-
内蒙古典型草原是中国北部地区重要的生态屏障,其功能的正常发挥对维持区域及全球性生态系统平衡具有极其重要的作用。近年来,由于长期过度放牧、刈割等人类活动的强烈干扰,草原生态环境恶化,初级生产力和生物多样性不断下降,草原退化现象日趋严重[1]。土壤微生物是草原生态系统的重要组成部分,是土壤物质循环和能量流动的主要参与者,在调节土壤养分循环、物质代谢、凋落物降解等方面起着重要作用[2]。土壤中微生物量越高,土壤基础呼吸强度越高,一定程度上反应该生态系统具有越强的物质循环能力和促进植被生长发育的能力[3]。赵彤等[4]研究发现:黄土丘陵区草地土壤微生物量碳、氮含量均高于人工灌木林、人工乔木林和农地。而在草地研究中发现,线叶菊Filifolium sibiricum草场的土壤微生物量碳、氮含量显著高于羊草Leymus chinensis草场、贝加尔针茅Stipa baicalensis草场、大针茅S. grandis草场和克氏针茅S. krylovii草场[5]。吴永胜等[6]研究发现:土壤微生物量碳含量随着草地退化程度增加而减少,而且在夏季高于春季和冬季。曹淑宝等[7]对草甸草原短期放牧处理后,发现轻度放牧增加了土壤微生物量碳、氮含量。土壤呼吸反映了土壤氧化和转化能力,是陆地生态系统碳循环的主要动力,是土壤中碳素以二氧化碳的形式返还大气的主要输出途径。胡诚等[8]对不同施肥管理措施下的土壤研究发现:有益微生物(effective microorganism,EM)菌液堆肥处理的土壤基础呼吸最高,土壤呼吸与土壤微生物量碳含量呈显著正相关。李香真等[9]对蒙古高原草原研究发现:草甸草原土壤呼吸最高,土壤呼吸与降水量呈显著正相关。李国辉等[10]研究发现:冷蒿Artemisia frigida等7种植物的根际土壤微生物量和基础呼吸强度均明显高于非根际土壤。冷蒿为菊科Compositae蒿属Artemisia的小半灌木,根系与不定根发达,可形成水平盘状根系,进而植株以“纯植株”丛分布。冷蒿耐牧性强,广泛分布于中国天然草场中,尤其是在退化草场中优势明显,被认为是过度放牧引起的退化草地的指示植物。冷蒿对草场的退化具有一定的阻击作用,并且是退化草原群落在恢复演替过程中重要的过渡者[11]。近年来,对冷蒿的研究主要集中于茎叶浸提液的化感作用[12]、对机械损伤的响应[13-14]、对低磷环境的响应[15]、根际土壤营养元素[16]、根际微生物区系及土壤酶活性[17]等方面。对不同放牧处理下冷蒿根际土壤微生物量和土壤呼吸的变化尚未见报道。本研究通过对比不同放牧梯度下冷蒿根际与非根际土壤微生物量以及土壤基础呼吸的差异,明确冷蒿根际土壤微生物量和基础呼吸对放牧干扰的响应规律,以期为揭示冷蒿耐牧性与土壤微生物之间的关系、冷蒿种群阻击草场退化原理以及草原生态系统保护提供依据。
HTML
-
研究区位于内蒙古锡林浩特市毛登牧场中的内蒙古大学草地生态学研究基地,其地理位置为44°10′02.4″N,116°28′56.8″E,海拔为1 160 m,属于半干旱大陆性气候,夏季在一定程度上受海洋季风气候影响,冬季寒冷干燥。全年平均气温为-0.4 ℃,7月(最热月)平均气温18.8 ℃,1月(最冷月)平均温度-22.3 ℃,≥0 ℃年积温为2 410.0 ℃,≥10 ℃积温为1 597.9 ℃,无霜期91.0 d,全年植物生长期为150 d左右。全年平均降水量为365.6 mm,在6-9月较为集中,占年降水量的80%左右。土壤为栗钙土。研究区植物主要有羊草,糙隐子草Cleistogenes squarrosa,克氏针茅,大针茅,冷蒿,防风Saposhnikovia divaricata,瓣蕊唐松草Thalictrum petaloideum,阿尔泰狗哇花Heteropappus altaicus等。
-
试验研究从2012年5月至2014年9月连续3 a对草场进行不同强度的放牧处理,每年放牧时间为5-9月。试验设置3个不同强度的放牧处理,对照处理(ck)即不放牧;轻度放牧(light grazing,LG)即5月、7月每月21日放牧1 d,全年利用2次;重度放牧(heavy grazing,HG)即连续放牧,5-9月每月21日放牧1 d;如遇恶劣天气,每次放牧时间顺延,设置3个重复·处理-1,共9个小区,小区面积为33.3 m × 33.3 m。试验用羊为当年生乌珠穆沁羊Caprahircus ujumqin,各放牧季节投放羊6只·小区-1。
-
于2015年7月中旬冷蒿生长旺盛期采集土壤样品。采用五点取样法,随机选取冷蒿5丛·小区-1,以冷蒿丛为中心,将冷蒿植株丛完整挖起(0~10 cm),先轻轻抖落大块不含根系的土壤,装入无菌塑料袋内,混匀,即为非根际土壤;然后用力抖落根系表面附着的土壤,即为根际土壤。将土样密封带回实验室仔细去除根系等杂质后,把5个点的土壤混匀作为此样地的土样。土样过2 mm筛后按四分法取一部分用于土壤理化性质见(表 1)及微生物量的测定;另一部分用于土壤呼吸的测定。待测土样保存在4 ℃的冰箱中。
土壤类型 处理 有机质/(g·kg-1) 全氮/(g·kg-1) 全磷/(g·kg-1) 全钾/(g·kg-1) 碱解氮/(g·kg-1) 速效磷/(g·kg-1) 速效钾/(g·kg-1) PH值 根际土壤 对照 15.63±0.52 B 1.03±0.10 B 0.34±0.01 B 1.14±0.02 B 96.12±6.74 C 1.17±0.04 B 21.22±0.08 B 8.05±0.10 A 轻度放牧 20.11±1.07 A 1.46±0.06 A 0.41±0.03 A 1.20±0.01 A 141.28±3.87 A 1.06±0.08 B 20.86±0.22 B 7.97±0.03 A 重度放牧 18.75±0.61A 1.33±0.02 A 0.4±0.02 A 1.14±0.02 B 108.62±2.86 B 1.21±0.04 A 22.65±0.31 A 7.77±0.04 B 非根际土壤 对照 14.20±0.08 ab 0.93±0.06 b 0.33±0.02 ab 1.14±0.01 b 64.62±6.29 a 0.37±0.04 b 14.03±0.08 c 8.43±0.04 a 轻度放牧 12.75±0.83 b 1.02±0.10 ab 0.31±0.01 b 1.19±0.04 a 73.45±2.03 a 0.58±0.08 a 15.52±0.10 b 8.14±0.02 c 重度放牧 15.79±0.86 a 1.11±0.04 a 0.36±0.02 a 1.13±0.02 b 72.37±3.68 a 0.65±0.15 a 16.11±0.04 a 8.31±0.09 b 说明:每个值均为平均值±标准误。根据最小显著差异法测验(P<0.05),不同大写字母表示冷蒿根际土壤的差异显著,不同小写字母表示冷蒿非根际土壤的差异显著。 Table 1. Soil chemical properties under different grazing intensity
-
取土样5 g经过氯仿熏蒸后用0.5 mol·L-1硫酸钾浸提,浸提液由岛津TOC-VCPH分析仪(日本)测定[18],设置重复3个·处理-1。计算公式为:wMBC=EC/KEC,wMBN=EN/KEN。其中:wMBC为微生物量碳(mg·kg-1);wMBN为微生物量氮(mg·kg-1);EC为熏蒸与未熏蒸土壤中有机碳的差值;EN为熏蒸与未熏蒸土壤中全氮的差值;KEC和KEN为转换系数。
-
采用LI-7000 CO2/H2O气体分析仪(美国)测定。称取15 g土壤样品,均匀置于样品室内,采用开路式连接方式隔2 s测定二氧化碳量,待数值稳定后选取5 min时段内数据进行计算。重复3次·土样-1。计算公式:R=ΔC/(m×Δt)。其中:R为呼吸强度(mg·kg-1·s-1);ΔC为2 s内的二氧化碳浓度差;m为土壤质量(kg);Δt为时间差。
-
测定的初始数据经Excel 2007整理后,采用Origin 8软件(美国Origin Lab公司)进行统计分析并作图,采用One-Way ANOVA的统计方法进行检验,并进行最小显著差法多重比较(P<0.05)。每个变量数值表示为平均值±标准误差。采用独立样本t检验进行冷蒿根际和非根际土壤与对照组之间的差异分析。相关性分析采用SPSS 19.0统计软件的Pearson’s相关分析方法。
1.1. 自然概况
1.2. 试验设计
1.3. 土壤样品的采集与保存
1.4. 试验方法
1.4.1. 土壤微生物量碳、微生物量氮测定
1.4.2. 土壤呼吸测定
1.5. 数据分析
-
由图 1可知:随着放牧强度的增加土壤微生物量碳呈现先升高后降低的趋势。冷蒿根际土壤微生物量碳质量分数在轻度放牧组最高,为665.08 mg·kg-1,与对照组相比,轻度、重度放牧后分别增加22.71%和7.04%;非根际土壤微生物量碳质量分数在3个处理组间均表现出显著差异(P<0.05),与对照组相比轻度放牧后增加42.92%,重度放牧处理后降低19.95%;3个处理组中根际土壤微生物量碳质量分数均高于非根际土壤,在对照和重度组中根际土壤微生物量碳质量分数高出非根际土壤29.30%和62.47%,差异极显著(P<0.01)。表明放牧处理可以增加冷蒿根际土壤微生物量碳质量分数,而非根际土壤微生物量碳质量分数只在轻度放牧处理后增加。
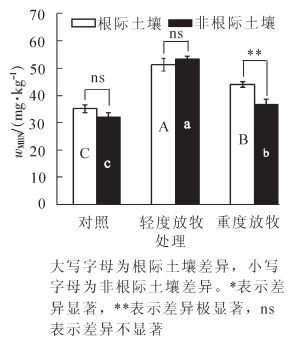
不同强度放牧处理后土壤微生物量氮质量分数(图 2)为32.06~53.25 mg·kg-1。放牧处理显著(P<0.05)提高了冷蒿根际土壤中微生物量氮质量分数,与对照组相比轻度、重度放牧后分别增加45.66%和25.17%;在非根际土壤中,与对照组相比,轻度、重度放牧后分别增加66.08%和13.51%。2类土壤的微生物量氮质量分数均表现为轻度放物组>重度放物组>对照组,放牧处理不同程度地增加了冷蒿根际与非根际土壤微生物量氮质量分数,而且微生物量氮的增加幅度大于微生物量碳。
-
由表 2可见:冷蒿根际与非根际土壤中,wMBC/wMBN为9~16,wMBC/wSOC(SOC为土壤有机质)为2%~4%,wMBN/wTN(TN为土壤全氮)为3%~5%。wMBC/wMBN在冷蒿根际土壤中对照组显著(P<0.05)高于其他2组,分别比轻度放牧、重度放牧组高18.61%和16.95%;在非根际土壤中3个处理组间差异均显著(P<0.05),对照组分别比轻度放牧组、重度放牧组高16.1%和38.19%;3个处理组中根际土壤的wMBC/wMBN值均高于非根际土壤。由wMBC/wMBN的变化可知,放牧处理更大程度地提高了土壤中的微生物量氮质量分数。土壤微生物量碳与土壤有机碳的百分比称为微生物熵[19]。在冷蒿根际土壤中轻度和重度放牧处理使微生物熵值分别提高了20.46%和4.17%;在非根际土壤中3个处理组间的微生物熵差异均显著(P<0.05),与对照组相比轻度放牧处理后增加19.15%,重度放牧处理后降低25.90%;在3个处理组中根际土壤的微生物熵均高于非根际土壤。表明轻度放牧促进了土壤微生物对有机碳的利用,而冷蒿根际环境更有助于微生物将有机碳转化成生物量碳。在冷蒿根际土壤中轻度放牧组wMBN/wTN值比对照组显著(P<0.05)高出15.95%,而重度放牧组与对照组差异不显著;在非根际土壤中轻度放牧组比对照顾组显著(P<0.05)高出22.62%,重度放牧组与对照组差异不显著。从微生物量的比值变化中可以看出放牧处理不同程度地增加了土壤微生物量,其中轻度放牧处理效果最为明显;也表明冷蒿根际微环境更可促进土壤微生物对土壤中碳素和氮素的利用。
土壤类型 处理 wMBC/wMBN 微生物熵/% wMBN/wTN/% 根际土壤 对照 15.41±0.31 A 2.86±0.10 B 3.72±0.04 B 轻度放牧 12.99±0.44 B 3.45±0.22 A 4.32±0.14 A 重度放牧 13.18±0.41 B 2.98±0.11 B 4.06±0.22 AB 非根际土壤 对照 13.40±0.33 a 2.80±0.17 b 3.65±0.08 b 轻度放牧 11.54±0.53 b 3.34±0.23 a 4.47±0.07 a 重度放牧 9.70±0.28 c 2.08±0.08 c 3.64±0.19 b 说明:表中数据为3个重复的平均值±标准误。根据最小显著差异法测验(P<0.05),不同大写字母表示冷蒿根际土壤的差异显著,不同小写字母表示冷蒿非根际土壤的差异显著。 Table 2. Ratio change of the soil microbial biomass under different grazing intensity
-
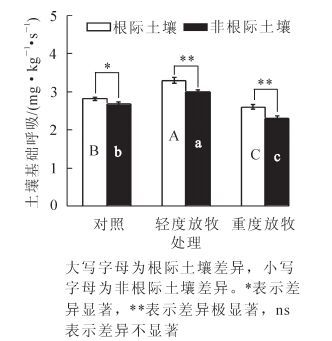
土壤呼吸作用释放的二氧化碳主要来源于微生物的呼吸,它可以用来衡量土壤微生物活性、评价土壤肥力。由图 3可知:放牧处理对冷蒿根际土壤基础呼吸的影响显著。与对照组相比,轻度放牧后增加17.27%,重度放牧处理后降低7.6%,差异显著(P<0.05);非根际土壤呼吸也受到了放牧处理的显著影响,与对照组相比轻度放牧后增加11.31%,重度放牧处理后降低14.61%,差异显著(P<0.05)。而在3个处理组中冷蒿根际土壤呼吸值均显著高于非根际土壤,在轻度放牧、重度放牧组中达到极显著(P<0.01)水平。表明轻度放牧处理加强了土壤呼吸作用而重度放牧处理减弱了土壤呼吸作用,冷蒿根际微环境有助于土壤呼吸作用的提高。
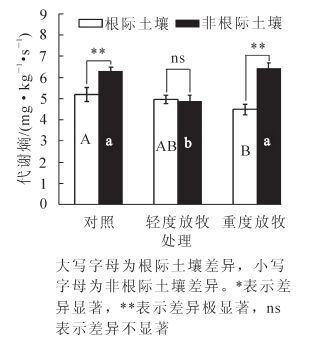
微生物代谢熵是指土壤基础呼吸强度与微生物量碳的比值[8]。比值的大小反应了单位微生物量碳的具体呼吸速率,它将微生物呼吸速率与微生物量有机结合起来,是对两者的一种有效调和。本研究中,与对照相比轻度放牧、重度放牧处理后冷蒿根际土壤的代谢熵分别降低了4.55%,13.78%(图 4);在非根际土壤中与对照组比轻度放牧组降低22.33%,重度放牧组升高2.56%;在对照组和重度放牧组中冷蒿非根际土壤代谢熵极显著(P<0.01)高于根际土壤。结果表明:冷蒿根际土壤微生物呼吸消耗的生物量碳相对较少,能更有效地将有机碳转化成微生物量碳。
-
不同放牧强度处理后,冷蒿根际、非根际土壤微生物量、基础呼吸、代谢熵与土壤化学性质的相关性见表 3。冷蒿根际土壤中微生物量碳与微生物量氮、全氮和碱解氮呈极显著(P<0.01)正相关,与有机质、全钾呈显著(P<0.05)正相关;微生物量氮与有机质、全氮、碱解氮呈极显著(P<0.01)正相关,与全磷、全钾呈显著(P<0.05)正相关,表明根际土壤中有机质、全氮、碱解氮、全磷、全钾质量分数的丰富提高了微生物量碳、氮质量分数;基础呼吸与微生物量碳、碱解氮、全钾呈显著或极显著(P<0.01)正相关,表明土壤中微生物量碳、碱解氮和全钾质量分数显著影响基础呼吸的强弱;代谢熵值与pH值呈显著(P<0.05)正相关,表明土壤弱碱性条件促进微生物生长。非根际土壤中微生物量碳与有机质呈极显著(P<0.01)负相关,与全磷、pH值呈显著(P<0.05)负相关;微生物量氮与pH值呈极显著(P<0.01)负相关,表明碱性环境土壤营养匮乏,不利于微生物生存,非根际土壤微生物量更容易受土壤pH值变化的影响;基础呼吸与微生物量碳呈极显著(P<0.01)正相关,与有机质、全磷呈极显著(P<0.01)负相关;代谢熵与微生物量碳、微生物量氮和全钾呈极显著(P<0.01)负相关,与pH值呈显著(P<0.05)正相关,表明土壤微生物量越高而代谢熵值越低,对比根际土壤代谢熵值可知,冷蒿根际微环境缓解了这种相关性。
土壤 指标 微生物量碳 微生物量氮 有机质 全氮 碱解氮 全磷 速效磷 全钾 速效钾 pH值 根际土壤 微生物量碳 1 0.929** 0.737* 0.798** 0.945** 0.485 -0.664 0.697* -0.344 -0.031 微生物量氮 0.929** 1 0.906** 0.915** 0.937** 0.761* -0.587 0.691* -0.145 -0.292 基础呼吸 0.728* 0.613 0.507 0.446 0.805** 0.264 -0.837** 0.898** -0.825** 0.457 代谢熵 -0.289 -0.3D7 -0.273 -0.424 -0.120 -0.299 -0.283 0.335 -0.706* 0.692* 非根际土壤 微生物量碳 1 0.875** -0.872** -0.258 0.165 -0.699* -0.002 0.741* -0.039 -0.700* 微生物量氮 0.875** 1 -0.654 0.132 0.492 -0.440 0.35 0.769* 0.434 -0.914** 基础呼吸 0.897** 0.663 -0.947** -0.502 0.069 -0.886** -0.293 0.553 -0.343 -0.490 代谢熵 -0.974** -0.927** 0.769* 0.069 -0.266 0.568 -0.182 -0.799** -0.130 0.745* 说明:*为显著相关P<0.05, **为极显著相关P<0.01。 Table 3. Correlative coeificients among microbial biomass, basal respiration rate and soil chemical properties under different grazing
2.1. 不同放牧强度下冷蒿根际与非根际土壤微生物量碳、氮的变化
2.2. 不同放牧强度下土壤微生物量碳、氮比值的变化
2.3. 不同放牧强度下土壤基础呼吸及代谢熵
2.4. 土壤微生物量碳、氮,土壤基础呼吸与土壤基本化学性质的相关性
-
土壤微生物量和土壤基础呼吸在一定程度上可反映土壤微生物活性和土壤中物质代谢强度,是土壤养分循环与转化的驱动力,也是衡量土壤生物学性状,揭示微生物群落状态的重要依据。研究表明:不同放牧强度可以改变草原土壤微生物的区系组成以及数量,轻度放牧能够增加草原土壤微生物总数和细菌、真菌、放线菌的数量,但重度放牧造成土壤理化性质发生过度改变,使得草原生态系统无法自我修复而表现为土壤肥力下降、微生物数量及活性下降[20]。
本研究中随着放牧强度的增加,冷蒿根际与非根际土壤微生物量碳、氮均呈现出先升高后降低的变化趋势,而且根际土壤高于非根际土壤,与张蕴薇等[21]研究人工草地土壤微生物量碳、氮结果一致。其主要原因有:① 轻度放牧处理中,动物的选择性啃食、践踏可以促使冷蒿不定根的形成与萌蘖的能力加强,为微生物生长提供更庞大的根系环境,并且排泄物的进入为微生物生存提供可持续利用的养分,加速了土壤营养元素的循环。② 过度放牧使土壤容重增加,密度变大,通气、透水性变差[22],导致理化性质变化剧烈,微生物生存环境恶化,不利于微生物的生长。③ 冷蒿发达的根系及丰富的根系分泌物[23]为微生物生长提供大量的营养物质和相对稳定的生存环境,而非根际土壤中由于没有庞大的根系及其分泌物的缓冲调节造成微生物量低于根际土壤。研究表明:在克氏针茅草原[24]和典型草原[25]土壤中与对照组相比放牧处理不同程度地增加了土壤中微生物量氮含量,其中轻度放牧处理后含量最高,与本研究结果一致。可能是由于放牧处理中动物的排泄物以及动物践踏促使植物凋落物混入土壤而加快分解,使得土壤中氮素含量增加[16],进而促进了土壤中氮素转化细菌的增加[26],土壤微生物量氮增加。
土壤中有机质、氮、磷、钾含量反映了土壤营养状况,是微生物生长的必需元素。当土壤中含有大量可被微生物利用的有机碳、氮、磷等元素时,可促进微生物迅速生长,代谢加强,微生物量碳、氮升高,基础呼吸强度增加。本研究中冷蒿根际土壤的营养状况优于非根际,微生物量碳、氮质量分数和基础呼吸强度也高于非根际。相关性分析表明,根际土壤微生物量碳、氮质量分数均与土壤中有机质、全氮、碱解氮呈极显著(P<0.01)正相关,与全钾、全磷呈显著(P<0.05)正相关,表明丰富的土壤营养物质可提高土壤微生物量碳、氮质量分数。李国辉等[10]研究表明,不同植物根际土壤微生物量碳、氮质量分数均与土壤有机质、全氮之间呈显著(P<0.05)或极显著(P<0.01)正相关,与本研究结果一致。在轻度放牧处理后冷蒿根际土壤养分最丰富,pH值呈弱碱性,产生最适微生物生长的环境,因此微生物量含量最高。
研究表明:wMBC/wMBN可以体现微生物群落结构信息,其显著变化预示着微生物群落结构的变化[19];土壤微生物量与土壤养分的比值可以反映土壤养分向微生物量转化的效率、土壤养分损失和土壤矿物对有机质的固定[27]。本研究中,土壤中wMBC/wMBN,wMBC/wSOC,wMBN/wTN的范围分别为9.0~15.5,2.0%~3.5%,3.0%~5.0%,与赵彤等[4]报道的7.0~11.0,2.7%~4.85%,2.56%~4.45%相比,wMBC/wMBN值略高,wMBC/wSOC与wMBN/wTN值相近。wMBC/wMBN值略高可能与植物生长特性、根系活动、凋落物的质量以及土壤有效养分差异有关[28]。放牧处理显著(P<0.05)降低了冷蒿根际与非根际土壤的wMBC/wMBN,而使土壤微生物量增加,这表明放牧处理改变了土壤的微生物群落结构,却增加了土壤中微生物的数量,可能是放牧减少了土壤中微生物类群,但增加了某些菌群的数量。蒲宁宁等[29]研究表明:昭苏草甸草原土壤的wMBC/wSOC值随着放牧强度的增加呈现先升高后降低的趋势,与本研究结果一致。表明轻度放牧条件可促进土壤有机碳的转化,加速土壤物质循环。本研究中轻度放牧处理组wMBN/wTN值均高于对照组和重度放牧,与李世卿等[30]研究结果一致,表明轻度放牧处理提高了土壤微生物活性和微生物量,促进土壤有机质分解转化,因此wMBN/wTN值相对较高。
土壤基础呼吸可反映土壤氧化、有机物转化及能量释放的能力。当土壤呼吸作用加强,其物质代谢加快可释放大量的能量供微生物生长利用[31]。目前,对草原土壤呼吸的研究结果不尽相同。陈海军等[32]研究表明:贝加尔针茅草原土壤呼吸强度随着放牧强度的增加而减少;而杨阳等[33]研究表明:随着放牧强度的增加土壤呼吸呈现先增高后降低的趋势,与本研究结果一致。出现不同的结果表明,土壤呼吸受多重因素的综合影响。本研究中轻度放牧处理后土壤基础呼吸强度显著(P<0.05)增加,根际土壤的基础呼吸强度均显著(P<0.05)高于非根际,可能由于轻度放牧处理后土壤微生物数量增加使得基础呼吸增强,相关分析也表明放牧处理后速效氮的增加可以提高基础呼吸强度,而冷蒿根际pH值呈弱碱性并且存在大量有机酸等分泌物适于微生物生存,表现出基础呼吸强度高于非根际土壤,与李国辉等[10]的研究一致。代谢熵是反映环境因素、利用方式等对微生物活性影响的一个敏感指标,可以作为微生物受胁迫的指标[34]。本研究中代谢熵在根际土壤中与有机质、全氮、碱解氮、全磷、速效磷、速效钾均呈一定程度的负相关,且随着放牧强度的增加逐渐降低,原因是轻度放牧处理增加了土壤有机质含量,营养元素的增加导致微生物数量和碳源利用效率的提高[35],而重度放牧处理使生物量碳增加,基础呼吸强度降低,导致代谢熵降低。非根际土壤中代谢熵随放牧强度增加呈现先降低后升高趋势,表明轻度放牧处理降低了单位微生物量碳的呼吸速率,而重度放牧处理组的升高体现了微生物受到胁迫后微生物量碳下降而单位时间的呼吸强度升高。
-
本研究结果表明:轻度放牧促使冷蒿根际与非根际土壤中微生物量、基础呼吸速率、wMBC/wSOC等升高,wMBC/wMBN和代谢熵下降;重度放牧使冷蒿根际土壤中微生物量等略有增加,基础呼吸速率、wMBC/wMBN和代谢熵显著下降,而非根际土壤中微生物量氮质量分数显著上升,生物量碳、基础呼吸速率等显著下降;放牧处理后冷蒿根际土壤中微生物量、基础呼吸速率和wMBC/wMBN等均高于非根际;冷蒿根际土壤中微生物量碳、氮与土壤有机质、全氮、碱解氮等呈显著或极显著正相关,基础呼吸与微生物量碳、碱解氮等呈显著或极显著正相关,代谢熵与pH值呈显著正相关。
综上所述,轻度放牧处理后冷蒿根际土壤微生物量及呼吸速率增加,重度放牧处理后有所下降;冷蒿根际土壤的微生物量及呼吸速率等均高于非根际土壤。冷蒿能够在一定程度上表现出抵御放牧压力的能力,是由于其根际微环境有利于微生物量及呼吸速率的增加,能够加快根际养分循环,从而为冷蒿生长提供更好的营养基础。














 DownLoad:
DownLoad: