-
芽孢杆菌Bacillus因能产生耐热、耐旱、抗紫外线和有机溶剂的芽孢[1],具有在不同环境下存活、定殖与繁殖等方面的优势[2],对植物病菌的作用机制多样[2-3],使得芽孢杆菌成为一种理想的生防菌。芽孢杆菌的作用机制主要包括竞争作用、拮抗作用和诱导植物抗性[2-3]等方面,其中拮抗作用是指生防菌株产生次生代谢产物抑制有害病原菌的生长、发展或直接杀灭病原菌,或是通过次生代谢产物改变自身周围的微环境,使之不利于病原微生物的生长繁殖[4]。芽孢杆菌产生的拮抗物质主要有抗生素、细菌素、细胞壁降解酶类和其他抗菌蛋白及挥发性抗菌物质[3]。脂肽类抗生素是一大类重要的拮抗物质[5],其理化性质稳定,对高温、酸和弱碱具有一定的耐受[6],已成为芽孢杆菌拮抗物质领域的研究重点。研究发现,解淀粉芽孢杆菌植物亚种Botryosphaeria amyloliquefaciens subsp. plantarun FZB42能够产生surfactin, fengycin等脂肽类物质抑制立枯丝核菌[7],解淀粉芽孢杆菌SWB16菌株产生的fengycin和iturin对球孢白僵菌分生孢子的发芽和菌丝生长具有显著的抑制作用[8]。借助扫描电子显微镜探究脂肽类抗生素对植物病原菌的抑菌机制表明,脂肽类抗生素导致真菌菌丝及孢子畸形,细胞膜破裂,从而使细胞代谢无法正常进行,最终导致细胞的死亡[9-11]。山核桃Carya cathayensis是投入产出效益很高的经济树种之一[12]。由于纯林化及片面追求产量的经营措施,导致山核桃林病虫害日趋严重[13],其中危害最大的是山核桃干腐病。山核桃干腐病又称山核桃溃疡病、墨汁病,病原菌为葡萄座腔菌Botryosphaeria dothidea。2009年以来,浙江省与安徽省将近90%的山核桃林感染山核桃干腐病,且病情继续加重。2015年仅临安市枯死的山核桃树累计达3.7万株,损失1 000万元之多。迄今为止对山核桃干腐病缺乏有效的防治方法,虽然某些化学农药有一定的效果,但化学农药的长期大量使用,产生了环境污染、病虫抗药性及农药残留等令人担忧的问题。因此,从生物防治角度来控制山核桃干腐病病情将是有效的途径。本研究应用实验室前期分离得到的微生物资源,通过平板对峙法筛选出1株对病原菌有明显抑制效果的解淀粉芽孢杆菌,经鉴定为解淀粉芽孢杆菌植物亚种(菌种保藏号:CGMCC 11640)。为初步了解CGMCC 11640产生的拮抗物质对山核桃干腐病病原菌的抑菌机制,推测拮抗物质中是否含有耐高温的脂肽类抗生素,本研究利用扫描电子显微镜观察CGMCC 11640菌体及其灭菌发酵上清液对山核桃干腐病病原菌菌丝生长及形态的影响,为山核桃干腐病生防菌的开发利用提供初步理论基础。
-
山核桃干腐病病原菌,解淀粉芽孢杆菌植物亚种(菌种保藏号为CGMCC 11640)。
-
平板对峙法共同培养病原菌与CGMCC 11640。在马铃薯葡萄糖琼脂培养基(PDA)平板中央接种病原菌圆饼,在距平板中心10 mm的两侧接CGMCC 11640圆饼,3个圆饼保持在一条水平线上,28 ℃培养。从抑菌带一侧的病原菌菌落边缘切正方形小块制备扫描电镜样品,观察对峙培养4,6,7 d后病原真菌的形态变化,以单独培养的病原菌作为对照。
-
选取CGMCC 11640菌的单菌落,接种于100 mL PDA液体培养基中,在37 ℃,摇瓶转速为180 r·min-1条件下培养14 h,得到CGMCC 11640菌株种子液。CGMCC 11640种子液按接种量为3%(体积分数)分别接种于500 mL PDA液体培养基中,在37 ℃,180 r·min-1条件下培养48 h,即得到CGMCC 11640菌株发酵液。将CGMCC 11640菌株发酵液3 000 r·min-1离心15 min后,去除菌体,得到CGMCC 11640灭菌上清液。将CGMCC 11640发酵上清液与水分别按V(上清液):V(水)=1 : 9(处理1),2 : 8(处理2),5 : 5(处理3)的比例配制成的含有CGMCC 11640灭菌发酵上清液的PDA混合培养基。
-
采用生长速率法测定菌丝抑制率。将含有CGMCC 11640灭菌发酵上清液的PDA混合培养基,在培养基中央分别接种直径10 mm的病原菌菌饼,重复3次·处理-1。28 ℃培养5 d后,十字交叉法测量病原菌菌落直径,计算菌丝抑制率。抑制率(%)=[(对照组菌落直径-处理组菌落直径)/对照组菌落直径]×100%。
-
在含有CGMCC 11640灭菌发酵上清液的PDA混合培养基中央接种直径10 mm的病原菌菌饼,28 ℃培养。重复3次·处理-1。从病原菌菌落边缘切正方体小块制备扫描电镜样品,观察培养3 d后病原真菌菌丝的形态变化,以在不含CGMCC 11640灭菌发酵上清液的PDA纯培养基上生长的病原菌作为对照。
-
将切取的菌落边缘正方体小块,浸没于体积分数为2.5%的戊二醛,4 ℃下静置过夜。0.1 mol·L-1磷酸缓冲盐溶液(PBS)浸洗样品15 min,重复3次。随后,将样品依次浸没于梯度体积分数为30%,50%,70%,80%,90%,95%的乙醇中15 min,再用体积分数为100%的乙醇洗脱2次,20 min·次-1。用V(乙醇):V(醋酸异戊酯)=1 : 1混合液处理样品30 min,再用体积分数为100%醋酸异戊酯处理样品2 h。样品自然风干后镀金,使用扫描电镜观察。
-
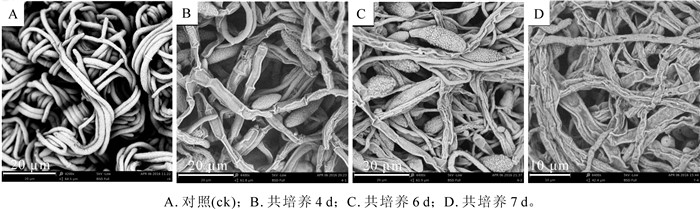
平板对峙培养时病原菌与CGMCC 11640形成抑菌带,说明CGMCC 11640抑制病原菌菌丝的生长。用扫描电镜观察病原菌单独培养(对照组)以及病原菌与CGMCC 11640对峙培养4,6,7 d时病原菌丝形态发现,对照组培养4,6,7 d的病原菌菌丝均通体完整饱满,粗细均匀,图 1A为对照组培养4 d时的扫描电镜图;对峙培养的病原菌菌落边缘菌丝明显异常,培养第4天时有部分菌丝出现萎缩干瘪现象(图 1B),第6天则绝大多数菌丝出现缩干瘪现象(图 1C),到培养第7天菌丝全部萎缩干瘪(图 1D),说明后期干瘪程度比前期严重。对照组的孢子形态饱满(图 2A),而对峙培养第4天时病原菌孢子正常(图 2B),而到第6天时部分病原菌孢子表现出凹陷、萎缩现象(图 2C),第7天异常孢子数量增加花板(图 2D)。畸形孢子的数量随之培养时间的延长而增多。
-
11640灭菌发酵上清液对病原菌菌丝生长的抑制效果测量含有不同比例CGMCC 11640灭菌发酵上清液的PDA混合培养基上生长5 d后的病原菌菌落直径,计算出菌丝抑制率如表 1。结果表明:CGMCC 11640灭菌发酵上清液对山核桃干腐病病原菌菌丝生长均具有较强的抑制作用,含灭菌发酵上清液与水的比例分别为V(上清液):V(水)=1 : 9,2 : 8,5 : 5的PDA混合培养基上生长的病原菌菌丝抑制率分别为75.81%,88.84%和94.30%,说明抑制效果在实验范围内随着发酵液含量的增加而增强。
表 1 CGMCC 11640灭菌发酵上清液对山核桃干腐病菌丝抑制率
Table 1. Hyphal inhibitory ratio of CGMCC 11640 sterilized supernatant liquor against B. dothidea
V(发酵上清液):V(水) 对照组菌落直径/mm 处理组菌落直径/mm 抑制率/% 1:9 86.0±0.1 20.8±2.3 a 75.81 2:8 86.0±0.1 9.6±1.4 b 88.84 5:5 86.0±0.1 4.9±0.1 c 94.30 说明:不同字母表示不同处理之间病原菌菌落直径在5%水平上差异显著,即P<0.05。 -
分别切取不含CGMCC 11640灭菌发酵上清液的PDA纯培养基(对照组)和含有CGMCC 11640灭菌发酵上清液PDA混合培养基(处理组)上生长3 d后的病原菌边缘菌落,制成扫描电镜样品,观察菌丝形态变化。结果发现:对照组病原菌丝菌丝整体圆润光滑,粗细均匀(图 3A),处理组病原菌菌丝出现萎缩干瘪现象(图 3 B),观察结果与2.1相同,说明耐高温的CGMCC 11640的代谢产物对病原菌起抑制作用。
-
本研究通过扫描电镜观察,结合其他研究者对芽孢杆菌抑真菌的机制成果推测,得出结论:CGMCC 11640菌株及其代谢物对病原菌的抑制作用主是通过破坏菌丝和孢子细胞壁和细胞膜,使细胞内原生质的泄露,从而导致菌丝和孢子萎缩,最终杀死病原菌细胞。
非核糖体合成的脂肽类抗生素是芽孢杆菌产生的拮抗物质中重要的一类。常见的三大家族脂肽类物质surfactins,iturins和fengycins在防治植物病害中最早开始研究[14-16]。因脂肽类稳定的理化性质及广泛的抗菌活性使其成为生防菌剂的研究热点。脂肽类的抗菌机制研究主要集中在作用于细胞膜,引起细胞膜的破裂和细胞质的泄露[17-19]。陶阳等[10]采用显微技术观察发现2种抗菌脂肽surfctin和fengycin能够导致桃软腐病菌菌丝体变形,细胞壁和细胞膜的破裂,原生质的泄露,细胞器、细胞核出现异常,使细胞代谢无法正常进行,最终导致细胞的死亡。胡陈云等[11]通过扫描电镜观察发现,被抗菌脂肽抑制的人参病原菌菌丝出现明显萎缩、皱缩现象。有研究表明:脂肽抗生素理化性质稳定,如较好的热稳定性,121 ℃高温处理仍有较高的活性[20]。本研究用含有CGMCC 11640灭菌发酵上清液的PDA混合培养基培养病原菌,发现病原菌菌丝生长受到抑制,说明CGMCC 11640发酵液中含有耐高温的抑菌物质。然后利用扫描电镜观察与CGMCC 11640菌株对峙培养以及在含有CGMCC 11640灭菌发酵上清液的培养基上生长的病原菌菌丝形态结构,发现病原菌菌丝形态均发生异常,主要表现为菌丝萎缩、干瘪、形成褶皱,但没有观察到菌丝体的外壁溶解现象;对峙培养6 d时观察病原菌孢子出现凹陷、萎缩现象,畸形孢子的数量随着培养时间的延长而增多。由此说明:CGMCC 11640通过代谢产物破坏细胞壁和细胞膜,使细胞内原生质的泄露,从而导致菌丝和孢子萎缩,与报道的有关脂肽抗生素的作用机制相一致。后续采用酸沉淀法提取脂肽类物质,经HPLC,LC-MS技术分析鉴定得出,CGMCC 11640菌株能够产生surfactins,iturins和fengycins三大类脂肽类物质。2015年5月进行初步林间试验,将脂肽粗提物与愈合剂混合后涂在山核桃干腐病斑处,2016年5月观察发现,伤口愈合,不再有黑色液体流出,表明CGMCC 11640产生的脂肽类物质对山核桃干腐有较好的抑制作用。因此,可以将CGMCC 11640菌株产生的脂肽类抗生素制成生物农药防治山核桃干腐病。
-
本研究承蒙浙江农林大学林业与生物技术学院王彦先生在扫描电镜使用方面的技术指导。谨此致谢!
Inhibitory mechanism of Bacillus amyloliquefaciens subsp. plantarum CGMCC 11640 against Botryosphaeria dothidea, the pathogen of canker disease of Carya cathayensis
-
摘要: 葡萄座腔菌Botryosphaeria dothidea引起的山核桃Carya cathayensis干腐病是导致山核桃树发病甚至死亡的主要病害之一,对山核桃产业带来了严重威胁。通过平板对峙法成功筛选出1株对病原菌有明显抑制效果的解淀粉芽孢杆菌植物亚种Bacillus amyloliquefaciens subsp. plantarum(菌种保藏号:CGMCC 11640)。试图通过扫描电镜探究该菌株对山核桃干腐病菌的抑制机制。对峙培养4,6和7 d后,比较菌丝和孢子形态,发现病原菌菌丝出现萎缩干瘪现象,培养时间越长,菌丝萎缩干瘪程度越大;对峙培养4 d时病原菌孢子正常,而到第6天时部分病原菌孢子表现出凹陷、萎缩现象,第7天异常孢子数量增加。用CGMCC 11640发酵上清液与水分别按V(上清液):V(水)=1:9,2:8,5:5的比例配制成的马铃薯葡萄糖琼脂(PDA)混合培养基培养病原菌,结果发现病原菌菌丝抑制率分别为75.81%,88.84%和94.30%,说明灭菌发酵上清液对病原菌有抑制作用。电镜观察发现,病原菌菌丝在含有灭菌发酵上清液的PDA混合培养基上出现萎缩干瘪现象。通过扫描电镜观察,推测CGMCC 11640菌株及其代谢物对病原菌的抑制作用主是通过破坏菌丝和孢子细胞壁和细胞膜,使细胞内原生质泄露,从而导致菌丝和孢子萎缩,最终杀死病原菌细胞。
-
关键词:
- 森林保护学 /
- 山核桃干腐病 /
- 解淀粉芽孢杆菌植物亚种 /
- 抑菌机制 /
- 扫描电镜
Abstract: The canker disease of Carya cathayensis caused by Botryosphaeria dothidea, is one of the diseases in China that has resulted in serious damage and even death for the tree. To overcome the serious disease threat to the C. cathayensis industry, Bacillus amyloliquefaciens subsp. plantarum CGMCC 11640, which has shown a strong inhibitive activity in vitro against Botryosphaeria dothidea, was obtained by screening with the dual-culture method and cultured in medium mixtures with ratios of sterilized fermentation supernate to PDA being 1:9, 2:8, and 5:5(volume ratio). Then, the inhibitory mechanisms of strain CGMCC 11640 against Botryosphaeria dothidea were further explored by scanning electron microscopy (SEM). Results revealed that the Botryosphaeria dothidea strain growth rate for the three volume ratio mediums were restrained by 75.8% for 1:9, 88.8% for 2:8, and 94.3% for 5:5. The SEM results showed that both bacterial cells and their sterilized fermentation supernate exhibited strong inhibition activity in vitro against Botryosphaeria dothidea and that the hyphae and spores of Botryosphaeria dothidea exhibited atrophy and introcession. Thus, CGMCC 11640 inhibited Botryosphaeria dothidea by destroying cell membranes (e.g. punching holes in them) and walls of hyphae and spores resulting in leakage of plasmatic material from the inside of the cells. -
表 1 CGMCC 11640灭菌发酵上清液对山核桃干腐病菌丝抑制率
Table 1. Hyphal inhibitory ratio of CGMCC 11640 sterilized supernatant liquor against B. dothidea
V(发酵上清液):V(水) 对照组菌落直径/mm 处理组菌落直径/mm 抑制率/% 1:9 86.0±0.1 20.8±2.3 a 75.81 2:8 86.0±0.1 9.6±1.4 b 88.84 5:5 86.0±0.1 4.9±0.1 c 94.30 说明:不同字母表示不同处理之间病原菌菌落直径在5%水平上差异显著,即P<0.05。 -
[1] 李晶, 杨谦.生防枯草芽孢杆菌的研究进展[J].安徽农业科学, 2008, 36(1):106-111. LI Jing, YANG Qian. Research progress on biocontrol Bacillus subtilis[J]. J Anhui Agric Sci, 2008, 36(1):106-111. [2] 陈中义, 张杰, 黄大昉.植物病害生防芽孢杆菌抗菌机制与遗传改良研究[J].植物病理学报, 2003, 33(2):97-103. CHEN Zhongyi, ZHANG Jie, HUANG Dafang. Research progress on antimicrobial mechanism and genetic engineering of Bacillus for plant diseases biocontrol[J]. Acta Phytopathol Sin, 2003, 33(2):97-103. [3] 彭研, 陈相艳, 裘纪莹, 等.生防芽孢杆菌的研究进展[J].山东农业科学, 2013, 45(7):138-140. PENG Yan, CHEN Xiangyan, QIU Jiying, et al. Research progress on biocontrol Bacillus[J]. Shandong Agric Sci, 2013, 45(7):138-140. [4] 赵东洋. 解淀粉芽孢杆菌SWB16脂肽类代谢产物对球孢白僵菌的拮抗作用及发酵条件的初步优化[D]. 重庆: 西南大学, 2014. ZHAO Dongyang. Antagonism of the Lipopeptide Metabolites Produced by Bacillus amyloliquefaciens strain SWBl6 Against Beauveria bassiana and Prelimary Optimization of Its Formation[D]. Chongqing:Southwest Universuty, 2014. [5] 王智文, 刘训理.芽孢杆菌非核糖体肽的研究进展[J].蚕业科学, 2006, 32(3):392-398. WANG Zhiwen, LIU Xunli. Research advances in nonribosomal peptides produced by Bacillus[J]. Sci Sericul, 2006, 32(3):392-398. [6] 徐杨, 王楠, 李伟, 等.海洋枯草芽孢杆菌3512A抗真菌脂肽的分离纯化及结构特性鉴定[J].中国生物防治, 2009, 25(4):328-333. XU Yang, WANG Nan, LI Wei, et al. Purification and structural identifications of the antifungal lipopeptides produced by Marine bacterium Bacillus subtilis 3512A[J]. ChinJ Biol Control, 2009, 25(4):328-333. [7] CHOWDHURY S P, HARTMANN A, GAO Xuewen, et al. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42:a review[J]. Front Microbiol, 2015, 6:780. doi:10.3389/fmicb. 2015. 00780. [8] 汪静杰, 赵东洋, 刘永贵, 等.解淀粉芽孢杆菌SWB16菌株脂肽类代谢产物对球孢白僵菌的拮抗作用[J].微生物学报, 2014, 54(7):778-785. WANG Jingjie, ZHAO Dongyang, LIU Yonggui, et al. Antagonism against Beauveria bassiana by lipopeptide metabolites produced by entophyte Bacillus amyloliquefaciens strain SWB16[J]. Acta Microbiol Sin, 2014, 54(7):778-785. [9] 孔建, 赵白鸽, 王文夕, 等.枯草芽孢杆菌抗菌物质对镰刀菌抑制机理的镜下研究[J].植物病理学报, 1998, 28(4):337-340. KONG Jian, ZHAO Baige, WANG Wengxi, et al. Survey on the antifungal mechanism of Bacillus subtilis cohen to Fusarium oxysporum under the microscope[J]. Acta Phytopathol Sin, 1998, 28(4):337-340. [10] 陶阳. Bacillus subtilis fmbJ抗菌脂肽对Rhizopus stolonifer作用机理研究[D]. 南京: 南京农业大学, 2010. TAO Yang. Antifungal Mechanism of Antimicrobial Lipopeptide Produced by Bacillus subtilis fmbJ against Rhizopus stolonifer[D]. Nanjing:Nanjing Agricultural University, 2010. [11] 胡陈云, 李勇, 刘敏, 等.枯草芽孢杆菌ge25对2种人参病原菌的抑制作用及脂肽类抑菌代谢产物的鉴定[J].中国生物防治学报, 2015, 31(3):386-393. HU Chenyun, LI Yong, LIU Min, et al. Antagonism of Bacillus subtilis ge25 against two kinds of ginseng pathogens and identification of antifungal lipopeptide metabolites[J]. Chin J Biological Control, 2015, 31(3):386-393. [12] 郑万钧.中国树木志[M].北京:中国林业出版社, 1985:23-79. [13] 杨淑贞, 丁立忠, 楼君芳, 等.山核桃干腐病发生发展规律及防治技术[J].浙江林学院学报, 2009, 26(2):228-232. YANG Shuzhen, DING Lizhong, LOU Junfang, et al. Occurrence regularity of Carya cathayensis canker disease and its control[J]. J Zhejiang For Coll, 2009, 26(2):228-232. [14] CHAN Y K, SAVARD M E, REID L M, et al. Identification of lipopeptide antibiotics of a Bacillus subtilis isolate and their control of Fusarium graminearum diseases in maize and wheat[J]. Biocontrol, 2009, 54(4):567-574. [15] ONGENA M, JOURDAN E, ADAM A, et al. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants[J]. Environ Microbiol, 2007, 9(4):1084-1090. [16] GANZ T, LEHRER R I. Defensin[J]. Curr Opin Immunol, 1994, 66(4):584-589. [17] DELEU M, BOUFFIOUX O, RAZAFINDRALAMBO H, et al. Interaction of surfactin with membranes:a computational approach[J]. Langmuir, 2003, 19(8):3377-3385. [18] DELEU M, PAQUOT M, NYLANDER T. Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes[J]. Biophys J, 2008, 94(7):2667-2679. [19] MAGET-DANA R, PEYPOUX F. Iturins, a special class of pore-forming lipopeptides:biological and physicochemical properties[J]. Toxicologyl, 1994, 87(1/3):151-174. [20] 裴炎, 李先碧, 彭红卫, 等.抗真菌多肽APS-1的分离纯化与特性[J].微生物学报, 1999, 39(4):344-349. PEI Yan, LI Xianbi, PENG Hongwei, et al. Purification and characterization of a novel antifungal peptide APS-1 produced by Bacillus cereus[J]. Acta Microbiol Sin, 1999, 39(4):344-349. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2017.02.017







 下载:
下载: