-
叶绿素是植物体中参与光合作用的重要色素,它能够捕捉光能转变成化学能用于植物生长和代谢过程。在植物生长发育过程中,其光合色素含量和叶绿素荧光参数均上升,光合效率增加。随叶片发育,板栗Castanea millissima[1]和紫丁香Syringa oblate[2]的叶绿素含量逐渐增加;在银杏Ginkgo biloba叶片展开过程中,叶绿素含量和净光合速率均增加[3];在绿豆Vigna radiata幼苗脱黄化初期,叶片叶绿素a和叶绿素b含量随光照时间增加而逐渐上升,而叶绿素a/b比值逐渐降低[4]。玉米Zea mays叶片展开过程中从叶基到叶尖光化学活性逐渐完善[5];杨树Populus叶片在春季发育过程中,光系统Ⅱ(PSⅡ)最大光化学效率、光能吸收、传递效率和反应中心数目均趋于稳定,且光系统也渐渐发育完善[6];与针叶相比,挪威云杉Picea abies营养芽的性能指标(PIABS)显著下降,PSⅡ最大光化学效率和光合效率也略有下降[7]。毛竹Phyllostachys edulis作为中国森林资源重要的组成部分,分布面积大,开发利用程度高,是集经济、生态和社会效益于一体的笋材两用竹种。目前,对毛竹的研究主要集中在毛竹林生态系统生物量及其碳通量的变化特征[8-9],毛竹叶片叶绿素含量的年动态变化[10],毛竹叶片的光合途径[11],毛竹发育过程中叶片的光合生理、茎秆的光合色素、光合酶活性的变化[12-13]等方面。但对毛竹快速生长期茎秆叶绿素荧光参数的研究较为匮乏。鉴于此,本研究以毛竹出笋后快速生长期高度为6 m的茎秆为研究对象,分析了毛竹生长发育过程中,不同节间的光合色素质量分数和叶绿素荧光参数的变化,为进一步研究毛竹快速生长机制提供理论依据。
HTML
-
试验地位于浙江省杭州市临安区(29°56′~30°27′N,118°51′~119°52′E)现代毛竹示范园内,毛竹林为纯林。该区属中亚热带季风气候,温暖湿润,四季分明。年平均气温15.8 ℃,7月气温最高,平均为28.1 ℃,1月气温最低,平均为3.4 ℃。极端最高气温为41.9 ℃,极端最低气温-13.3 ℃。年平均降水量为1 628.6 mm,年平均日照时数为1 939.0 h,无霜期为234.0 d,森林覆盖率达76.5%。毛竹林土壤属山地红壤,土层深度60 cm以上。每年3,7和11月施肥,来年从毛竹密度大的区域收获竹笋。
-
试验材料为2017年当年生毛竹笋竹,2017年4月末,选取生境条件一致,生长状况良好,株高6 m左右,基径约15 cm的自然状态下的毛竹笋竹,从茎秆地上部分的基部将其伐倒,将节间按照从基部至顶部的顺序编号,每间隔2个节间选取测定点,从基部至顶部依次选取编号为1,4,7,10,13,16,19,22的节间,测定时间为10:00-12:00。选取5株笋竹,每株作为1个独立实验,共5次重复。
-
称取0.5 g茎秆外层绿色组织,取样厚度为2 mm,将其迅速剪成碎块后放入具塞的试管中,加入体积分数为80%的丙酮5 mL,室温下遮光萃取48 h,至茎秆外层绿色组织完全变白,取上清液分别在波长为663,646和470 nm处测定其光密度值(D)。参照LICHTENTHALER[14]的公式分别计算光合色素的质量分数。
-
采用非调制式叶绿素荧光仪(YZQ-500,中国)进行快速叶绿素荧光诱导曲线的测定。测定前暗适应20 min,然后暴露在饱和脉冲光(3 000 μmol·m-2·s-1蓝光)下1 s,以10 μs(2 ms之前)和1 ms(2 ms之后)的间隔记录荧光信号,测得快速叶绿素荧光诱导动力学曲线(OJIP)。
-
依照STRASSER等[15-16]提出的能量流动模型,天线色素吸收的能量(ABS)其中一小部分主要以热能和荧光的形式耗散掉,大部分则被反应中心(RC)所捕获,在反应中心激发能被转化为还原能,将初级醌受体(QA)还原为QA-,QA-又可以被重新氧化,从而产生电子传递(ET)用于固定二氧化碳或其他途径,以此为基础发展起来的数据处理就被称为JIP-test。根据JIP-test[15-16]计算得到的部分参数见表 1。
参数缩写 描述 Fo 在暗适应后的最小荧光强度 Fp 在暗适应后的最大荧光强度 φpo 最大光化学效率 ψo 捕获激子将电子传递到电子传递链QA-下游其他电子受体的概率 φEo PSⅡ反应中心吸收光能用于电子传递的量子产额 φDo 用于热耗散的量子比率 ABS/CSo 单位面积吸收的光能 TRo/CSo 单位面积捕获的光能 ETo/CSo 单位面积电子传递的量子产额 DIo/CSo 单位面积的热耗散 ABS/RC 单位反应中心吸收的光能 TRo/RC 单位反应中心捕获的用于还原QA的能量 ETo/RC 单位反应中心捕获的用于电子传递的能量 DIo/RC 单位反应中心耗散掉的能量 RC/CSo 单位面积内反应中心的数量 PIABS 以吸收光能为基础的性能指数 Table 1. Formulae and glossary of terms for the analysis of the fluorescence transient OJIP
-
所有数据均为5次重复的平均值±标准差,利用Origin 9.0统计分析和作图。采用one-way ANOVA,进行Turkey多重比较(P<0.05)。
1.1. 试验地概况
1.2. 试验材料
1.3. 测定方法
1.3.1. 光合色素质量分数的测定
1.3.2. 快速叶绿素荧光诱导曲线的测定
1.4. 叶绿素荧光动力学参数分析
1.5. 数据分析
-
从表 2可看出:随着毛竹茎秆节间升高,叶绿素a,叶绿素b和类胡萝卜素质量分数整体呈逐渐下降的趋势。在第1~7节中3种光合色素质量分数均基本保持不变;从第7节往上不断下降,第16节与第7节相比,叶绿素a,叶绿素b和类胡萝卜素质量分数分别降低了58.9%,67.4%和56.6%(P<00.05),叶绿素总量降低了61.0%(P<00.05);叶绿素a/b比值整体呈上升趋势,第22节比第7节比值升高了59.1%(P<00.05)。
节间 光合色素质量分数/(μg·g-1) 叶绿素a/b 叶绿素a 叶绿素b 总叶绿素 类胡萝卜素 1 15.19 ± 0.28 a 5.07 ± 0.04 a 20.26 ± 0.29 a 6.37 ± 0.27 a 3.00 ± 0.24 c 4 16.22 ± 0.27 a 5.27 ± 0.40 a 21.49 ± 0.39 a 7.00 ± 0.28 a 3.10 ± 0.27 c 7 15.80 ± 1.09 a 5.21 ± 0.20 a 21.01 ± 1.28 a 6.66 ± 0.48 a 3.03 ± 0.10 c 10 13.98 ± 0.42 b 3.91 ± 0.11 b 17.89 ± 0.53 b 6.27 ± 0.32 a 3.58 ± 0.02 c 13 10.01 ± 0.63 c 2.71 ± 0.03 c 12.22 ± 0.61 c 4.27 ± 0.19 b 3.69 ± 0.25 c 16 6.49 ± 0.29 d 1.70 ± 0.09 d 8.19 ± 0.23 d 2.89 ± 0.17 c 3.83 ± 0.35 bc 19 2.43 ± 0.07 e 0.53 ± 0.02 e 2.96 ± 0.09e 1.23 ± 0.09 d 4.59 ± 0.1 ab 22 1.83 ± 0.15 e 0.38 ± 0.03 e 2.22 ± 0.16e 0.97 ± 0.05 d 4.82 ± 0.55 a 说明:数值为平均值±标准差。同列不同字母表示差异显著(P<0.05),同列相同字母表示差异不显著(P>0.05) Table 2. Differences of pigment contents in the Phyllostachys edulis stems of different internodes
-
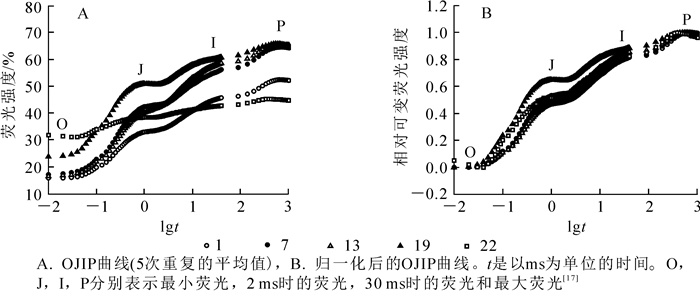
毛竹茎秆在暗适应后的最小荧光O点,PSⅡ处于“完全开放”状态。O点荧光强度随着茎秆节间的升高而上升(图 1A),且随着节间的升高上升越明显。P点表示PSⅡ反应中心完全关闭,不接受光量子,此时荧光产量最高。中下部节间的P点荧光强度变化不显著,从第22节开始下降(图 1A)。在O~P点变化过程中,还存在2个拐点(J点和I点)。J点和I点相对可变荧光强度均随节间升高而上升,从22节开始下降(图 1B)。由上可知,毛竹茎中下部的O相,J相和I相均高于上部,而P相变化不明显。
-
从表 3可以看出:从16节Fo逐渐上升,第16节比第10节上升了22.6%(P<00.05)。FP随着节间的升高而下降,第16节比第10节下降了6.7%(P<00.05)。RC/CSo呈先上升后下降的趋势,第16节比第10节上升了12.0%、第22节比第16节下降了44.7%(P<00.05)。1~13节PIABS变化不显著,从第16节往上逐渐下降,比第10节下降了81.9%(P<00.05)。
节间 Fo FP RC/CSo PIABS 1 15.83 ± 0.85 c 52.50 ± 1.00 c 4.40 ± 0.10 bc 0.69 ± 0.17 a 4 16.47 ± 0.75 c 60.97 ± 0.81 b 5.21 ± 0.57 ab 0.68 ± 0.20 a 7 17.43 ± 2.27 c 64.60 ± 1.15 ab 5.12 ± 0.16 ab 0.69 ± 0.20 a 10 17.67 ± 0.23 c 69.00 ± 2.15 a 5.07 ± 0.27 ab 0.72 ± 0.15 a 13 17.80 ± 0.72 c 65.90 ± 2.51 ab 5.86 ± 0.46 a 0.88 ± 0.16 a 16 21.67 ± 2.15 b 64.37 ± 0.95 ab 5.68 ± 0.24 ab 0.13 ± 0.03 b 19 24.10 ± 0.72 b 64.30 ± 1.51 b 4.71 ± 0.12 b 0.17 ± 0.04 b 22 31.40 ± 0.56 a 43.77 ± 3.81 d 3.14 ± 0.96 c 0.07 ± 0.04 b 说明:数值为平均值±标准差。同列不同字母表示差异显著(P<00.05),同列相同字母表示差异不显著(P>0.05) Table 3. Chlorophyll fluorescence parameters in the Phyllostachys edulis stems of different internodes
-
从表 4可以看出:1~13节φPo,φEo,ψo和φDo变化不显著,从16节φPo,φEo和ψo逐渐下降,分别比第13节下降了9.6%,36.1%和22.0%(P<00.05);φDo比13节上升了36.0%(P<00.05)。
节间 φPo φEo ψo φDo 1 0.70 ± 0.02 a 0.36 ± 0.03 a 0.51 ± 0.04 a 0.30 ± 0.02 bc 4 0.73 ± 0.01 a 0.32 ± 0.04 a 0.44 ± 0.05 a 0.27 ± 0.01 c 7 0.73 ± 0.03 a 0.33 ± 0.02 a 0.45 ± 0.03 a 0.27 ± 0.03 c 10 0.74 ± 0.01 a 0.34 ± 0.03 a 0.46 ± 0.04 a 0.26 ± 0.01 e 13 0.73 ± 0.02 a 0.36 ± 0.03 a 0.50 ± 0.05 a 0.25 ± 0.01 c 16 0.66 ± 0.03 a 0.23 ± 0.01 b 0.39 ± 0.01 b 0.34 ± 0.03 bc 19 0.63 ± 0.01 b 0.21 ± 0.03 b 0.34 ± 0.04 b 0.37 ± 0.01 b 22 0.28 ± 0.07 c 0.15 ± 0.01 c 0.26 ± 0.16 c 0.72 ± 0.07 a 说明:数值为平均值±标准差。同列不同字母表示差异显著(P<00.05),同列相同字母表示差异不显著(P>0.05) Table 4. Changes of quantum yield in the Phyllostachys edulis stems of different internodes
-
从表 5可以看出:ABS/CSo和DIo/CSo从第16节逐渐上升,分别比第13节上升了21.7%和52.1%(P<00.05)。TRo/CSo和ETo/CSo从第16节逐渐下降,分别比第13节降低了20.4%和18.4%(P<00.05)。
节间 ABS/CSo TRo/CSo ETo/CSo DIo/CSo 1 15.83 ± 0.85 c 11.05 ± 0.33 ab 5.63 ± 0.27 ab 4.79 ± 0.54 c 4 16.47 ± 0.75 c 12.01 ± 0.38 ab 5.26 ± 0.77 ab 4.45 ± 0.38 c 7 17.43 ± 2.27 c 12.96 ± 1.15 ab 5.75 ± 0.56 ab 4.75 ± 1.13 c 10 17.67 ± 0.23 c 13.14 ± 0.25 a 6.04 ± 0.64 ab 4.52 ± 0.06 c 13 17.80 ± 0.72 c 12.98 ± 0.24 a 6.42 ± 0.63 a 4.82 ± 0.54 c 16 21.67 ± 2.15 b 10.33 ± 0.31 c 5.24 ± 0.04 ab 7.33 ± 1.42 bc 19 24.10 ± 0.72 b 10.07 ± 0.05 c 5.01 ± 0.05 b 9.03 ± 0.40 b 22 31.40 ± 0.56 a 8.75 ± 1.98 d 4.73 ± 0.43 b 22.65 ± 2.52 a 说明(数值为平均值±标准差。同列不同字母表示差异显著(P < 0.05),同列相同字母表示差异不显著(P>0.05) Table 5. Changes of specific activity per internode area in the Phyllostachys edulis stems of different internodes
-
从表 6可以看出:ABS/RC和DIo/RC从第16节逐渐上升,分别比第10节上升了9.2%和44.9%(P<00.05)。随着节间的升高,ETo/RC和TRo/RC变化不显著。
节间 ABS/RC TRo/RC ETo/RC DIo/RC 1 3.60 ± 0.16 bc 2.51 ± 0.08 a 1.28 ± 0.07 a 1.09 ± 0.11 bc 4 3.18 ± 0.23 c 2.32 ± 0.19 a 1.01 ± 0.04 a 0.86 ± 0.06 c 7 3.40 ± 0.35 bc 2.48 ± 0.16 a 1.12 ± 0.10 a 0.92 ± 0.20 c 10 3.49 ± 0.14 bc 2.60 ± 0.10 a 1.19 ± 0.07 a 0.89 ± 0.05 c 13 3.04 ± 0.16 c 2.22 ± 0.16 a 1.09 ± 0.05 a 0.82 ± 0.04 c 16 3.81 ± 0.21 bc 2.52 ± 0.03 a 0.48 ± 0.03 a 1.29 ± 0.19 bc 19 5.11 ± 0.13 b 3.20 ± 0.04 a 1.08 ± 0.12 a 1.92 ± 0.09 b 22 8.72 ± 1.84 a 2.52 ± 1.03 a 1.32 ± 0.33 a 6.20 ± 0.81 a 说明(数值为平均值±标准差。同列不同字母表示差异显著(P < 0.05),同列相同字母表示差异不显著(P>0.05) Table 6. Change of specific activity of reflecting center in the Phyllostachys edulis stems of different internodes
2.1. 不同节间色素质量分数差异分析
2.2. 不同节间快速叶绿素荧光诱导动力学曲线的变化
2.3. 不同节间最小荧光Fo,最大荧光Fm,反应密度RC/CSo和性能指数PIABS的变化
2.4. 不同节间量子产额的变化
2.5. 不同节间单位面积能量吸收和分配的变化
2.6. 不同节间反应中心能量吸收和分配的变化
-
叶绿素含量的变化对植物光合作用具有一定影响,可作为植物表现光合生理状态的良好指示剂[18]。在毛竹出笋后的快速生长期,不同部位叶绿素a,叶绿素b,类胡萝卜素和总叶绿素质量分数均增加,60 d时最高[19]。随着毛竹年龄的增加,茎秆和叶片中的叶绿素a,叶绿素b和类胡萝卜素质量分数均增加[20]。本研究中,毛竹茎秆中部及下部发育较为成熟,其表面包被的笋箨已逐渐脱落,茎秆外层由下至上接受的光照面积和光照时间逐渐减少,进而导致茎秆皮层绿色组织中光合色素质量分数逐渐减小,叶绿素a/b逐渐增加(表 2)。在毛竹快速生长期,茎秆中下部较高的叶绿素质量分数及其较强的吸收光能和同化二氧化碳能力可能是维持毛竹笋竹快速生长的原因之一。
植物光合原初反应和叶绿素荧光存在着密切的关系,快速叶绿素荧光诱导动力学曲线(OJIP)能够提供关于PSⅡ供体侧、受体侧及PSⅡ反应中心电子氧化还原状态等光化学信息[21-22]。在毛竹快速生长期,上部节间的ψO和φPo均减小,故而φEo下降,且归一化处理后上部节间的J相和I相荧光强度仍下降,表明上部节间PSⅡ反应中心吸收的光能用于电子传递较少,受体侧的传递电子能力较弱,电子在PSⅡ反应中心受体侧QA-处大量积累,QA到QB的电子传递链过程受到了抑制[23],同时其QA和QB被还原的能力相对较弱。PIABS性能指数可以准确地反映植物光合机构的整体状态[5]。上部节间ABS/RC较高,TRo/RC和ETo/RC变化不明显(表 6),同样表明上部节间单位反应中心所吸收的能量并没有更多地用于还原QA和电子传递。PIABS和φPo整体均减小,但PIABS的变化幅度比φPo大,表明这与PSⅡ有活性反应中心的数目和在PSⅡ和PSⅠ之间的传递电子数量有着极大关系。
快速叶绿素荧光诱导动力学曲线(OJIP)还可反映植物光合器官对光能的吸收、转化和耗散等状况[17]。OJIP曲线上的O点(Fo)可理解为植物PSⅠ的活性[24],毛竹茎秆上部节间的ABS/CSo和Fo均高于中下部,表明上部单位面积吸收的光能更多地分配给了PSⅠ,使PSⅠ活性增强,而PSⅡ获得的激发能较少。上部的TRo/CSo和ETo/CSo低于中下部,表明上部节间单位面积吸收的光能较多,但单位面积光合利用率较低,PSⅡ反应中心活性和光合能力也相对较弱。上部节间FP低于中下部,表明上部节间有活性的反应中心数量较少,这与RC/CSO的变化相一致。中下部节间RC/CSo和φPo均高于上部,表明中下部PSⅡ反映中心活性较强,色素所吸收和捕获的能量用于光化学部分的比例较高,优先推动了PSⅡ电子传递(ETo/RC)[25],通过光化学反应转化成与光合作用有关的化学能,有效地发挥了光合机构的功能,提高了茎秆中PSⅡ反应中心内原初的转化效率,这与温星等[12]对毛竹叶片的研究结果一致。在光合机构捕获光能发生电子传递的同时,其中一部分以热能和荧光的形式耗散掉,而光能利用和耗散方式之间存在着相互竞争的关系[26]。上部节间φDo,DIo/CSo和DIo/RC均比中、下部高,这是因为茎秆发育过程中,上部节间的光合系统还未发育成熟,实际用于光合作用的能量较少,PSⅡ和PSⅠ之间电子传递较慢,光合效率较低,通过耗散更多的能量来维持整个能量的合理分配,避免过剩的激发能对光合系统的破坏,这与孙山等[1]对板栗的研究结果一致。
综上所述,在毛竹快速生长期,茎秆不同节间的叶绿素荧光参数存在明显差异,中下部PSⅡ的结构已经基本发育完整,此时PSⅡ反应中心活性较强,电子传递速率较快,能量耗散较少,光能利用效率较高,生长较快;上部节间则与中下部相反,生长比较缓慢。研究成果对明确毛竹快速生长机制具有参考价值。










 DownLoad:
DownLoad: