-
国槐Sophora japonica又名豆槐、白槐、家槐,属于豆科Leguminosae槐属Sophora乔木,喜光而稍耐荫,在中国各省区广泛栽培。槐角即国槐的果实,荚果,念珠状,其性寒、味苦,有凉血止血、清肝明目的功效。槐角含有多种化学成分,主要成分为黄酮类化合物[1],具有抑菌、抗病毒、降血压等一系列功能[2-5],并可作为质量鉴定、药材及其制剂的质量控制指标[6],引起了国内外的广泛研究。虽然国槐原产于中国并在中国广泛分布,但多用作行道树或庭院树种,并且容易受到环境污染及病虫害影响,药用资源开发利用率较低,难以满足需求[7]。目前,从国槐槐角中获取具有极高药用价值的黄酮产物,通常是从槐角中直接提取,由于经常受到季节、地区等因素影响,限制了黄酮作为新型药剂的推广[8]。液体悬浮培养是植物细胞生产次生代谢产物最简单有效并被广泛应用的方式[9],因此,采用槐角愈伤组织悬浮培养技术生产黄酮类化合物可以有效解决药用植物资源有限的问题。利用细胞培养技术生产黄酮类化合物是一个比较常见的方法,如采用杜仲Eucommia ulmoides,金荞麦Fagopyrum dibotrys,甘草Glycyrrhiza uralensis[10-12]等植物作为外植体进行细胞培养生产黄酮类化合物已有了一定的研究,但是采用国槐槐角种胚进行细胞培养生产黄酮类化合物的研究报道还相对较少,将国槐槐角种胚细胞悬浮培养生产的黄酮类化合物进行抗氧化性研究尚无报道。因此,笔者在本实验室研究的基础上,将国槐槐角种胚细胞悬浮培养后,对其生产的代谢产物进行抗氧化特性研究,采用了5种不同方法,测定提取液对不同自由基、亚硝酸盐的清除能力及抑制脂肪、脂肪酸自氧化的能力,可以为黄酮类化合物的活性成分分析和开发利用提供理论依据,也能为其抗氧化活性的构效理论提供依据。
HTML
-
实验所用国槐槐角采自北京八家郊野公园内,国槐槐角愈伤组织来自国槐种胚的诱导,筛选生长快、分散性好的槐角愈伤组织,将它们在B5固体培养基上继代5次以上,获得稳定的愈伤组织后将其转接到B5液体培养基中,在液体培养基中进行悬浮培养,继代6次后得到愈伤组织即为本实验所需材料[8, 13]。
-
固体培养基为:B5 + 1.00 mg·L-1 2, 4-D + 0.20 mg·L-1 6-BA(6-苄氨基嘌呤),蔗糖30.00 g·L-1,琼脂6.00 g·L-1,灭菌前pH 6.60。液体培养基为:B5 + 2.00 mg·L-1 2, 4-D + 0.50 mg·L-1 6-BA+ 0.05 mg·L-1 NAA(萘乙酸),蔗糖30.00 g·L-1,灭菌前pH 6.17。培养基均在121 ℃高压条件下灭菌30 min。固体培养基放置于温度为(25±1)℃的环境下培养,30 d继代1次。获得稳定愈伤组织后,接种到装液量为50.00 mL B5液体培养基的三角瓶中培养,接种量为40.00 g·L-1,该液体培养基悬浮体系在温度为(25±1)℃,转速为110 r·min-1的摇床上全程暗培养,25 d继代1次[7-8, 13]。
-
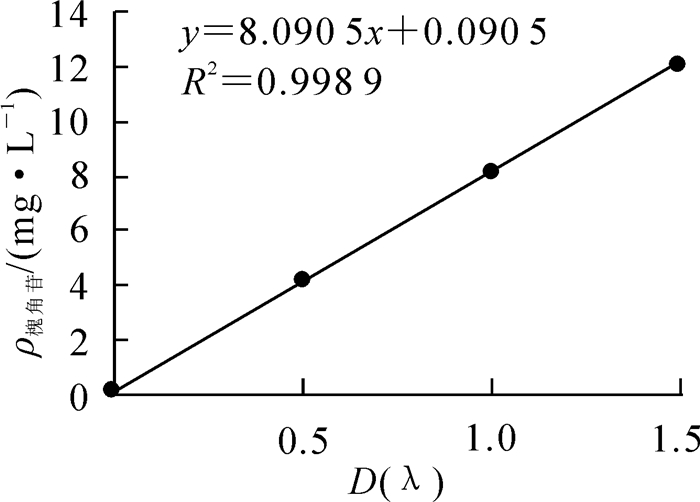
黄酮类化合物含量的测定采用紫外分光光度法[14],选用槐角苷为标准品,绘制标准曲线。精确称取2.00 mg槐角苷标准品,用体积分数70%乙醇溶解后定容至100.00 mL容量瓶中,得到质量浓度为0.02 mg·L-1的槐角苷标准溶液;依次量取配制好的槐角苷标准溶液1.00,2.00,3.00,4.00,5.00,6.00 mL定容至10.00 mL,得到质量浓度分别为2.00,4.00,6.00,8.00,10.00,12.00 mg·L-1的槐角苷溶液,以体积分数为70%乙醇溶液为空白对照,在261.5 nm处测定槐角苷的吸光度。以吸光度为横坐标,槐角苷质量浓度为纵坐标绘制标准曲线(之前已测定过仪器的精密度、稳定性、重复性)(图 1)。槐角苷标准品在0~12.00 mg·L-1范围内线性关系良好,线性回归方程为y=8.090 5x+0.090 5,R2=0.998 9,x为不同质量浓度溶液的吸光度,y为槐角苷溶液质量浓度。
-
将烘干后的愈伤组织研磨,过80目筛,备用。称取0.10 g粉末于10.00 mL离心管中,加入4.00 mL体积分数为70%乙醇,室温下超声提取40 min。提取后离心5 min,收集其上清液,合并到50.00 mL的鸡心瓶,在(40 ± 1)℃,60 r·min-1的条件下进行旋蒸,得到槐角细胞黄酮粗提液。
-
取上述提取液稀释30倍后测定在261.5 nm处的吸光度,重复3次。总黄酮的含量(X)以槐角苷计,用Y代表黄酮化合物产量。计算公式如下:
式(1)和式(2)中:c为稀释后的提取液中槐角苷的质量浓度(mg·L-1);v为所提取槐角苷溶液的体积(mL);m为精确称取的供试材料的质量(g);wDW为收获的细胞干质量浓度(g·L-1)。结合槐角苷标准曲线,经计算,样品乙醇提取液中黄酮化合物的产量为354.00 mg·L-1。
-
采用二倍稀释法[15]将黄酮化合物质量浓度为354.00 mg·L-1的细胞提取液进行稀释,最终得到质量浓度分别为354.00,177.00,88.50,44.25,22.13,11.06,5.53 mg·L-1等7个质量浓度的黄酮粗提液。
-
称取0.083 4 g七水合硫酸亚铁溶于少量蒸馏水中,然后转移到50.00 mL容量瓶中,定容,得到6.00 mmol·L-1硫酸亚铁溶液;称取0.041 4 g水杨酸,加入少量乙醇,搅拌,然后转移到50.00 mL容量瓶中用无水乙醇准确定容,得到6.00 mmol·L-1的水杨酸-乙醇体系(避光保存);用移液枪量取333.40 μL质量分数为30%的过氧化氢用蒸馏水定容至100.00 mL容量瓶中,得到质量分数为0.1%的过氧化氢。在10.00 mL具塞试管中先后加入质量浓度为6.00 mmol·L-1硫酸亚铁溶液、6.00 mmol·L-1的水杨酸-乙醇、不同质量浓度样品,最后加入过氧化氢启动反应,设置8.00 mL反应体系(表 1)。37 ℃水浴反应30 min后,在510.0 nm处测定吸光度。计算公式为:清除率(%)= [1-(A-B)/C]×100%。其中:A为加入抗氧化剂后溶液的吸光度,B为提取样液在测定波长下的吸光度(消除提取液本身的颜色对结果的干扰),C为未加入抗氧化剂时溶液的吸光度。
添加物 添加量/mL A组 B组 C组 6.00 mmol.L-1硫酸亚铁(FeSO4)溶液 2.00 2.00 2.00 水杨酸-乙醇 2.00 2.00 2.00 不同质量浓度样品 2.00 2.00 0 体积分数为70%乙醇 0 0 2.00 质量分数为0.1%过氧化氢 2.00 0 2.00 蒸馏水 0 2.00 0 Table 1. Dosage of each solution in the reaction system
-
称取三羟甲基氨基甲烷12.11 g,用蒸馏水溶解定容至1 000.00 mL,得到0.10 mol·L-1的三羟甲基氨基甲烷溶液;量取50.00 mL浓度为0.10 mol·L-1的三羟甲基氨基甲烷溶液与22.90 mL,0.10 mol·L-1的氯化氢溶液混匀并用蒸馏水定容至100.0 mL,得到pH 8.2(25 ℃)浓度为50.00 mmol·L-1的三羟甲基氨基甲烷盐酸盐缓冲液;称取0.315 0 g邻苯三酚固体,用10.00 mmol·L-1的氯化氢溶液定容至100.00 mL,得到25.00 mmol·L-1的邻苯三酚溶液(避光保存)。在10.00 mL具塞试管中设置9.00 mL反应体系,先后加入50.00 mL浓度为0.10 mol·L-1的三羟甲基氨基甲烷溶液、不同质量浓度样品,混匀后在37 ℃水浴中保温20 min,取出后立即加入在37 ℃预热过的25.00 mmol·L-1邻苯三酚溶液,混匀后于37 ℃水浴中反应5 min后加入1.00 mol·L-1的盐酸终止反应(表 2),在420.0 nm处测定吸光度。计算公式为:清除率(%)= [1-(A-B)/C]×100%。其中:A为加入抗氧化剂后溶液的吸光度,B为提取样液在测定波长下的吸光度(消除提取液本身的颜色对结果的干扰),C为未加入抗氧化剂时溶液的吸光度。
添加物 添加量/mL A组 B组 C组 50.0 mmol·L-1三羟甲基氨基甲烷盐酸盐(Tris-HCl)缓冲液 5.50 5.50 5.50 不同质量浓度样品 2.00 2.00 0 25.00 mmol·L-1邻苯三酚溶液 0.50 0 0.50 10.00 mmol·L-1氯化氢(HCl) 0 0.50 0 体积分数为70%乙醇 0 0 2.00 1.00 mmol·L-1氯化氢(HCl) 1.00 1.00 1.00 Table 2. Dosage of each solution in the reaction system
-
分别量取1.00 mL不同质量浓度样品移入25.00 mL的容量瓶中,各加入5.00 mg·L-1的亚硝酸钠标准溶液5.00 mL,在室温下反应60 min;加2.00 mL质量分数为0.4%的对氨基苯磺酸溶液,摇匀,静置5 min;加入1.00 mL质量分数为0.2%的盐酸萘乙二胺溶液,摇匀,定容至刻度线,静置15 min;另用以上相同步骤做一空白对照;在540.0 nm处测定吸光度,计算亚硝酸盐的清除率。计算公式为:清除率(%)=[(B空白对比吸光度-B加入黄酮吸光度)/B空白对比吸光度]×100%。
-
称取愈伤组织粉末0.05,0.10,0.20,0.50 g,各用4.00 mL无水乙醇溶解;用无水乙醇配制体积分数为0.5%的亚油酸乙醇液,将愈伤组织粉末分别加入50.00 mL体积分数为0.5%的亚油酸乙醇液中混匀,以仅在50.00 mL体积分数为0.5%的亚油酸乙醇液中加4.00 mL无水乙醇为对照;在(40 ± 1)℃烘箱中强化保存,定时搅拌,采用硫代巴比妥酸法测定吸光度;以相同质量的VC做阳性对照,重复以上步骤。
-
将猪油放在烧杯中,用文火熬炼;称取研磨后的愈伤组织粉末0.05,0.10,0.20,0.50 g,各用8.00 mL无水乙醇溶解,分别加入50.00 g温热猪油中,搅匀,以仅在猪油中加8.00 mL无水乙醇为对照;在(70 ± 1)℃烘箱中强化保存,定时搅拌,测其过氧化值;以相同质量的二丁基羟基甲苯做阳性对照,重复以上步骤。
-
采用Excel 2007和SPSS 17.0软件对试验数据进行分析。
1.1. 试验材料
1.2. 试验方法
1.2.1. 培养条件
1.2.2. 槐角苷标准曲线的绘制
1.2.3. 槐角细胞黄酮粗提液的提取
1.2.4. 黄酮化合物产量的测定
1.2.5. 不同质量浓度梯度细胞提取液的配制
1.2.6. 水杨酸fenton试剂法测定羟自由基清除率
1.2.7. 邻苯三酚自氧化法测定超氧自由基清除率
1.2.8. 盐酸萘乙二胺法测定亚硝酸盐的清除率
1.2.9. 硫代巴比妥酸法测定亚油酸自氧化抑制作用
1.2.10. 碘量法测定猪油自氧化抑制作用
1.3. 数据分析
-
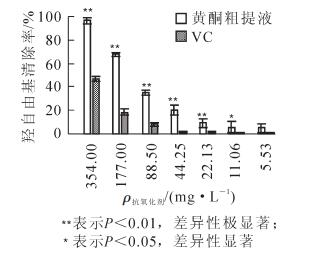
图 2显示了不同质量浓度的槐角种胚愈伤组织黄酮粗提液和VC溶液对羟自由基的清除能力。由图 2可知:这2种抗氧化剂对羟自由基都具有清除作用,且随着质量浓度的增大,清除能力增强,说明对羟基自由基的清除能力与样品的质量浓度呈现较好的量效关系。两者对羟自由基的清除能力有一定的差距:当质量浓度为354.00 mg·L-1(即未稀释)时,黄酮粗提液对羟自由基的清除能力达到了96.58%,而VC溶液只有46.79%;当质量浓度较小,为11.06和5.53 mg·L-1时,黄酮粗提液对羟自由基的清除率仍然在5.00%左右,而VC溶液却不到1.00%。对其抗氧化性进行显著性分析发现,在354.0~22.13 mg·L-1质量浓度范围内,黄酮粗提液对羟自由基的清除率极显著高于相同质量浓度的VC溶液;在质量浓度为11.06 mg·L-1时,黄酮粗提液对羟自由基的清除率也显著高于VC溶液;在质量浓度为5.53 mg·L-1时,黄酮粗提液和VC溶液对羟自由基的清除率差异性不显著,但高于VC溶液。
-
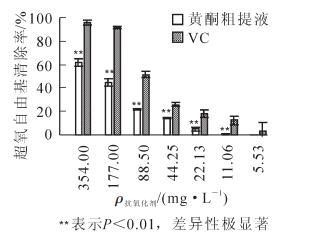
不同质量浓度的槐角种胚愈伤组织黄酮粗提液和VC溶液对超氧自由基的清除作用见图 3,与清除羟自由基的作用相似,随着黄酮粗提液和VC溶液质量浓度的升高,对超氧自由基的清除能力也越强。但是2种抗氧化剂对超氧自由基的清除能力不同,当质量浓度为354.00 mg·L-1(即未稀释)时,黄酮粗提液对超氧自由基的清除能力为61.54%,而VC达到了95.75%;当质量浓度较小,为11.06和5.53 mg·L-1时,黄酮粗提液对超氧自由基的清除率不到1.00%,而VC溶液的清除率仍在3.00%以上。对其抗氧化性进行显著性分析发现,在354.00 ~11.06 mg·L-1质量浓度范围内,VC溶液对超氧自由基的清除率极显著高于相同质量浓度的黄酮粗提液;在质量浓度为5.53 mg·L-1时,黄酮粗提液和VC溶液对超氧自由基的清除率虽然差异性不显著,但低于VC溶液。
-
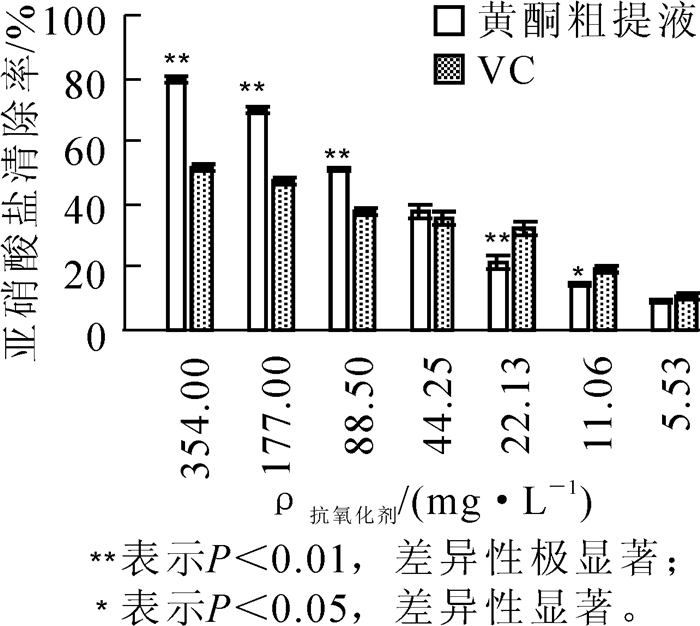
图 4显示了不同质量浓度的槐角种胚愈伤组织黄酮粗提液和VC溶液对亚硝酸盐的清除作用。由图 4可知:这2种抗氧化剂对亚硝酸盐都具有清除作用,与清除羟自由基、超氧自由基的作用相似,随着黄酮粗提液和VC溶液质量浓度的升高,对亚硝酸盐的清除能力也随之增强。但是2种抗氧化剂对亚硝酸盐的清除能力不同,当质量浓度为354.00 mg·L-1(即未稀释)时,黄酮粗提液对亚硝酸盐的清除能力为79.91%,而VC溶液只有51.60%;在354.00~44.25 mg·L-1质量浓度范围内,黄酮粗提液对亚硝酸盐的清除作用要高于相同质量浓度的VC溶液;而当质量浓度继续减小至5.53 mg·L-1时,2种抗氧化剂对亚硝酸盐的清除作用均在10.00%左右,但VC溶液对亚硝酸盐的清除作用更强。对其抗氧化性进行显著性分析发现在354.00~88.50 mg·L-1质量浓度范围内,黄酮粗提液对亚硝酸盐的清除率极显著高于相同质量浓度的VC溶液;在质量浓度为22.13 mg·L-1时,黄酮粗提液对亚硝酸盐的清除率极显著低于相同质量浓度的VC溶液;当质量浓度低于22.13 mg·L-1时,黄酮粗提液和VC溶液对亚硝酸盐的清除率差异性不显著,黄酮粗提液对亚硝酸盐的清除率稍低于VC溶液。
-
黄酮类化合物抑制亚油酸的自氧化作用是基于不饱和脂肪酸的自由基反应, 反应过程中产生过氧化自由基, 进而氧化生成环氧化物, 环氧化物生成丙二醛, 丙二醛与硫代巴比妥酸(TBA)作用生成TBA染料, 其最大吸收波长为532.0 nm。因此, 生成TBA染料的多少反映了532.0 nm处吸光度的大小, 是衡量自由基链反应进程的标志。抗氧化剂可抑制自由基链反应, 最终表现为TBA染料生成较少, 吸光度变小[16]。表 3显示了不同质量的槐角种胚愈伤组织黄酮粗提物和VC对亚油酸自氧化的抑制作用,添加抗氧化剂后,样品的吸光度与对照组相比均减小。从实验结果看:这2种抗氧化剂对亚油酸自氧化都具有抑制作用,且随着抗氧化剂添加量增加,吸光度越小,抗氧化性越好。从实验数据可以看出:黄酮粗提物及VC抑制亚油酸自氧化的能力相差不大,而黄酮粗提物抑制亚油酸自氧化的能力没有相同质量的VC强。
编号 处理 D(λ)吸光度 4 d 8 d 1 黄酮粗提物0.05 g 0.047 ± 0.002 0.077 ± 0.002 2 黄酮粗提物0.10 g 0.044 ± 0.003 0.074 ± 0.003 3 黄酮粗提物0.20 g 0.033 ± 0.005 0.059 ± 0.004 4 黄酮粗提物0.50 g 0.024 ± 0.003 0.041 ± 0.003 5 VC 0.05 g 0.042 ± 0.003 0.054 ± 0.003 6 VC 0.10 g 0.036 ± 0.001 0.039 ± 0.002 7 VC 0.20 g 0.028 ± 0.002 0.035 ± 0.002 8 VC 0.50 g 0.023 ± 0.002 0.030 ± 0.002 9 空白对照(ck) 0.054 ± 0.003 0.082 ± 0.002 Table 3. Inhibition of self oxidation of linoleic acid by two kinds of antioxidants
-
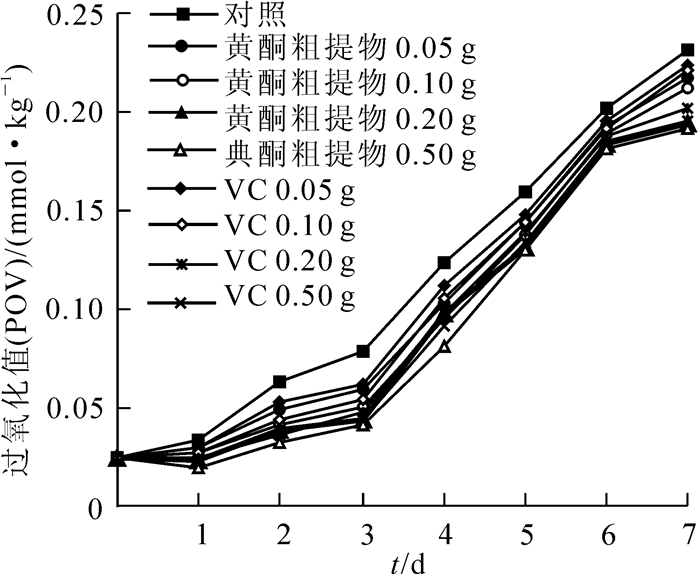
猪油在腐败过程中,会产生过氧化物,过氧化值的大小,反映了过氧化物的多少。抗氧化剂可以消除过氧化物的存在,最终表现为过氧化值的下降[16]。不同质量的黄酮粗提物和二丁基羟基甲苯对猪油自氧化的抑制作用见图 5,与抑制亚油酸自氧化的作用相似,不同质量的黄酮粗提物和二丁基羟基甲苯可消除猪油腐败过程中产生的过氧化物,对猪油自氧化均具有明显的抑制作用,随着黄酮粗提物和二丁基羟基甲苯质量的增加,过氧化值的下降的程度也增大,即对猪油自氧化的抑制作用也越强。另外从实验结果看,愈伤组织的黄酮粗提物抑制猪油自氧化的效果优于相同质量的二丁基羟基甲苯。
2.1. 愈伤组织黄酮粗提液对羟自由基的清除能力
2.2. 愈伤组织黄酮粗提液对超氧自由基的清除能力
2.3. 愈伤组织黄酮粗提液对亚硝酸盐的清除能力
2.4. 黄酮粗提物对亚油酸自氧化的抑制作用
2.5. 黄酮粗提物对猪油自氧化的抑制作用
-
羟基自由基被认为是具有较强毒性的自由基[17],得电子能力极强,可以和大部分细胞内物质发生反应,甚至能氧化损伤基因,对衰老和癌症有着极大的影响。超氧自由基是代谢过程中生成的第1个自由基,同时也是其他部分氧自由基的来源,因为它可以进一步转化成其他自由基[18]。亚硝酸盐广泛存在于环境中,但较多亚硝酸盐摄入,可将人体血红蛋白中的二价铁离子氧化成三价铁离子,使血红蛋白失去携氧能力,同时它还是形成强致癌物亚硝胺的前体物质,及时清除亚硝酸盐是食品安全的保障[19]。因此,本实验测定槐角种胚细胞黄酮粗提液对羟自由基、超氧自由基、亚硝酸盐的清除能力是研究其抗氧化活性必不可少的。油脂或油脂食品在储存过程中,由于长时间接触空气,会产生危害人体健康的氢过氧化物,另外油脂在炸制过程中,既受热又氧化,其过氧化物形成及分解成自由基的速度加快,对人体危害程度还会大大增加[20],而人工合成的抗氧剂有一定毒性,所以有必要选择合适的抗氧化剂削弱过氧化物的产生。本实验选择槐角种胚细胞黄酮提物液测定其对亚油酸和猪油自氧化的抑制作用,既能反应黄酮粗提物抗氧化性活性的强弱,又能为食品安全提供必要保障。
结果显示:槐角种胚细胞黄酮粗提液对羟自由基、超氧自由基、亚硝酸盐都具有清除能力,具有较强的抗氧化活性,且在一定质量浓度范围内,清除能力随着黄酮粗提液质量浓度的增大而增强,但是对各种自由基的清除能力有所不同。黄酮粗提液对羟自由基的清除能力极强,最高可达到96.58%,极显著高于相同质量浓度的VC溶液;对超氧自由基的清除能力也较强,最高可达到61.54%,但是其清除率极显著低于相同质量浓度的VC溶液,这说明槐角种胚细胞黄酮粗提液对羟自由基的清除能力大于对超氧自由基的清除能力。而对于亚硝酸盐的清除能力,最高可达到79.91%,极显著高于相同质量浓度的VC溶液,但较低质量浓度的黄酮粗提液对亚硝酸盐的清除能力却不及VC溶液。同时,槐角种胚细胞提取物可以去除亚油酸和猪油在自氧化过程中产生的过氧化物,对其自氧化有明显的抑制作用,可见槐角种胚细胞提取物的应用范围较广泛,可以在食品生产中有独特的用处。这些抗氧化能力作用的发挥,主要是由于国槐种胚细胞悬浮培养后黄酮粗提液中所含的黄酮类化合物产生作用的结果,对不同物质的清除能力的不同可能与提取液所含黄酮类化合物的种类和结构不同有关[21],测定它们对不同自由基的清除能力及对亚油酸过氧化和猪油自氧化的抑制能力可以为研究黄酮粗提液的成分及其抗氧化活性的构效理论提供依据。
结合前人研究,在不同植物体内提取的黄酮类化合物有着不同强度的抗氧化活性,其中在杜仲雄花、银杏Ginkgo biloba叶、玫瑰茄Hibiscus sabdariffa花萼等提取的黄酮粗提物分别对羟基自由基、1, 1-二苯基-2-三硝基苯肼(DPPH)自由基等有较强的清除效果,并且其抗氧化活性均优于相同质量浓度的VC溶液[22-24],与本实验中国槐种胚细胞悬浮液黄酮粗提物的抗氧化性研究结果相似。在相同质量浓度下,黄酮粗提物具有更优的抗氧化活性,可能与黄酮类化合物本身的化学结构和特性有关,一方面黄酮类化合物电子自旋密度分布的均匀性,同时在分子内形成半醌式自由基时所需能量较低,其形成的自由基较稳定,从而具有较高的抗氧化活性[25];另一方面,黄酮类化合物可以直接清除反应链中的自由基,切断自由基链反应,从而起到防止和断裂链反应的两重功效[23]。
总体来看,国槐槐角种胚细胞悬浮培养生产的黄酮类化合物具有明显的抗氧化活性,且在一定质量浓度范围内,黄酮提取物质量浓度越大,其抗氧化性越好。国槐槐角种胚细胞悬浮培养生产的黄酮类化合物是细胞培养技术的产物,是一种具有抗氧化活性的天然绿色食品抗氧化剂,具有用量少、安全无毒等优点。当今,食品工业发展迅速,含有天然活性成分的保健食品越来越受到现代人的推崇,而同时国槐槐角作为药材存在原料不足、易受环境污染、病虫害等影响,难以满足需求,运用细胞培养技术生产的槐角种胚细胞具有明显的抗氧化活性,可以有效解决药用植物资源有限的问题,应用前景广阔。














 DownLoad:
DownLoad: