下部分生物量 总生物量 干质量/g 增幅/% 干质量/g 增幅/% 干质量/g 增幅/% ck 0.213±0.003 A 0.0 0.183±0.027 A 0.0 0.396±0.030 A 0.0 TY02 0.335±0.001 D 57.3 0.210±0.011 ABCDE 14.4 0.544±0.010 E 37.4 TY07 0.267±0.004 C 25.3 0.212±0.008 DCDE 15.8 0.479±0.004 D 21.0 TY12 0.365±0.001 E 71.4 0.223±0.004 CDE 21.6 0.588±0.004 E 48.5 TY14 0.219±0.007 AB 2.7 0.203±0.006 ABCD 10.7 0.411±0.010 AB 3.8 TY18 0.425±0.009 G 99.7 0.214±0.012 BCDE 16.5 0.639±0.021 F 61.4 TY19 0.275±0.006 C 29.3 0.197±0.002 ABC 7.6 0.473±0.005CD 19.4 TY21 0.260±0.002C 21.9 0.206±0.006 ABCDE 12.5 0.466±0.004 CD 17.7 TY24 0.235±0.005 B 10.3 0.201±0.010 ABCD 9.8 0.436±0.008 BC 10.1 TY29 0.397±0.001 F 86.4 0.233±0.015 CD 27.3 0.630±0.014 F 59.1 TY34 0.259±0.030 C 21.6 0.204±0.006 ABCD 11.3 0.463±0.033 CD 16.9 TY35 0.377±0.002 EF 76.8 0.227±0.006 DE 23.8 0.604±0.008 EF 52.5 TY41 0.218±0.002 AB 2.5 0.194±0.002 AB 6.0 0.413±0.004 AB 4.3 说明:同列不同大写字母表示差异达到极显著水平(P < 0.01)。 表 2 菌根侵染率、生物量、氮吸收量及硝酸还原酶活性Pearson相关系数
Table 2. Pearson correlation coefficient of mycorrhizal colonize, biomass, nitrogen content and nitrate reductase activity
项目 侵染率 地下部分干质量 地下部分干质量 总生物量 根部氮量分数 叶部氮质量分数 根部硝酸还原酶活性 叶部硝酸还原酶活性 侵染率 1 地下部分干质量 0.706** 1 地上部分干质量 0.584 0.831** 1 总生物量 0.613* 0.872** 0.996** 1 根部氮质量分数 0.595 0.308 0.119 0.144 1 叶部氮质量分数 0.565 0.451 0.490 0.496 0.360 1 根部硝酸还原酶活性 0.515 0.505 0.647* 0.642* 0.207 0.423 1 叶部硝酸还原酶活性 0.579* 0.653 0.815** 0.806** 0.194 0.272** 0.763** 1 说明:*差异达到显著水平(P < 0.05);**差异达到极显著水平(P < 0.01)。 -
[1] FENG Gu, ZHANG Fusun, LI Xiaolin, et al. Uptake of nitrogen from indigenous soil pool by cotton plant inoculated with arbuscular mycorrhizal fungi[J]. Commun Soil Sci Plant Anal, 2002, 33(19/20):3825-3836. [2] HODGE A. Plant nitrogen capture from organic matter as affected by spatial dispersion, interspecific competition and mycorrhizal colonization[J]. New Phytol, 2003, 157(2):303-314. [3] 高悦, 吴小芹. 6种外生菌根菌对3种松苗叶绿素含量及叶绿素荧光参数的影响[J].南京林业大学学报:自然科学版, 2010, 34(6):9-12. GAO Yue, WU Xiaoqin. Efects of several ectomycorrhizal fungi on the chlorophyll content and chlorophyll fluorescence parameters in different pine seedlings[J]. J Nanjing For Univ Nat Sci Ed, 2010, 34(6):9-12. [4] 闫明, 钟章成.铝胁迫对感染丛枝菌根真菌的樟树幼苗生长的影响[J].林业科学, 2007, 43(4):59-65. YAN Ming, ZHONG Zhangcheng. Effects of aluminum stress on growth of Cinnamomum camphora seedlings inoculatde with AMF[J]. Sci SilvSin, 2007, 43(4):59-65. [5] 王如岩.菌根真菌对喀斯特地区幼苗生长状况的影响[D].南京:南京林业大学, 2011. WANG Ruyan. Effects of Mycorrhizal Fungal on Growth Status of Seedings in Karst Areas[D]. Nanjing:Nanjing Forestry University, 2011. [6] CAIRNEY J W G, MEHARG A A. Ericoid mycorrhiza:a partnership that exploits harsh edaphic conditions[J]. Eur J Soil Sci, 2003, 54(4):735-740. [7] SOKOLOVSKI S G, MEHARG Y A, MAATHUIS F J M. Calluna vulgaris root cells show increased capacity for amino acid uptake when colonized with the mycorrhizal fungus Hymenoscyphus ericae[J]. New Phytol, 2002, 155(3):525-530. [8] 陈真, 杨兵, 张春英, 等.锦绣杜鹃菌根真菌rDNA ITS序列分析及接种效应研究[J].菌物学报, 2011, 30(5):729-737. CHEN Zhen, YANG Bing, ZHANG Chunying, et al. Molecular analysis and inoculation effect of mycorrhizal fungi isolated from hair roots of Rhododendron pulchrum[J]. Mycosystema, 2011, 30(5):729-737. [9] BENDING G D, READ D J. Nitrogen mobilisation from protein polyphenol complex by ericoid and ectomycorrhizal fungi[J]. Soil Biol Biochem, 1996, 28(12):1603-1612. [10] BURKE R M, CAIRNEY J W G. Carbohydrolase production by the ericoid mycorthizal fungus Hymenoscyphus ericae under solid state fermentation conditions[J]. Mycol Res, 1997, 101(9):1135-1139. [11] 张春英, 戴思兰.杜鹃花类菌根研究进展[J].北京林业大学学报, 2008, 30(3):113-119. ZHANG Chunying, DAI Silan. Research advances on ericoid myeorrhiza[J]. J Beijing For Univ, 2008, 30(3):113-119. [12] 欧静, 刘仁阳, 陈训.桃叶杜鹃菌根显微结构及侵染情况[J].中南林业科技大学学报, 2012, 32(11):28-33. OU Jing, LIU Renyang, CHEN Xun. Study on microstructure and infections of Rhododendron annae mycorrhiza[J]. J Cent South Univ For & Technol, 2012, 32(11):28-33. [13] 欧静, 韦小丽, 何跃军, 等.接种ERM真菌对桃叶杜鹃幼苗的促生效应及生理生化影响[J].林业科学, 2013, 49(7):48-56. OU Jing, WEI Xiaoli, HE Yuejun, et al. Effects of Inoculation with ERM fungi isolates on the growth and physio-biochemical properties of Rhododendron annae seedlings[J]. Sci Silv Sin, 2013, 49(7):48-56. [14] 欧静, 何跃军, 刘仁阳, 等. ERM真菌对桃叶杜鹃幼苗光合性能及叶绿素荧光参数的影响[J].微生物学通报2013, 40(8):1423-1436. OU Jing, HE Yuejun, LIU Renyang, et al. Effects of inoculation with different ERM isolates on photosynthesis and chlorophyll fluorescence parameter of Rhododendron annae Franch. seedlings[J]. Microbiol China, 2013, 40(8):1423-1436. [15] PHILLIPS J M, HAYRNAN D S. Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection[J]. Transac Br Mycol Soc, 1970, 55(1):158-161. [16] 刘润进, 陈应龙.菌根学[M].北京:科学出版社, 2007:161-162. [17] 尹丽娟, 张春英, 杨兵.云锦杜鹃菌根真菌吸收氮源特性及其接种效应[J].中国农业科学, 2010, 43(4):868-872. YIN Lijuan, ZHANG Chunying, YANG Bing. Characteristics of nitrogen absorbed by ericoid mycorrhizal fungi and impact on growth of Rhododendron fortunei[J]. Sci Agric Sin, 2010, 43(4):868-872. [18] 宋福强, 杨国亭, 孟繁荣, 等.丛枝菌根对大青杨苗木生长的影响[J].林业科学研究, 2004, 17(6):770-776. SONG Fuqiang, YANG Guoting, MENG Fanrong, et al. Effect of albuscular mycorrhizal fungi on the growth of Populus ussuriensis[J]. For Res, 2004, 17(6):770-776. [19] 付淑清, 屈庆秋, 唐明, 等.施氮和接种AM真菌对刺槐生长及营养代谢的影响[J].林业科学, 2011, 47(1):95-100. FU Shuqing, QU Qingqiu, TANG Ming, et al. Effects of nitrogen and AM fungi on the growth and nutrition metabolism of Robinia pseudoacacia[J]. Sci Silv Sin, 2011, 47(1):95-100. [20] FOISSNER I, WENDEHENNE D, LANGEBARTELS C, et al. In vivo imaging of an elicitor induced nitric oxide burst in tobcco[J]. Plant J, 2000, 23(6):817-824. [21] KAISER W M, HUBER S C. Post translational regulation of nitrate reductase mechanism, physiological relevance and environmental triggers[J]. J Experi Bot, 2001, 52(363):1981-1989. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2014.06.015







 下载:
下载: