-
云斑天牛Batocera horsfieldi属鞘翅目Coleoptera天牛科Cerambycidae沟胫天牛亚科Lamiinae白条天牛属Batocera,又名白条天牛,是在中国广泛分布的一种丰富且具有破坏性的天牛。幼虫蛀食活树树干的内皮和边材部分,导致树容易断裂,其基本生物学特性与亚科其他天牛相似,生命周期通常在2 a内完成[1]。成虫出现在初夏,主要以山核桃Carya cathayensis的枝条为食。云斑天牛性成熟,雌雄成虫交配后,雌虫将卵存放在寄主树的树皮下度过整个夏天。孵化后的幼虫立即进入内部树皮,化蛹前至少喂食20个月[2]。据报道,在浙江省杭州市临安区,多数山核桃被天牛钻蛀,其种类主要有桑天牛Apriona germari,星天牛Anoplophora chinensis和云斑天牛[3]。云斑天牛幼虫主要生活在寄主植物树干内,而成虫会飞行,鞘翅坚硬且耐药性强,一般的物理和化学方法很难防控。许多植食性昆虫利用气味作为寄主定位的线索,无论是营养、配偶地点还是存放其后代[4-8]。有研究显示:植物挥发性气体能引起天牛显著的行为反应[9-10]。植物挥发性气体是多种微浓度的挥发性化合物组成的混合物[11],可以调控天牛取食、产卵和交配等行为[12]。如ZHUGE等[13]发现:云斑天牛可能依赖特定比例的挥发物来寻找定位寄主植物;寄主植物挥发物中的壬醛对云斑天牛有较好的林间诱捕效果[14]。植物在自然状态、受到天牛攻击或机械损伤时释放的挥发物在种类和浓度上存在差异[15]。天牛利用识别植物挥发物的化学成分和相对含量来甄别植物的状态,进而做出反应[16],如光肩星天牛Anoplophora glabripennis对受到天牛攻击后的复叶槭Acer negundo的挥发物有显著驱避效果,而对健康复叶槭的挥发物有显著引诱效果[17]。近年来,许多国内外学者通过研究昆虫和植物挥发物之间的化学联系,明确昆虫和植物挥发物互作机制,探究如何利用有益昆虫或防控有害昆虫,可以采用类似方法来探索防控云斑天牛的防治方法。本研究运用动态顶空套袋吸附法法收集不同受害状态下山核桃释放的挥发物,并通过气相色谱-触角电位测量系统(GC-EAD)技术和Y型嗅觉仪技术,检测能引起云斑天牛显著电生理和行为反应的挥发物,为筛选云斑天牛的植物源引诱剂或驱避剂的成分和比例提供理论基础。
HTML
-
试验所用云斑天牛成虫和山核桃样树来自于浙江省杭州市临安区玲珑街道雅园村的山核桃苗圃基地(30°12′N,119°38′E,平均海拔113 m)。山核桃苗圃基地有少量苦楝Melia azedarach,林下除杂草外无其他植被,林地地势较平坦。其中山核桃树龄5~20年生,树高为3~15 m,胸径为8~25 cm,林冠郁闭度约0.5。虫源利用云斑天牛的假死性进行人工捕捉。
-
在山核桃林中随机选取健康状态以及被天牛取食、产卵和钻蛀受害状态的山核桃(树龄为5~10年生,树高为5~15 m,胸径为10~15 cm)各3株并标记,其中各株样树仅1种受害状。采用活体植物动态顶空吸附法在同一时间收集样树不同受害状态下山核桃释放的挥发物,采样6 h(9:00-16:00),重复3次。为消除空气中的气体对采集样品的污染,各种受害状态设空白对照样2份。具体的收集方法和步骤参见文献[18]。
-
在中国科学院动物研究所实验室进行雌、雄云斑天牛成虫对不同受害状态下山核桃枝条挥发物洗脱液的触角电位反应实验。GC-EAD分析采用Agilent 6890N气相色谱,色谱柱为HP-5MS,载气:高纯度氮气,流速1.0 mL·min-1;进样量2.0 μL,无分流进样,进样口温度220 ℃,氢火焰离子检测器,检测器温度280 ℃。柱温升温程序:40 ℃恒温1 min,然后以4 ℃·min-1的速度升温到220 ℃恒温3 min,共运行49 min。测试的样品为不同受害状态下山核桃枝条挥发物洗脱液和对照正己烷溶剂,试验用虫均为活体,重复5次,GC-EAD与色谱图上的化合物的峰形和保留时间进行对比,从而鉴定出对雌、雄云斑天牛触角有触角电位反应的物质。具体测定方法和步骤参见文献[18]。
-
据云斑天牛成虫对不同受害状态下山核桃挥发物的触角电位反应,选择有强烈电生理反应的8种挥发物(表 1),用1.0 mL液体石蜡作溶剂将各化合物配制成浓度为1.0 mol·L-1的溶液[19]。Y型嗅觉仪生物测定装置的规格和嗅觉反应的实验方法和步骤参见文献[18]。
化合物 纯度/% CAS号 来源 蒎烯(1R)-(+)-alpha-pinene 99 7785-70-8 阿拉丁 萜品烯g-terpinene 98 99-85-4 南京草本源生物科技有限公司 壬醛nonanal 96 124-19-6 阿拉丁 α-萜品醇α-terpineol ≥96 98-55-5 上海源叶生物科技有限公司 五甲基苯pentamethyl phenyl ≥98 700-12-9 阿拉丁 丙烯酸异辛酯2-ethylhexyl acrylate ≥99 103-11-7 阿拉丁 2, 6-二甲基萘2, 6-dimethylnaphthalene 98 581-42-0 阿拉丁 正十六烷hexadecane 98 544-76-3 阿拉丁 Table 1. Name, purity and source of the eight standard volatiles
-
试验数据用SPSS 19.0软件进行统计分析。比较云斑天牛成虫对不同化合物行为反应的差异显著性通过卡方检验来分析[20-21],其中选择率计算公式如下:选择率=(气味臂内的总虫数或对照臂的总虫数/测试的总虫数)×100%。
1.1. 供试植物和虫源
1.2. 采用动态顶空采集法收集山核桃挥发物
1.3. 云斑天牛成虫对不同受害状态下山核桃挥发物的触角电位反应
1.4. 云斑天牛成虫对8种标准挥发物的嗅觉反应
1.5. 数据分析
-
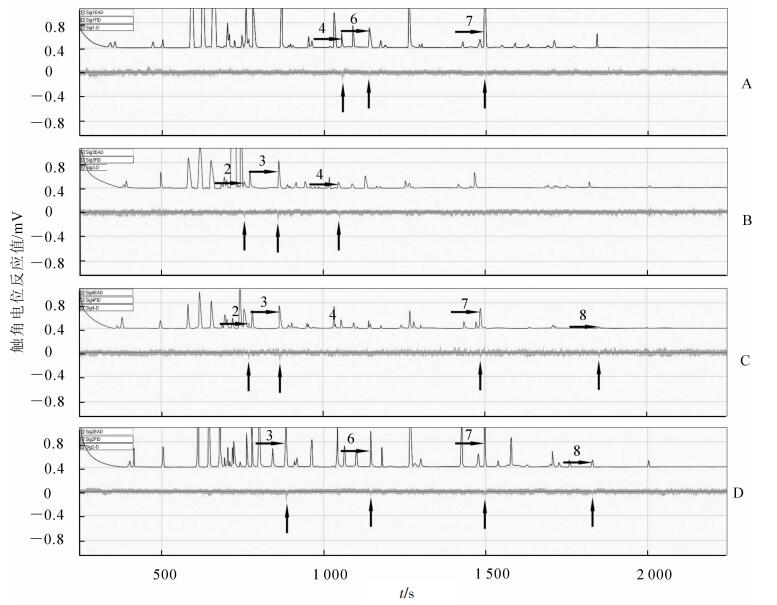
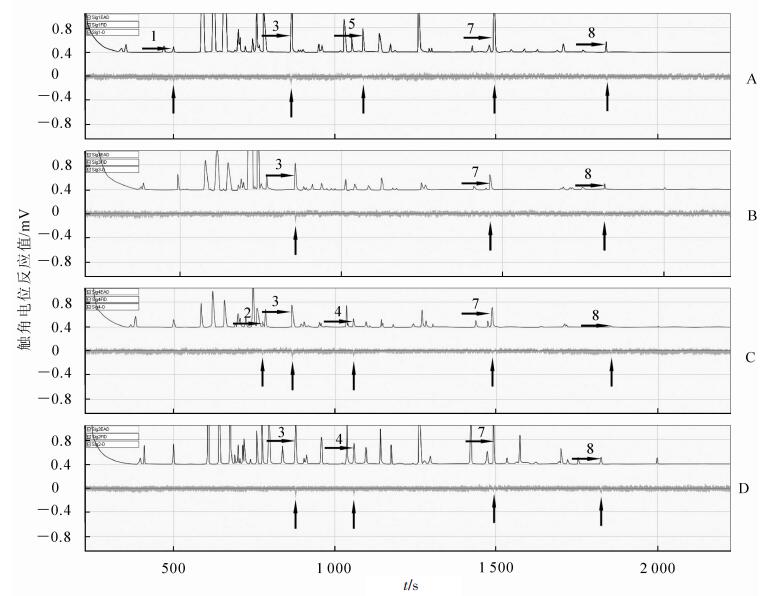
每种受害状态选择1个电生理反应明显且重复的图谱(图 1和图 2)。从图 1和图 2可知:云斑天牛雌雄成虫对不同受害状态下山核桃挥发物的电生理反应是不同的,其中雌成虫触角仅对健康山核桃挥发物中的蒎烯、壬醛、五甲基苯、2, 6-二甲基萘及正十六烷有电生理反应,雌成虫触角仅对取食受害的山核桃挥发物中的壬醛、2, 6-二甲基萘及正十六烷有电生理反应,雌成虫触角仅对产卵受害的山核桃挥发物中的萜品烯、壬醛、α-萜品醇、2, 6-二甲基萘及正十六烷有电生理反应,雌成虫触角仅对钻蛀受害的山核桃挥发物中的壬醛、α-萜品醇、2, 6-二甲基萘及正十六烷有电生理反应(图 1);雄成虫触角仅对健康的山核桃挥发物中的α-萜品醇、丙烯酸异辛酯及2, 6-二甲基萘有电生理反应,雄成虫触角仅对取食受害的山核桃挥发物中的萜品烯、壬醛及α-萜品醇有电生理反应,雄成虫触角仅对产卵受害的山核桃挥发物中的萜品烯、壬醛、2, 6-二甲基萘及正十六烷有电生理反应,雄成虫触角仅对钻蛀受害的山核桃挥发物中的壬醛、丙烯酸异辛酯、2, 6-二甲基萘及正十六烷有电生理反应(图 2)。

Figure 1. Electroantennogram responses pattern of female Batocera horsfieldi adults to volatiles released from the Carya cathayensis in different damaged states

Figure 2. Electroantennogram responses pattern of male Batocera horsfieldi adults to volatiles released from the Carya cathayensis in different damaged states
综上所述,云斑天牛雌雄成虫触角仅对蒎烯、萜品烯、壬醛、α-萜品醇、五甲基苯、丙烯酸异辛酯、2, 6-二甲基萘及正十六烷有电生理反应。
-
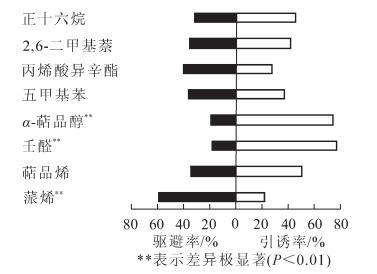
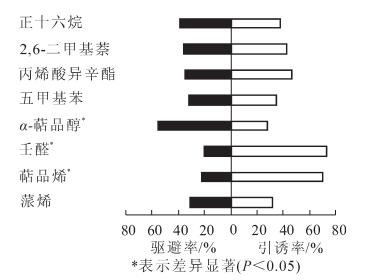
卡方检验结果表明:云斑天牛雌雄成虫对这8种化合物显示出的行为反应是存在差异的。其中壬醛和α-萜品醇对云斑天牛雌成虫有极显著引诱作用(P<0.01),雌成虫对这2种化合物的选择率分别是76.67%和74.44%,而蒎烯对云斑天牛雌成虫有极显著的驱避作用(P<0.01),雌成虫对其选择率仅22.22%,其余5种化合物对云斑天牛雌成虫没有明显的行为反应(图 3);萜品烯、壬醛和α-萜品醇能引起云斑天牛雄成虫显著的行为反应(P<0.05),其中萜品烯和壬醛对其有引诱作用,雄成虫对它们的选择率分别是70.00%和73.33%,而α-萜品醇对其有驱避作用,雄成虫对其选择率仅27.78%,其余5种化合物不能引起云斑天牛雄成虫显著的行为反应(图 4);雌雄虫对这8种化合物显示出的行为反应差别主要是α-萜品醇对雌成虫有极显著的引诱作用,而对雄成虫有显著的驱避作用。结果表明:引起云斑天牛成虫触角产生电生理反应的挥发物不一定会对云斑天牛产生显著的引诱或趋避作用。
2.1. 云斑天牛成虫对不同受害状态下山核桃挥发物的触角电位反应
2.2. 云斑天牛成虫对6种化合物的行为反应
-
有报道显示:植食性昆虫对不同浓度的同一化合物产生的电生理反应有差异[22]。触角电位实验结果表明:云斑天牛成虫对在不同受害状态下山核桃释放的挥发物中的蒎烯、萜品烯、壬醛、α-萜品醇、五甲基苯、丙烯酸异辛酯、2, 6-二甲基萘及正十六烷8种挥发物产生显著的电生理反应,但云斑天牛成虫对不同受害状态下山核桃中这8种挥发物存在不同的电生理反应。这可能是因为这8种挥发物虽在不同受害状态下山核桃挥发物中都存在,但比例和浓度不同。
卡方检验结果表明:壬醛和α-萜品醇对云斑天牛雌成虫均存在极显著引诱作用(P<0.01),萜品烯和壬醛对云斑天牛雄成虫产生显著引诱作用(P<0.05);而蒎烯对云斑天牛雌虫产生极显著驱避作用(P<0.01),α-萜品醇对云斑天牛雄成虫产生显著驱避作用(P<0.05)。张冬勇[14]报道,壬醛引起云斑天牛产生显著的电生理反应,将其作为诱芯时诱捕到了云斑天牛;且YANG等[23]报道中m(顺-3-己烯醇):m(壬醛):m(三氯乙烯):m(β-月桂烯)=57:15:17:11引起云斑天牛产生最强的电生理和行为反应,其中壬醛是混合物的一种成分。
云斑天牛雌雄成虫对挥发物的电生理和行为反应有差异。据报道:天牛在定位寄主时主要通过触角上的感受器来识别寄主植物[24],说明这可能是天牛触角的性二型导致的差异。TOOKER等[21]研究发现:香草烯不能引起黄峰Antisrophus rufus显著的行为反应,但它可以加强其他挥发物的作用,说明植物挥发物中某一挥发物本身不能引起植食性昆虫显著的引诱或趋避反应,但其存在可能会加强或遏制其他挥发物的作用。如嗅觉反应实验中的五甲基苯、丙烯酸异辛酯、2, 6-二甲基萘和十六烷,虽不能引起云斑天牛产生显著的行为反应,但其作用不容忽略,在后期的研究中需加以重视。
供试天牛是从野外人工捕捉的,无法确保天牛成虫虫龄的完全一致,这可能会对实验结果产生一定的影响,因为不同虫龄的天牛成虫对挥发物的反应可能不同。天牛对植物化学指纹图谱(chemical fingerpint)[25]的识别是极其复杂的,研究单一挥发物对云斑天牛的作用仅是第一步,还需要进一步的研究,即将不同有效成分按特定比例混配进行嗅觉反应实验和林间诱捕试验。











 DownLoad:
DownLoad: