-
干旱是影响植物生长和发育的主要因素之一,干旱胁迫对植物造成的伤害在所有非生物胁迫中居于首位[1]。干旱胁迫不仅会打破植物体内水分代谢的平衡,引起植物细胞的失水,而且会导致植物体外部形态和内部生理生化特征均发生显著变化[2-3],这些变化可以表现在植物生长发育的各个时期,又可以表现在光合蒸腾作用、物质的合成与运输以及酶活性等具体的生理代谢过程中[4]。植物受到干旱胁迫后,体内活性氧增加、细胞渗透调节物质增多、个体及群体光合能力显著降低,最终导致植物个体或群体生长受到抑制,形态结构也随之发生变化[5]。当前,国内外关于植物抗旱性特征的研究相当多,如干旱胁迫下植物根冠比、干物质胁迫指数等一系列生长、形态和生理指标的变化及其与抗性之间的关系等[6-9]。正常情况下,植物体内活性氧的产生和清除会处于一个相对平衡的状态,当植物受到干旱胁迫后该平衡将被打破,这时植物体内的氧会活化成对植物细胞有着伤害作用的活性氧,活性氧增多会造成膜的稳定性降低,使生理功能紊乱,严重时甚至导致细胞的死亡[10-11]。因此,探究植物在干旱胁迫环境下的生理生化的变化及其抗旱机制,对改善植物的抗旱性和提高植物对环境的适应能力均具有重要的现实意义。毛竹Phyllostachys edulis具有生长快、产量高、效益好等特点,是中国分布最广泛、面积最大、利用价值最高的竹种[12],但干旱现象频繁发生,已经对毛竹的生长造成了不利影响。目前,关于毛竹干旱胁迫下生理方面的研究大部分采用盆栽试验[13-17],而对大田中干旱胁迫下毛竹生理方面的研究较少,盆栽试验虽然对阐明干旱胁迫下毛竹的一些机制较为合理,但并不能反应大田毛竹在受到干旱后的生理响应。毛竹在经营过程中多实行选择性采伐,采伐量达600株· hm-2·a-1,但是毛竹伐桩的腐烂却非常缓慢,通常伐桩完全腐烂需8 a甚至更长的时间,竹林内未腐烂的伐桩就会占据大量的林地空间,妨碍竹鞭的穿透生长,进而影响林地资源利用率和毛竹林经济的产出[18]。对于毛竹伐桩方面的研究主要为伐桩促腐[19-20],关于利用毛竹伐桩来对林内立竹进行灌溉方面的研究较少。毛竹多生长在高山上,采用喷灌滴灌等灌溉方式就需要投入大量的人力和财力,而采用毛竹伐桩进行灌溉既可以降低灌溉成本,又可以提高林地利用率。毛竹作为一种克隆植物,它能够通过地下鞭在克隆分株之间进行水分的运输与分享,从而实现毛竹林的水分生理整合。本研究采用模拟持续干旱环境,并增加注水伐桩的方法,对毛竹叶片的光合蒸腾作用、光合色素、抗氧化系统及膜脂过氧化程度的变化等进行分析研究,探讨模拟干旱环境下伐桩注水对毛竹生长可能产生的影响,为气候变化背景下毛竹的适应性经营及节水灌溉提供理论参考。
HTML
-
试验毛竹林位于浙江省杭州市富阳区庙山坞自然保护区(30°03′~30°06′N,119°56′~120°02′E)内。该保护区属于亚热带湿润季风气候,冬春季降水较少。海拔为290~530 m,年均降水量为1 100.0 mm,年均气温为16.1 ℃,年均无霜期237.0 d,土壤为微酸性红壤。试验所选材料为1年生标准竹,对各样地1年生毛竹进行每竹检尺,以平均胸径作为选择标准竹的依据,所选标准竹均要求生长良好,生长状况基本一致且无病虫害。处理前ck,T1和T2处理的土壤相对含水量分别为65.59%±2.79%,63.94%±4.08%和64.67%±3.69%;处理30 d时,ck,T1和T2处理的土壤相对含水量分别为54.37%±3.09%,52.17%±5.13%和53.97%±4.01%;处理60 d时,ck,T1和T2处理的土壤相对含水量分别为41.29%±2.63%,40.29%±3.86%和42.29%±1.95%;处理90 d时,ck,T1和T2处理的土壤相对含水量分别为27.11%±1.56%,25.11%±3.21%和26.11%±2.09%。各时间段的降水量分别为51.4,73.2和76.4 mm。
-
2016年1月在试验毛竹林中选取立地条件、地形和毛竹生长状况等均基本相同的10 m×20 m样地9块,各块样地之间间隔5 m。为防止周围土壤中的水分通过水平方式输送到相邻样地,在各块样地四周挖深约50 cm宽约20 cm的土沟,同时为了试验具有一致性,对各块样地内3年生以上及部分3年生毛竹进行砍伐,保证各块样地在砍伐后毛竹株数(3 000株·hm-2)完全相同,砍伐毛竹25株·样地-1左右,砍伐后各试验毛竹林立竹平均胸径分别为12.24,12.11,12.09,12.37,12.15,12.38,12.21,12.03,和12.23 cm,立竹年龄结构(3度竹:2度竹:1度竹)分别为1.00:0.63:1.34,1.00:0.58:1.25,1.00:0.75:1.23,1.00:0.81:1.14,1.00:0.56:1.19,1.00:0.74:1.29,1.00:0.61:1.42,1.00:0.83:1.52和1.00:0:59:1.33。试验设置ck(0个伐桩注水),T1(12个伐桩注水)和T2(18个伐桩注水),各块样地内的注水伐桩散布尽量均匀,重复3次·处理-1,对每块相同处理样地设置相同的注水伐桩并用毛竹打桩机将伐桩内部的蜡质层和竹隔打破,伐桩按地径来选取,且按1.00 cm一个径阶进行划分,所选伐桩的径阶分别为10.00,11.00,12.00,13.00,14.00和15.00 cm,T1处理伐桩选2个·径阶-1,T2处理的伐桩选3个·径阶-1,每个相同径阶伐桩的加水量控制相同(各径阶伐桩加水量分别约为800,1 000,1 300,1 600,1 800和2 000 mL),其中每块样地在选择注水伐桩后,其余伐桩不处理,随后同一时间对各样地进行薄膜覆盖模拟干旱环境,用胶水将塑胶薄膜与毛竹靠近地面茎部结合处存在的缝隙粘合牢固,所有样地均在1月28日设置完毕,以保证各样地土壤含水量尽量相同,同一天对T1和T2处理样地内已经打通的伐桩进行加水,并将伐桩口用塑料薄膜包扎,以防止水分的蒸发,每周对伐桩内水量进行观察,保证伐桩中始终有水。试验分别于处理结束后30,60和90 d时进行,测定各处理下1年生标准竹的净光合速率(Pn)与蒸腾速率(Tr),同时在竹冠的上部、中部和下部取成熟叶片混合样,测定其主要生理指标。
-
使用便携式光合仪(Li-Cor 6400,美国Li-CorInc.)发光二极管(LED)红蓝光源叶室对1年生标准竹叶片的净光合速率(Pn)与蒸腾速率(Tr)进行活体测定,光强设为900 μmol·m-2·s-1,并保持二氧化碳摩尔分数为400 μmol·mol-1。试验在天气晴朗的早上9:00-11:00进行,选取标准竹3株·样地-1,分别在各标准竹上部、中部和下部各选取3个叶片进行测定,其中根据光合参数可计算毛竹的水分利用效率EWUE=Pn /Tr。由于毛竹的叶片不能充满叶室,试验结束后摘下所测叶片,用拍照法计算所测部分叶面积[21],随后对每个叶片毛竹的光合测定值进行调整。
-
采用混合取样法取0.2 g新鲜的成熟毛竹叶片于预冷的研钵中,加入5 mL预冷的50 mmol·L-1磷酸缓冲液(pH 7.8)冰浴研磨,然后用磷酸缓冲液定容至10 mL。并于4 ℃下10 500 r· min-1离心15 min,取上清液(粗酶液)于4 ℃保存。光合色素采用V(丙酮):V(乙醇)=1:1混合液提取后用紫外可见分光光度计(UV-1800PC,中国上海美普达仪器有限公司)测定;丙二醛(MDA)用硫代巴比妥酸法测定;相对电导率用叶片组织浸出液初始电导值和煮沸后的电导值的比值表示,用DDSJ-308A型电导仪(中国上海精密科学仪器有限公司)测定;超氧化物歧化酶(SOD)活性用氮蓝四唑(NBT)光化还原法测定;过氧化物酶(POD)用愈创木酚氧化法测定;过氧化氢酶(CAT)用紫外吸收法测定。
试验数据用Excel 2003进行统计和制作图表,用SPSS 19.0软件进行单因素方差分析(one-way ANOVA)和最小显著差法(LSD)多重比较,设定P<0.05为显著性水平,比较不同处理毛竹各项生理指标的差异。本试验所有数据都采用均值±标准差表示。
1.1. 试验材料
1.2. 试验设计
1.3. 测定指标、测定方法及数据处理
1.3.1. 光合指标的测定
1.3.2. 生理指标测定
-
由表 1可知:在整个模拟干旱环境的过程中,3个处理时间点毛竹的净光合速率与蒸腾速率均表现为T2>T1>ck,处理30 d时,水分利用效率表现为ck>T2>T1,而处理60 d和90 d时,水分利用效率表现为ck>T1>T2。处理30 d时,各处理下毛竹净光合速率与蒸腾速率均表现为ck和T2处理差异显著(P<0.05),而T1处理与ck和T2处理差异均不显著,水分利用效率表现为T1和T2处理差异不显著,但均与ck差异相显著;处理60 d时,各处理下毛竹净光合速率与蒸腾速率均表现为T1和ck处理差异不显著,但均与T2处理差异显著,水分利用效率则表现为ck和T2处理差异显著,但均与T1处理差异不显著;处理90 d时,3个处理之间毛竹的净光合速率和蒸腾速率均达到了差异显著水平,T1和T2处理与ck相比,增幅分别达到了32.27%和43.55%,各处理之间的水分利用效率表现为T1和T2处理差异不显著,但均与ck差异显著。
t/d 净光合速率(Pn)/(μmol·m-2·s-1) 蒸腾速率(Tr)/(mmol·m-2·s-1) 水分利用效率(EWUE)/(mmol·mol-1) ck T1 T2 ck T1 T2 ck T1 T2 30 2.38±0.10b 2.48±0.03ab 2.62±0.13a 0.47±0.06b 0.54±0.04ab 0.56±0.01a 5.12±0.08a 4.58±0.26b 4.69±0.26b 60 2.79±0.03b 3.01±0.43b 4.02±0.39a 0.53±0.01b 0.60±0.05b 0.90±0.05a 5.29±0.18a 5.01±0.32ab 4.69±0.46b 90 3.44±0.10c 3.83±0.10b 4.55±0.31a 0.62±0.04c 0.74±0.02b 0.89±0.03a 5.63±0.25a 5.17±0.08b 5.15±0.21b 说明:不同小写字母表示处理间差异显著(P<0.05)。 Table 1. Photosynthesis and transpiration of Phyllostachys edulis with different number of water storage in stumps under simulated drought environment
-
由表 2可知:在处理的3个时间点上,毛竹叶片各光合色素质量分数均表现为T2>T1>ck。处理30 d时,3个处理间毛竹叶片叶绿素a差异不显著,ck和T2毛竹叶片叶绿素b差异显著(P<0.05),但均与T1差异不显著,而T1和ck之间毛竹叶片类胡萝卜素差异不显著,但均与T2差异显著。处理60 d时,ck和T1毛竹叶片叶绿素a、叶绿素b和类胡萝卜素差异不显著,但均与T2达到差异显著水平。处理90 d时,3个处理间毛竹叶片叶绿素a差异达到显著水平,T1和T2分别比ck增加了14.83%和20.57%,T1和T2间毛竹叶片叶绿素b差异不显著,但均与ck差异显著,T1和T2分别比ck增加了18.46%和21.54%,毛竹叶片类胡萝卜素质量分数仍然表现为T1和ck差异不显著,但均与T2差异显著。整个试验处理过程中,各时间点不同处理间毛竹叶绿素a/b差异均不显著。
t/d 叶绿素a/(mg·g-1) 叶绿素b/(mg·g-1) ck T1 T2 ck T1 T2 30 1.92±0.12 a 1.93±0.11a 1.96±0.06 a 0.59±0.01b 0.61±0.02ab 0.62±0.01 a 60 2.10±0.00 b 2.29±0.08 b 2.54±0.09 a 0.69±0.03 b 0.00±0.05 b 0.81±0.06 a 90 2.09±0.04 c 2.40±0.11b 2.52±0.02 a 0.65±0.03 b 0.00±0.01 a 0.09±0.01 a t/d 类胡萝卜素/(mg·g-1) 叶绿素a/b ck T1 T2 ck T1 T2 30 0.40±0.01b 0.41±0.02 b 0.56±0.01 a 3.22±0.32 a 3.16±0.06 a 3.18±0.11a 60 0.55±0.05 b 0.60±0.03 b 0.68±0.03 a 3.14±0.01a 3.25±0.01a 3.19±0.16 a 90 0.45±0.02 b 0.40±0.01b 0.49±0.01a 3.18±0.00 a 3.09±0.23 a 3.10±0.08 a 说明:不同小写字母表示处理间差异显著(P<0.05)。 Table 2. Contents of chlorophyll and carotenoid in leaves of Phyllostachys edulis with different number of water storage in stumps under simulated drought environment
-
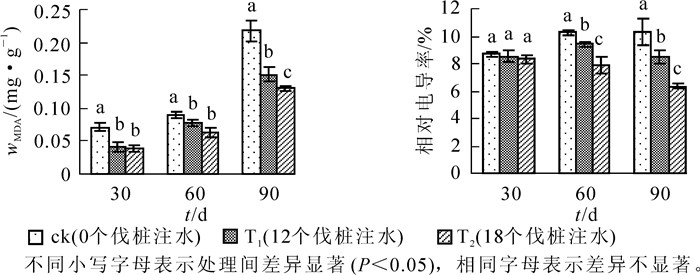
由图 1可知:处理3个时间点,毛竹叶片丙二醛(MDA)质量摩尔浓度随着注水伐桩数量的增加而降低。处理30 d时,T1和T2处理毛竹叶片MDA都显著低于ck,但T2处理略低于T1处理,两者无显著差异;处理60 d时,T1与T2处理毛竹叶片MDA无显著差异,但均与ck差异显著(P<0.05),同时T1和T2处理毛竹叶片MDA质量摩尔浓度的差距逐渐拉大。当处理时间达到90 d时,T1和T2处理毛竹叶片MDA较ck均有显著的降低,且3个处理之间差异达到显著水平,T1和T2处理毛竹叶片MDA与ck相比降幅分别达到了43.54%和66.49%。毛竹叶片相对电导率在整个处理的各时间点表现为随着注水伐桩数量的增加而降低,处理30 d时各处理间毛竹叶片相对电导率无显著差异;处理60 d和90 d时,3个处理之间毛竹叶片相对电导率均达到差异显著水平,且处理达到90 d时,T1和T2处理毛竹叶片相对电导率较ck均有大幅度的降低,降幅分别达到了21.63%和61.79%。
-
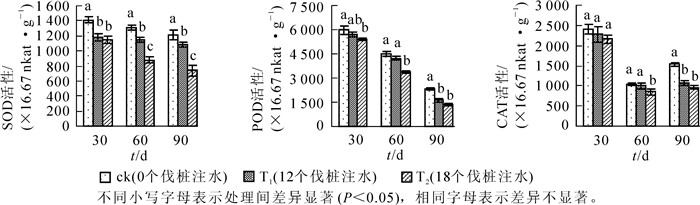
由图 2可知:毛竹叶片超氧化物歧化酶(SOD)活性在整个处理期间均随着注水伐桩数量的增加而降低。处理30 d时,T1和T2处理毛竹叶片SOD活性均显著低于ck,但T2处理略低于T1处理,两者无显著差异;处理达到60 d和90 d时,各处理之间毛竹叶片SOD活性差异均达到显著水平(P<0.05);在处理90 d时,T2处理与ck相比下降幅度达到最大,此时T1和T2处理毛竹叶片SOD活性与ck相比下降幅度分别达到了11.24%和63.99%。毛竹叶片POD活性表现为在整个处理期间均随着注水伐桩数量的增加而降低。T1处理毛竹叶片过氧化物酶(POD)活性在模拟干旱环境30 d和60 d时均略微低于同时期的ck,无显著差异,直到处理90 d时T1处理显著低于同期的ck,T2处理在各时间点毛竹叶片POD活性均显著低于ck,此时,T1和T2处理与ck毛竹叶片POD活性相比下降幅度分别达到了43.62%和71.18%。毛竹叶片过氧化氢酶(CAT)活性在整个处理期间均与注水伐桩数量负相关。处理30 d时,各处理间毛竹叶片CAT活性无显著差异;处理60 d时,T1与ck处理无显著差异,T2处理较ck和T1处理均有显著下降;处理达到90 d时,T1和T2处理毛竹叶片CAT活性无显著差异,较ck均有显著下降,T1和T2处理较ck毛竹叶片CAT活性的分别降低了44.00%和59.71%。
2.1. 模拟干旱环境下不同注水伐桩处理毛竹光合蒸腾作用
2.2. 模拟干旱环境下不同注水伐桩处理对毛竹叶片光合色素的影响
2.3. 模拟干旱环境下不同注水伐桩处理毛竹叶片丙二醛(MDA)质量摩尔浓度和相对电导率
2.4. 模拟干旱环境下不同注水伐桩处理毛竹叶片抗氧化酶活性
-
在所有影响植物光合作用的因子中,干旱胁迫一直以来都受到很大重视。随着全球气候变化的不断加剧,干旱胁迫对植物的影响也越来越突显[22]。光合作用不仅是植物重要的生命活动,同时也是植物生长的生理基础,植物在干旱胁迫下光合生产力的高低也常用来判断其耐旱能力的强弱[23]。因此,研究干旱胁迫下植物光合作用的变化对理论和生产实践均具有重要的指导意义。植物在受到干旱胁迫后,光合作用显著下降,即使是轻度的干旱胁迫也能够使植物的光合蒸腾速率下降,生长受到明显的抑制[24-25]。本研究同样发现,试验各时间点毛竹净光合速率与蒸腾速率均表现为T2>T1>ck,说明随着干旱胁迫的加剧(注水伐桩数量的减少),毛竹净光合速率与蒸腾速率显著下降,增加注水伐桩可以显著提高毛竹的光合与蒸腾能力,从而使毛竹光合产物的积累增加。在各时间点T2处理毛竹净光合速率和蒸腾速率均与T1和ck处理差异均达到显著水平(P<0.05),处理30 d和60 d时,ck和T1处理毛竹净光合速率与蒸腾速率差异均不显著,直到处理90 d时,3个处理之间毛竹净光合速率与蒸腾速率均达到差异显著水平(P<0.05),说明干旱环境下,毛竹净光合速率和蒸腾速率的高低与注水伐桩数量有着密切的关系,干旱胁迫持续时间越长,注水伐桩作用也越显著。各时间点,不同处理间均表现为ck处理毛竹的水分利效率最高,说明干旱胁迫可以在一定程度上提高毛竹的水分利用效率。随着干旱胁迫时间的进一步延长和注水伐桩数量的继续增加,毛竹净光合速率与蒸腾速率还需要深入的研究。
叶绿素在植物光合作用过程中对光能的传递与转换起着非常重要的作用。相关研究表明,随着干旱程度的加剧植物叶绿素质量分数降低[26]。也有研究发现,干旱胁迫可使植物叶片叶绿素a和叶绿素b有不同程度的增加[27],如银中杨Populus alba × Populus berlinensis在受到干旱胁迫后,不同处理之间随着干旱胁迫程度的加剧,其叶片叶绿素质量分数反而出现了升高的趋势[28]。在本研究中,各时间点不同处理间均未出现叶绿素质量分数升高的现象,这可能是各时间点的干旱胁迫均未达到出现这种现象的干旱程度,也可能是不同植物之间叶绿素对干旱胁迫的响应存在差异。各时间点不同处理下毛竹叶片叶绿素a,叶绿素b和类胡萝卜素质量分数均表现为T2>T1>ck,这可能是随着注水伐桩数量的减少,毛竹叶绿体片层中捕光叶绿素a/b-Pro复合体的合成受到抑制或伤害,从而阻止了其叶片叶绿素积累和原叶绿素酯的形成,进而引起叶绿素的下降,也可能是毛竹叶片叶绿素自身发生氧化降解[29],同时,脂质过氧化作用诱导也可能使毛竹叶片叶绿素的降低[30]。各处理之间叶绿素a和叶绿素b也随着干旱胁迫时间的延长T1和T2处理均与ck处理达到差异显著水平(P<0.05),说明增加注水伐桩可以显著提高毛竹叶片叶绿素a和叶绿素b质量分数,类胡萝卜素质量分数各时间点均表现为T1和ck处理差异不显著,但均与T2处理差异显著(P<0.05),说明增加注水伐桩的数量与毛竹叶片类胡萝卜素质量分数关系密切。通常情况下,植物叶片叶绿素a/b值的大小是衡量植物对水分胁迫的敏感性和植物抗旱性的强弱的一个重要指标[31]。本研究中各时间点不同处理间毛竹叶片叶绿素a/b差异均不显著(P>0.05),表明毛竹具有较强的抗旱能力。
干旱胁迫会对植物造成多方面伤害,植物细胞质膜结构遭到破坏或者发生膜脂过氧化均会造成细胞膜透性的增大,引起细胞内电解质的外渗,从而使植物叶片相对电导率升高。本研究显示,处理各时间点,ck处理毛竹叶片细胞膜受到破坏程度均最大,相对电导率也最高,T1处理次之,T2处理最低,这与史燕山等[32]的研究结果一致。随着干旱胁迫时间的延长,ck处理与T1和T2处理毛竹叶片相对电导率差距逐渐增大,这说明干旱环境下,增加注水伐桩可以改善毛竹的生长状况。丙二醛(MDA)是植物在干旱胁迫下遭受伤害后膜脂过氧化最重要的产物之一。它能够与细胞内各种物质发生强烈地反应,造成酶和膜的严重损伤,进而引起膜电阻及膜的流动性的降低,最终造成膜结构和生理完整性的破坏[33]。本研究同样发现,各时间点毛竹叶片MDA质量摩尔浓度均表现为ck>T1>T2,这表明随着注水伐桩数量的减少,膜脂过氧化反应在加重,细胞原生质膜破坏程度不断加重。随着干旱胁迫时间的延长不同处理间达到差异显著水平,各处理间毛竹叶片MDA质量摩尔浓度差距拉大,注水伐桩数量越少,毛竹叶片MDA越高,毛竹在干旱胁迫下受损程度也越重。
植物遭受干旱胁迫后会引发活性氧水平升高,同时也会主动或被动地启动自身的抗氧化防御系统进行防御,减缓细胞伤害,提高植物对干旱胁迫的适应性。而防御系统中的超氧化物歧化酶(SOD)、过氧化物酶(POD)以及过氧化氢酶(CAT)活性的高低就成为控制伤害的关键因素[34]。抗氧化系统防御能力的高低取决于这几种酶彼此间的协调作用[35]。相关研究显示,随着干旱胁迫强度逐渐增加,SOD,POD和CAT酶活性均呈升高趋势[36]。本研究同样发现各时间点毛竹叶片SOD,POD和CAT活性均表现为ck处理最高,T1处理次之,T2处理最低。各时间点毛竹叶片CAT和POD活性均随着模拟干旱程度的增加,活性增大,说明CAT和POD等2种酶在清除SOD转化的过氧化氢的能力还在增强,其在将过氧化氢变成为水的过程中起着重要作用,同时毛竹叶片SOD和CAT酶活性均呈现上升趋势,这可能和两者是协同保护酶的原因[37]。随着干旱胁迫时间的延长,ck处理毛竹叶片各氧化酶活性与T1和T2处理差距逐渐增大,在干旱胁迫处理后期,T1和T2处理与ck处理毛竹叶片各氧化酶活性差异均达到显著水平(P<0.05),说明土壤相对含水量越低,注水伐桩的效果越显著。干旱胁迫下注水伐桩与毛竹叶片各氧化酶的关系还有待进一步研究,因为黄条金刚竹Pleioblastus kongosanensis f. aureostriaus等地被竹叶片SOD,POD和CAT活性随干旱胁迫程度的增加均呈先升高后降低的趋势[38]。而本研究中,各时间点毛竹叶片中保护酶均未出现先升高后降低的现象,这可能是模拟干旱还未达到能够引起毛竹产生这种生理现象的干旱程度,也可能是不同竹种间抗氧化防御系统对干旱胁迫响应方式存在差异。
综上所述,模拟干旱环境下,增加注水伐桩可以显著提高毛竹光合蒸腾能力和抗氧化能力,并且各时间点毛竹净光合速率、蒸腾速率和叶片光合色素质量分数均与注水伐桩数量正相关,而叶片MDA质量摩尔浓度,相对电导率和SOD,CAT和POD活性均与注水伐桩数量负相关。干旱胁迫时间越长,土壤相对含水量越低,注水伐桩对毛竹林的影响作用也越显著。注水伐桩为极端干旱天气条件下毛竹的正常生长提供了有力保障,同时也为高山地区毛竹林的灌溉创造了条件,大大降低了毛竹林的灌溉成本。故此在今后毛竹林经营过程中可以充分利用毛竹伐桩,使其在毛竹林培育生产及灌溉方面发挥作用。极端干旱环境下毛竹各项生理指标随着干旱胁迫时间的进一步延长和注水伐桩数量的继续增加该如何变化还需要进一步深入的研究,同时在干旱环境下增加注水伐桩后不同年龄毛竹的生理响应也有待进一步研究。











 DownLoad:
DownLoad: