-
目前,环境污染得到广泛重视,水体污染为受关注的热点领域[1]。在水污染物的重金属成分中,铅(Pb)是一种典型的重金属污染物,严重威胁着生态系统和人体健康[2-3],是水污染控制的重点。在众多的水体污染处理技术中[4-5],水凝胶作为一种高含水量,具有良好生物相容性和环保吸附性能的软材料,已被广泛应用于水污染物去除领域[6]。水凝胶基材的选择决定着凝胶吸附性能的强弱。生物质有着廉价易得、生产成本、不易造成二次污染等优点,可作为理想的改性基材。选择生物质作为基材构筑新型高效、绿色吸附材料是发展趋势。在众多生物质基材原料中,海藻酸钠(SA)作为天然的生物质高分子有机物质,由于其亲水性很强,与钙离子(Ca2+)在水溶液中通过离子交换反应聚合形成凝胶球[7-8],能够有效地去除水中重金属[9]。该特性使其成为制备复合吸附剂的理想框架。在传统的化学吸附材料中添加磁性四氧化三铁(Fe3O4)纳米颗粒可以使传统的化学吸附剂具有物理磁性。吸附剂可以在外部磁场下快速从水体中分离,同时不会在环境中产生二次污染,解决了传统吸附剂不容易与水体分离,难以回收的缺点。因此,制备磁性可回收生物质基水凝胶是一项极有新意的工作。结合海藻酸钠和Fe3O4的优良特性,海藻酸钠和烷基化磁性 Fe3O4纳米粒子制备出新型的复合吸附剂,可以达到增强吸附性能的效果,具有低成本、绿色安全和其他优势。具体工作内容是通过硅烷化反应制备含有端氨基的磁性纳米颗粒,进而利用生物质海藻酸钠包覆磁性颗粒,获得生物基磁性凝胶微球吸附材料。纳米级粒度更有利于凝胶球的包覆行为,并使结构更紧密。本研究制备了1种富含氨基、羟基和羧基多官能团的新型磁性复合凝胶球,同时,研究了复合凝胶球的吸附性能。新型磁性复合凝胶球对铅离子(Pb2+)的吸附效果显著,并具有良好的重复利用性能,为绿色零污染吸附剂的制备提供了基础。
HTML
-
海藻酸钠[(C6H7O6Na)n,980 g·kg−1],七水合硫酸亚铁(Ⅱ)(FeSO 4·7H2O,980~1020 g·kg−1),氢氧化钠(NaOH,960 g·kg−1),氯化钙(CaCl2,990 g·kg−1)购自杭州安耐尔技术有限公司;3-氨丙基-三甲氧基硅烷(APTS,纯度970 g·kg−1)购自阿拉丁制药有限公司;氯化铁(Ⅲ)六水合物(FeCl3·6H2O,980 g·kg−1)、无水乙醇(999 g·kg−1)、氢氧化钠(NaOH,250~280 g·kg−1)、氯乙酸(980 g·kg−1)、氯化铅(分析级)、甲醇(995 g·kg−1)、异丙醇丙二醇(995 g·kg−1)购自国药股份化学试剂有限公司;实验水为蒸馏水。
-
磁性粒子Fe3O4(MNP)的制备方法为离子共沉淀法[10]。将5.9 g FeCl3·6H2O和3.0 g FeSO4·7H2O溶解分散于100 mL蒸馏水中,1 mol·L−1NaOH调节溶液pH至11.0,获得磁性粒子(MNP),75 ℃ 超声搅拌40 min,蒸馏水和甲醇交替洗涤数次。采用强磁分离产物,在甲醇溶液储存合成的MNP,备用。
在200 mL甲醇中加入上述制备得到的MNP,搅拌分散。加入10 mL APTS,氮气(N2)保护下(60 ℃)回流搅拌12 h。甲醇洗涤数次,得到端氨基修饰磁性颗粒(AM),磁力法分离产物,储存在甲醇溶液中备用。参考文献[7],取20 mL 10 g·kg−1海藻酸钠溶液在烧杯中,加入氨基修饰AM,搅拌混合后超声波处理30 min;配置20 g·kg−1氯化钙溶液,将混合悬浮液缓慢匀速滴入其中。悬浮液在水中发生交联形成颜色偏黑、粒度均匀的复合磁性凝胶球。凝胶球在CaCl2溶液中充分浸泡48 h后,用超纯水洗涤数次,即得新型磁性海藻酸钠复合凝胶球(SA@AM)。冻干备用。
-
反应物、中间产物及终产物的红外光谱(IR)谱采用傅立叶变换红外光谱仪(FTIR)测定,扫描波数为4 000~400 cm−1,溴化钾(KBr)压片法制做样品。产物的X射线衍射分析采用粉末衍射仪测试,扫描范围为2θ=20.0°~80.0°,扫描速度为2°·min−1。采用扫描电子显微仪和透射电子显微镜进行样品表观形貌分析。透射电镜采用铜网为载体制样,观察比较MNP、AM和SA@AM复合凝胶球表面形貌。振动样品磁强计(0~±1 T)测量磁化曲线,测试条件为300 K;元素分析仪测试产物元素的分布情况。
-
分别配制以下系列质量浓度10、20、30、40、50、60、70、80、90 mg·L−1的Pb2+标准溶液(pH 6.0),加入1 g的SA@AM凝胶球置于50 mL溶液中,在25 ℃持续吸附3 h。取上清液,确定滤液中离子的浓度,绘制等温吸附曲线,得到产物中Pb2+的最大吸附量。通过模型拟合离子吸附剂的热力学特性并研究吸附曲线。
-
取1 g的SA@AM微球于50 mL质量浓度为80 mg·L−1的Pb2+标准溶液。在25 ℃振动吸附,并在5、10、15、30、45、60、75、90、105、120 min的间隔取出上清液,并测量上清液中的离子浓度,绘制吸附量随时间变化的曲线,得到离子吸附的动力学曲线。通过动力学模型拟合离子吸附动力学曲线来研究离子的吸附动力学。
1.1. 实验材料
1.2. 新型磁性海藻酸钠SA@AM复合凝胶球的制备
1.3. 原料、中间产物及终产物的表征分析
1.4. SA@AM吸附重金属离子的性能测试
1.4.1. 平衡吸附实验
1.4.2. 动力学吸附实验
-
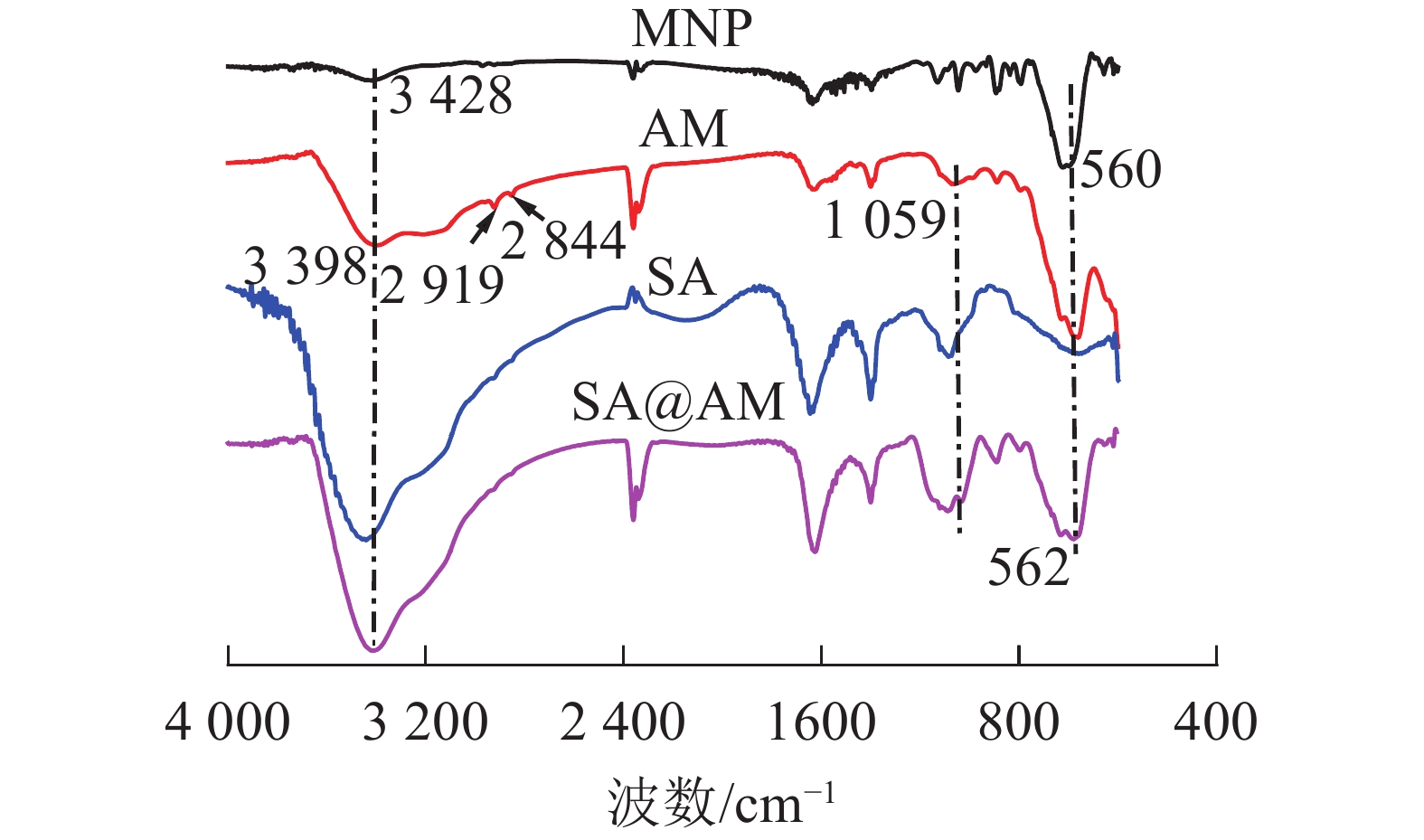
由图1可知:在560 cm−1的吸收峰归属于纳米磁性粒子Fe3O4(MNP)的Fe—O伸缩振动峰,3 428 cm−1的吸收峰归属于Fe3O4的—OH的伸缩振动峰[11]。与MNP的红外光谱相比可知:在3 398 cm−1的吸收峰归属于端氨基AM的N—H伸缩振动峰;亚甲基的对称和不对称伸缩振动峰归属于2 919和2 844 cm−1的吸收峰[12];Si—O—C的伸缩振动吸收峰在1 059 cm−1处显现,Fe3O4中Fe—O键的特征吸收峰在560 cm−1出现。比较产物MNP、AM、SA@AM的红外谱可知:在N—H伸缩振动峰归属于3 397 cm−1的吸收峰;制备的产物AM、SA@AM都含有Fe3O4中Fe—O键的特征吸收峰562 cm−1。综上分析可得:图谱所表示的特征峰基本与产物的结构相一致,可以判定目标产物的成功制备。
-
NMR结构分析是产物表征常用的有效方法。由于强磁材料的特殊性干扰核磁场强,无法获得较为准确核磁谱图,因此,利用元素分析结合X射线衍射仪(XRD)等表征方法对材料结构进行分析。由表1可知:各样品中均可以检测出碳、硅、氧、氮、铁等元素。MNP经硅烷化反应制备得到的富含端氨基AM,AM中氮和硅的质量分数(4.72%和4.98%)有所增加。氨基改性的AM被包覆在SA@AM中,SA@AM与磁性粒子MNP、AM相比,其碳、氧元素的质量分数增加,硅、氮、铁元素的质量分数减少,SA@AM的组成特征与分析结果相符合。综上所述,元素分析质量分数检测结果与产物实际特征相符合,进一步验证了预期产物的成功合成。
磁性材料 元素质量分数/% 碳 硅 氧 氮 铁 MNP 13.27 0.01 16.12 0.37 53.25 AM 14.74 4.98 15.97 4.72 47.34 SA@AM 32.96 1.92 20.21 2.34 26.46 Table 1. The mass fraction of each element in the product sample
-
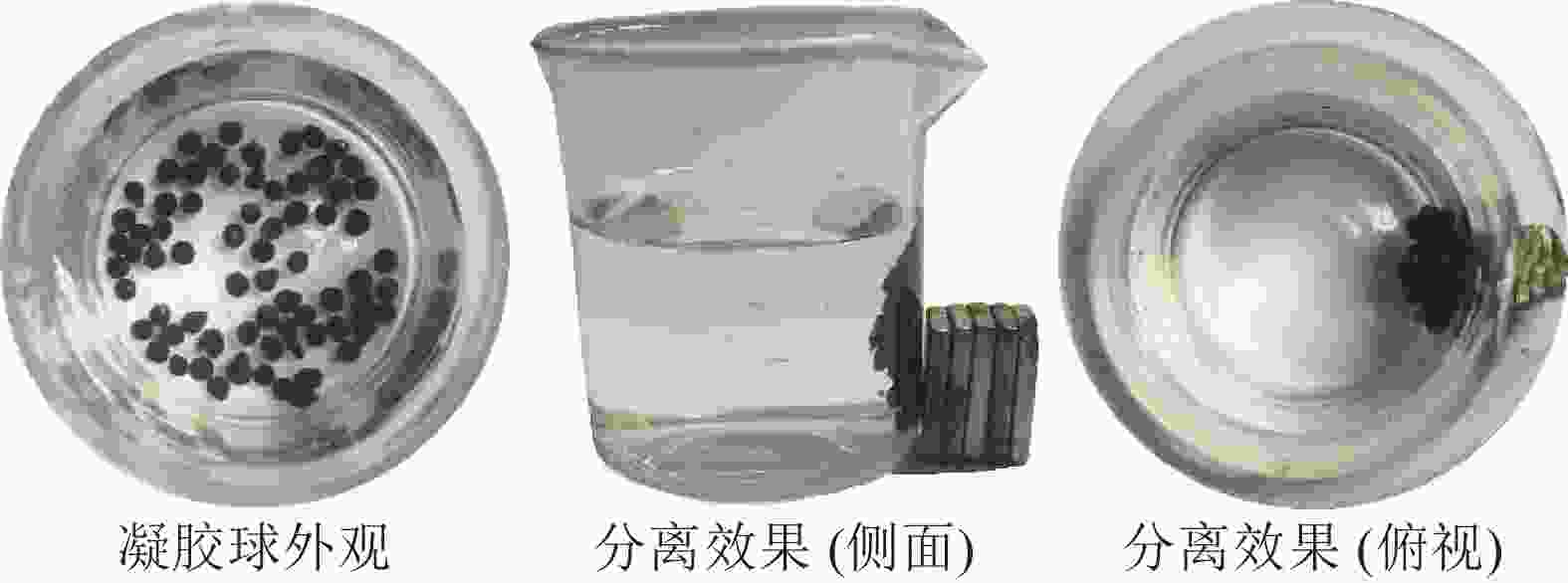
产物MNP、中间产物(AM)和终产物(SA@AM)的扫描电镜(TEM)、透射电镜(SEM电镜)照片和复合凝胶球外观照片如图2的所示。从图2可见:氨基改性AM的扫描电镜(SEM)图2A看出其结构单一,大小较为均匀,呈圆球状,颗粒堆积成簇。AM的透射电镜(TEM)图(图2B)看出:端氨基AM表面紧致,为不规则、无定形态,尺寸为15~20 nm,中心部分是磁性核粒子为黑色,边缘部分较为松散,为白色透明状,说明氨基硅烷在磁性核粒子上包覆成功。同时,图2显示,AM发生微量堆积而且相互吸附,可能是由于AM自带的磁力使其发生团聚成簇现象[13]。SA@AM的冻干凝胶球SEM图2C看出:AM颗粒附着包覆在海藻酸钠凝胶上,凝胶球含有较多褶皱和大的空隙。这是凝胶球高吸附性能的基础。SA@AM的外观图2D看出:凝胶球为黑色球型,表面光滑而且具有一定的弹性。因此,从表面形貌定性分析,验证得到了目标复合产物。
-
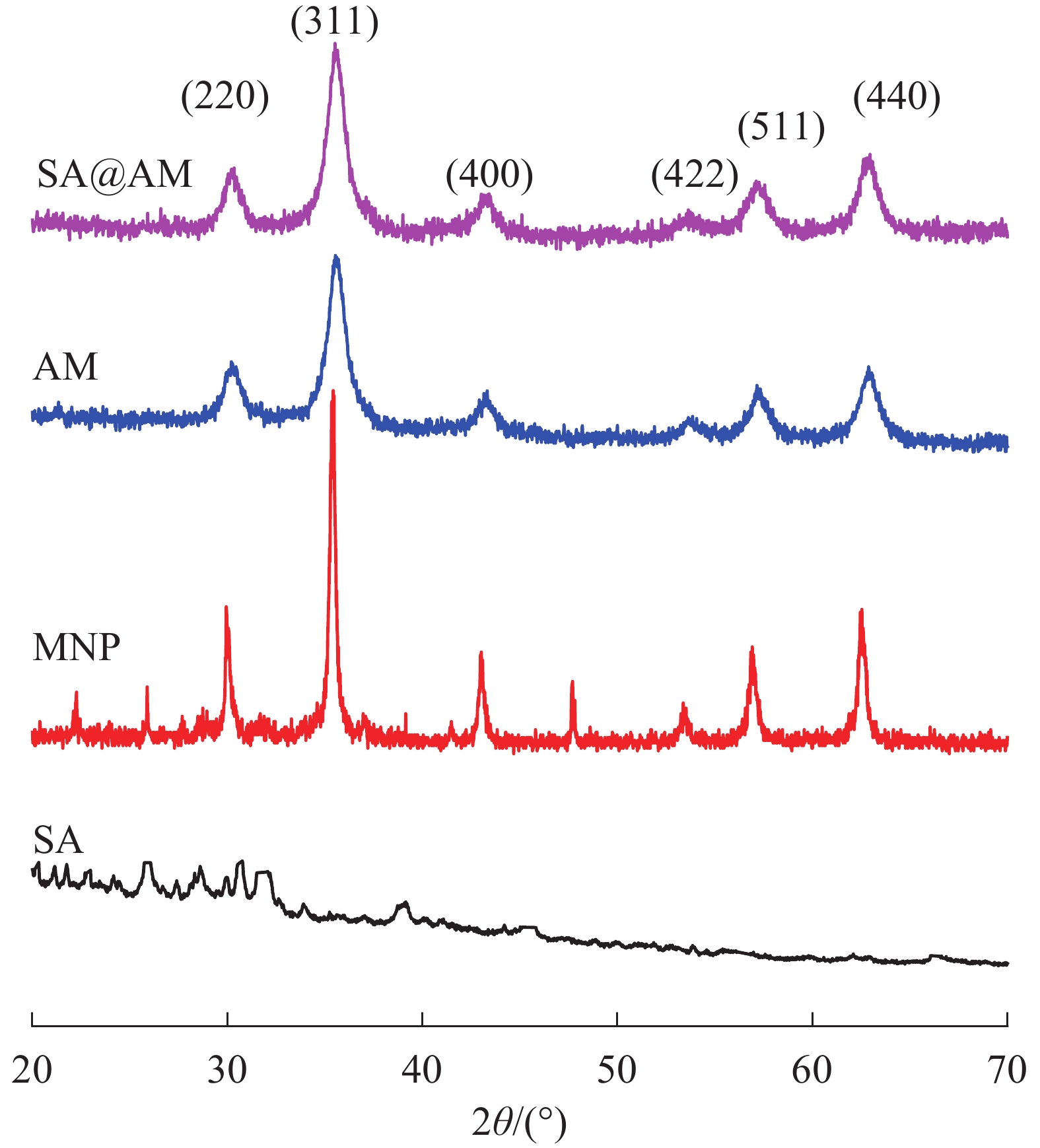
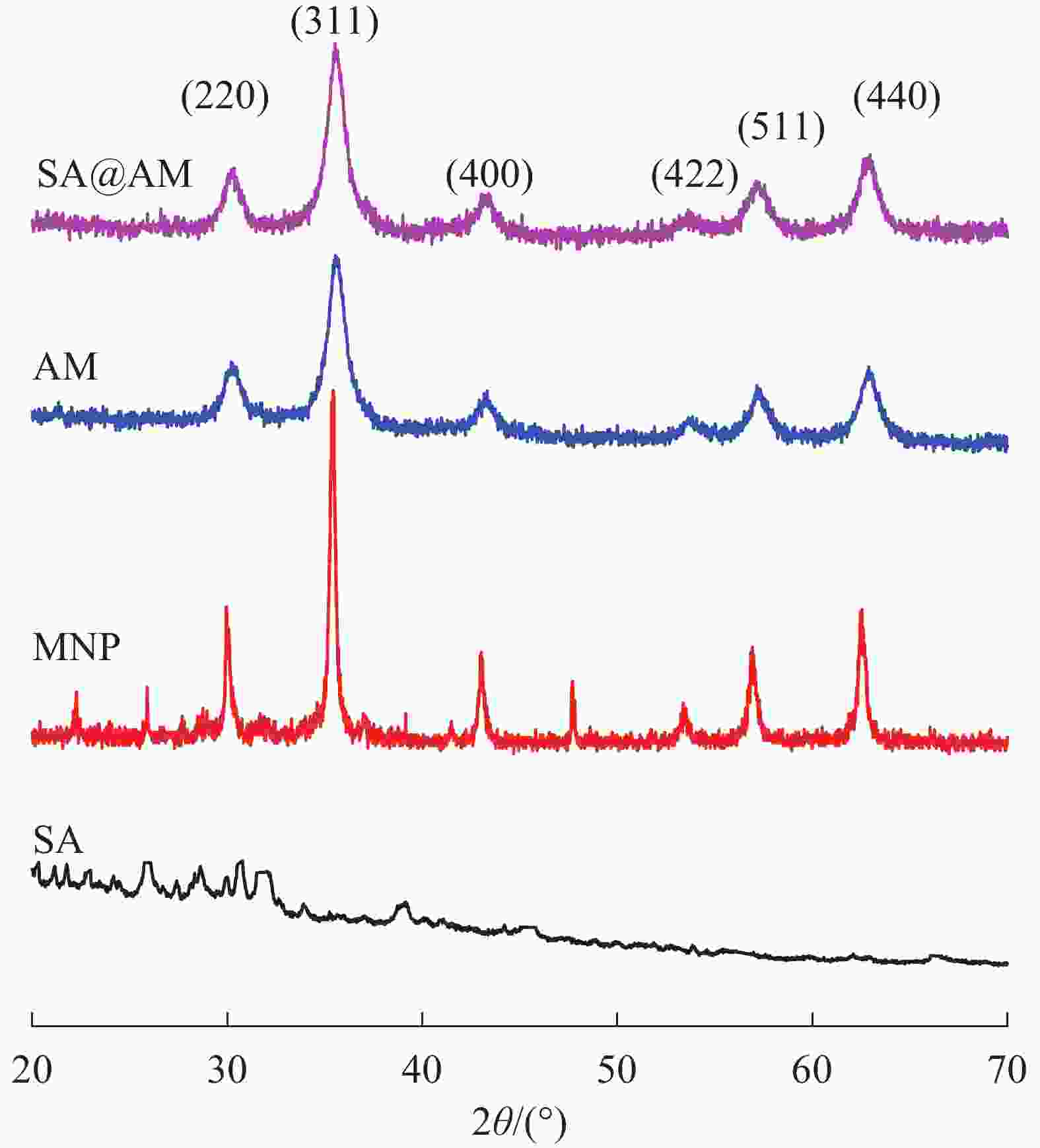
原料SA、原始产物MNP、AM和终产物SA@AM的XRD谱如图3所示。在图3各样品的XRD图谱中可以得到:MNP、AM和SA@AM在相同位置出现6个典型特征峰,分别为2θ=30.3°、35.4°、43.2°、53.9°、57.2°和 62.8°,分别对应(220)、(311)、(400)、(422)、(511)和(440)6种Fe3O4在X射线中的晶面[14]。其中特征峰的位置和强度均没有发生变化,结构为Fe3O4反尖晶石,磁铁矿晶型表现为顺磁性[15]。表明在改性磁性纳米粒子过程均未改变磁性材料的晶型结构。此外,图3中AM与MNP的XRD曲线比较,非晶相衍射峰在2θ=20.0°~30.0°内出现。此峰是由于非晶相的氨基硅烷包裹在MNP表面所致。终产物SA@AM与SA、AM的XRD曲线图相比,在2θ=20.0°~27.0°内出现SA的非晶相衍射峰;另外,由于SA包覆在MNP表面,使得终产物中Fe3O4在2θ=53.9°的衍射峰变宽。这些结果进一步证明产物磁性粒子MNP、AM和SA@AM的成功合成。
-
原始产物MNP、AM和终产物SA@AM的VSM分析磁化曲线如图4所示。产物的磁场饱和强度采用磁强度计进行测定[16]。在300 K、外部磁场为−1~1 T条件下,各产物几乎无剩磁产生,因为其磁滞曲线为S型而且过原点,同时矫顽力和剩余磁场强度都接近0[17],表明所制备的MNP、AM和SA@AM具有超顺磁性而且有良好的磁力响应性能[18]。测试的结果表示:MNP、AM和SA@AM的磁化值分别为13.8、13.4和6.85 A·m2·kg−1,SA@AM比MNP和AM的磁化值高。粒子表面被硅烷官能团覆盖可能影响产物AM的磁化值,这或许是AM的磁性比MNP稍低的原因。AM被海藻酸钠包覆影响SA@AM的磁化性能,造成其磁性减弱。
-
样品的吸光度采用原子分光光度计检测,吸附量根据吸光度结合公式(1)计算得出 [19]。
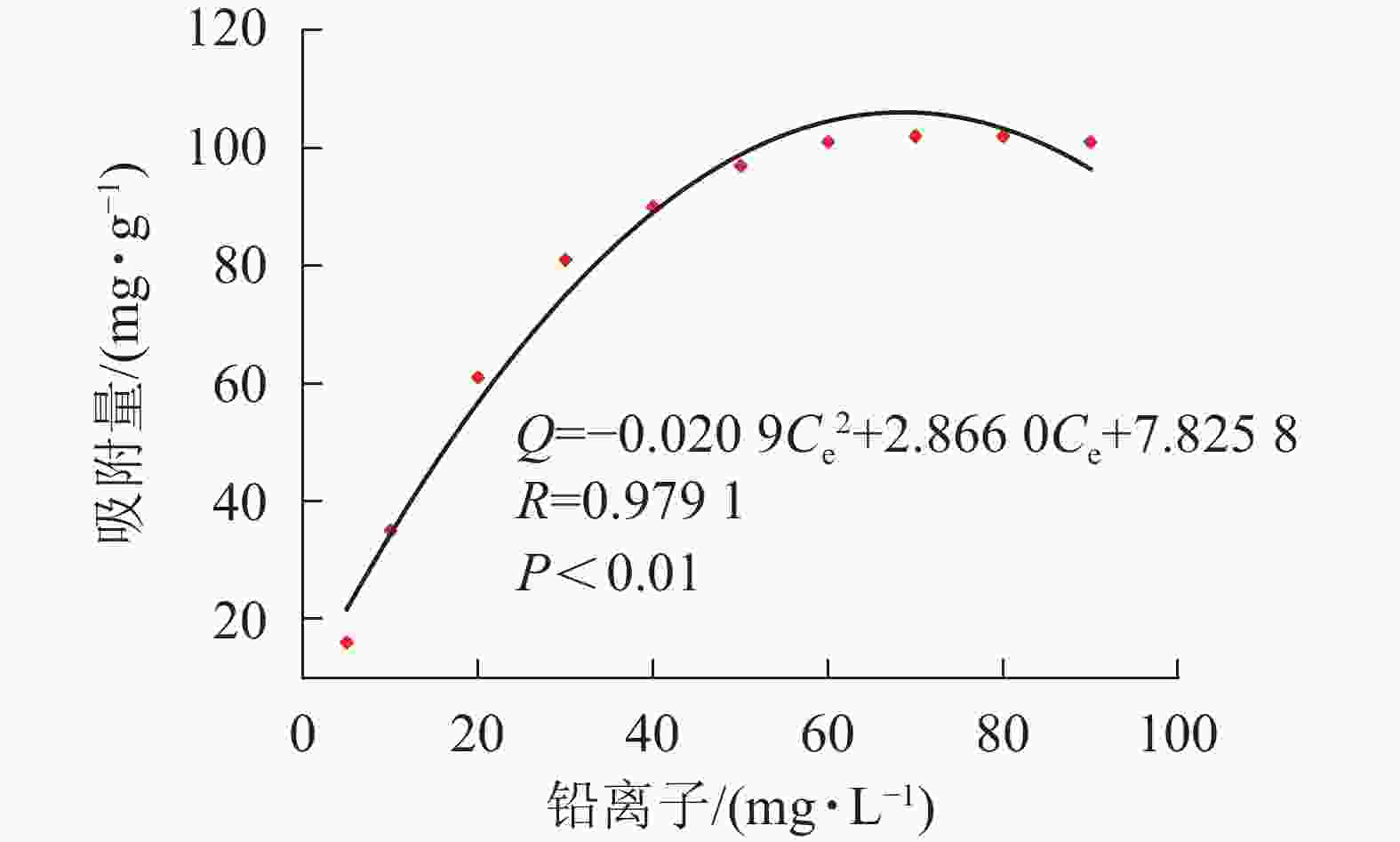
式(1)中:Q表示吸附量(mg·g−1),W表示凝胶的质量(g),C0表示重金属离子的初始质量浓度(mg·L−1),Ce表示金属离子的最终质量浓度(mg·L−1),V表示吸附溶液的体积(mL)。根据公式(1)计算新型磁性微球SA@AM对Pb2+的吸附量,绘制等温吸附性曲线,如图5所示。吸附等温线选择常用的Freundlich和Langmuir模型,其线性方程表示分别如下:
式(2)和式(3)中:
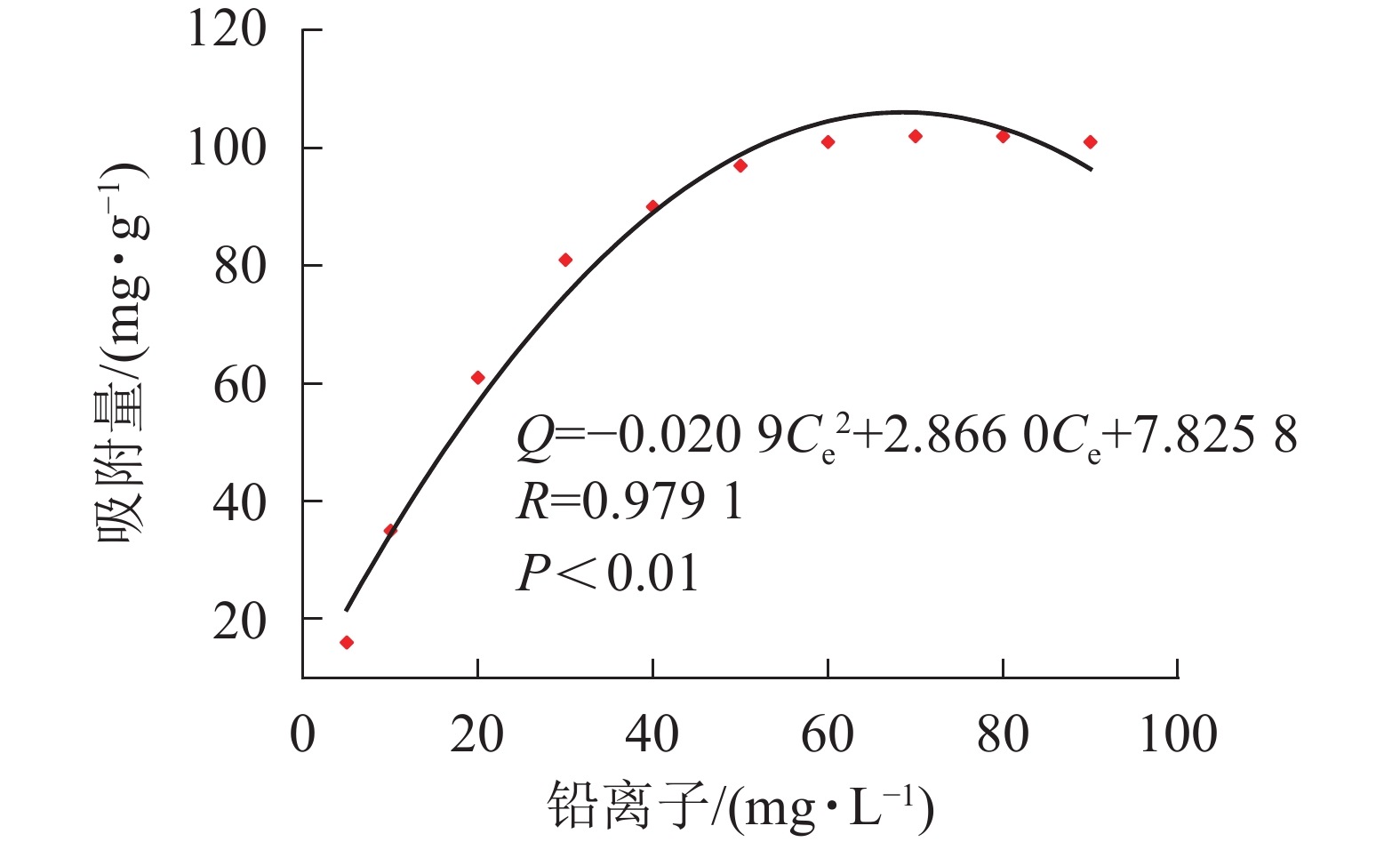
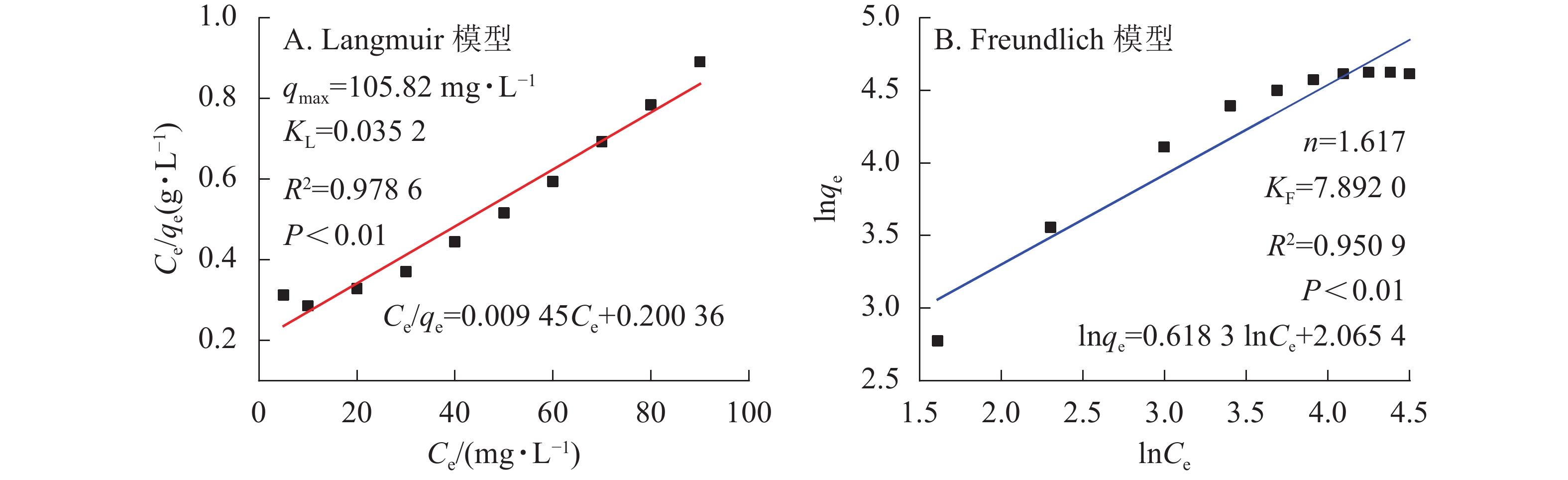
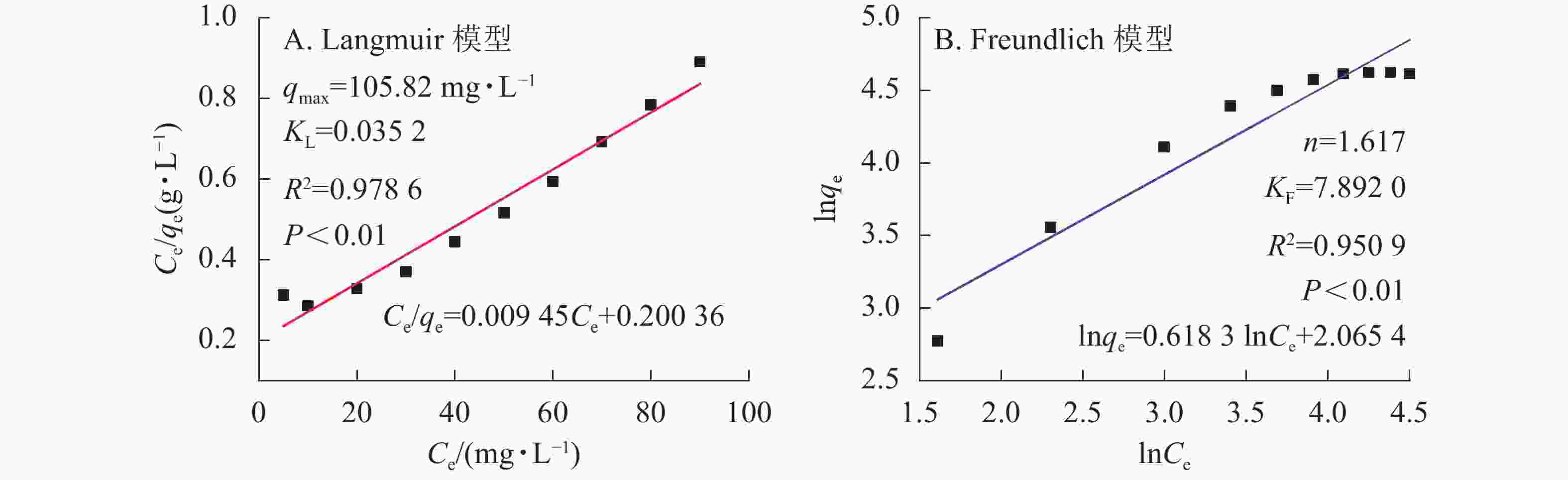
${C}_{\rm{e}}'$ 表示平衡时离子质量浓度(mg·L−1);qe为平衡时的吸附量(mg·g−1);qmax为饱和时的吸附量(mg·g−1);KF和n是Freundlich的吸附常数; KL是Langmuir的吸附常数。材料等温吸附特性分别采用Freundlich和Langmuir模型[20]进行拟合,拟合曲线和结果如图6所示。由图5凝胶等温吸附性曲线可知:SA@AM凝胶对Pb2+的最大饱和吸附量为102.12 mg·g−1。随着Pb2+溶液质量浓度的增加,吸附剂对其吸附量也明显增加直至饱和。凝胶对Pb2+的吸附过程具有规律性,显著性检验系数P<0.01表明呈极显著相关。由图6可知:Langmuir模型和Freundlich模型的线性相关系数分别为0.9786和0.9509,对2个相关系数进行显著性检验,均为极显著正相关(P<0.01)。由数值大小可见:Langmuir模型较Freundlich模型线性相关高。Langmuir模型线性拟合结果与实际测试结果较为相近,SA@AM对Pb2+的最大吸附量为105.82 mg·g−1,故凝胶吸附材料对Pb2+的吸附热力学行为更符合Langmuir吸附等温模型。
-
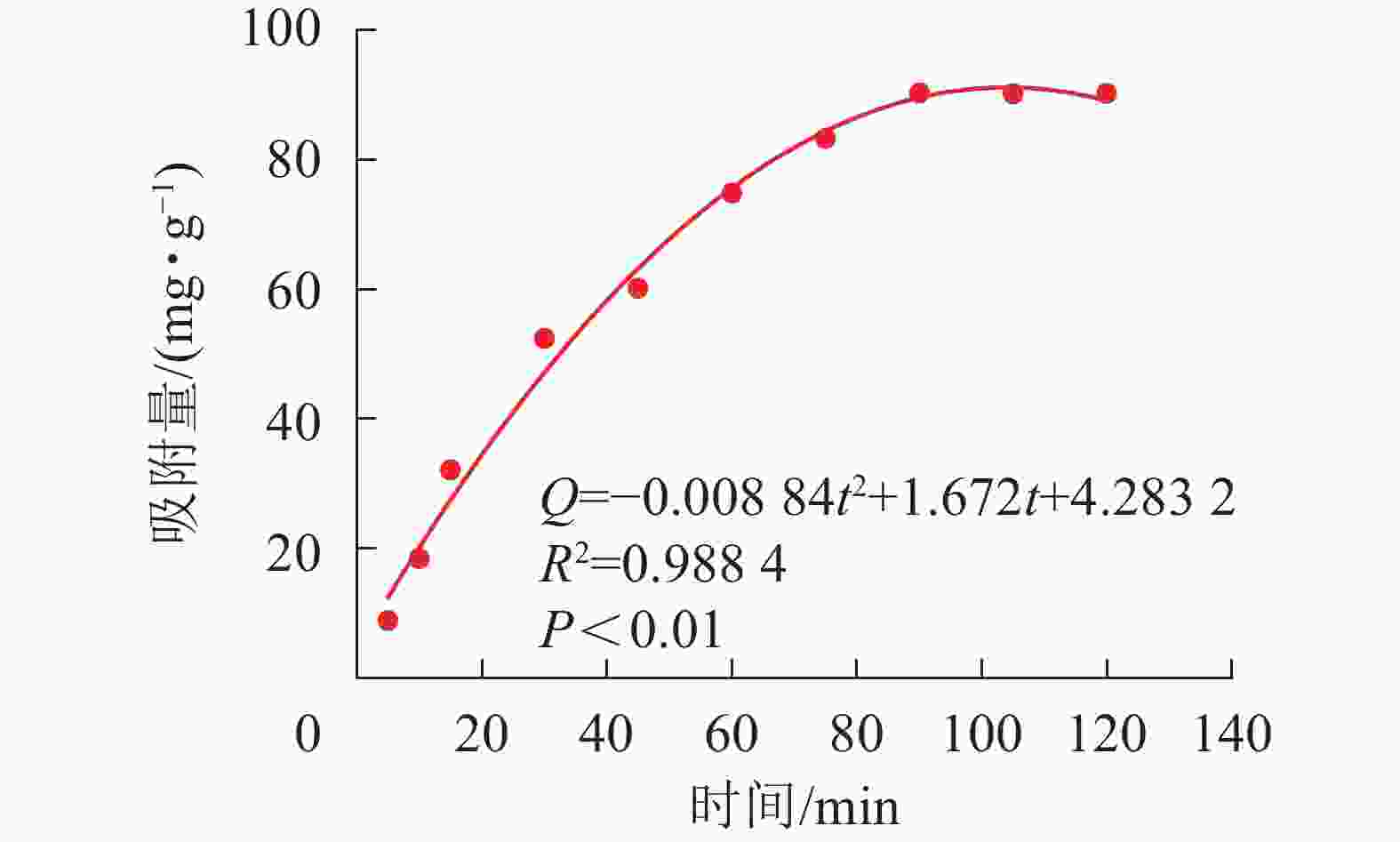
SA@AM对Pb2+的吸附量根据公式(1)计算,动力学吸附曲线如图7所示。常采用准一级反应动力学模型
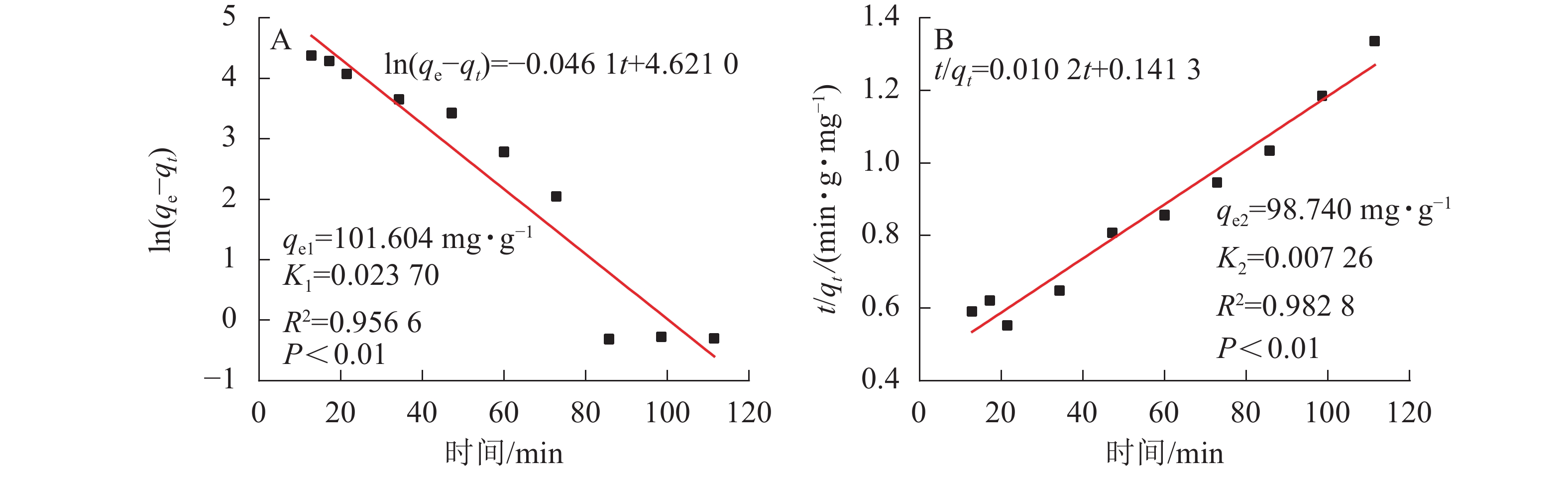
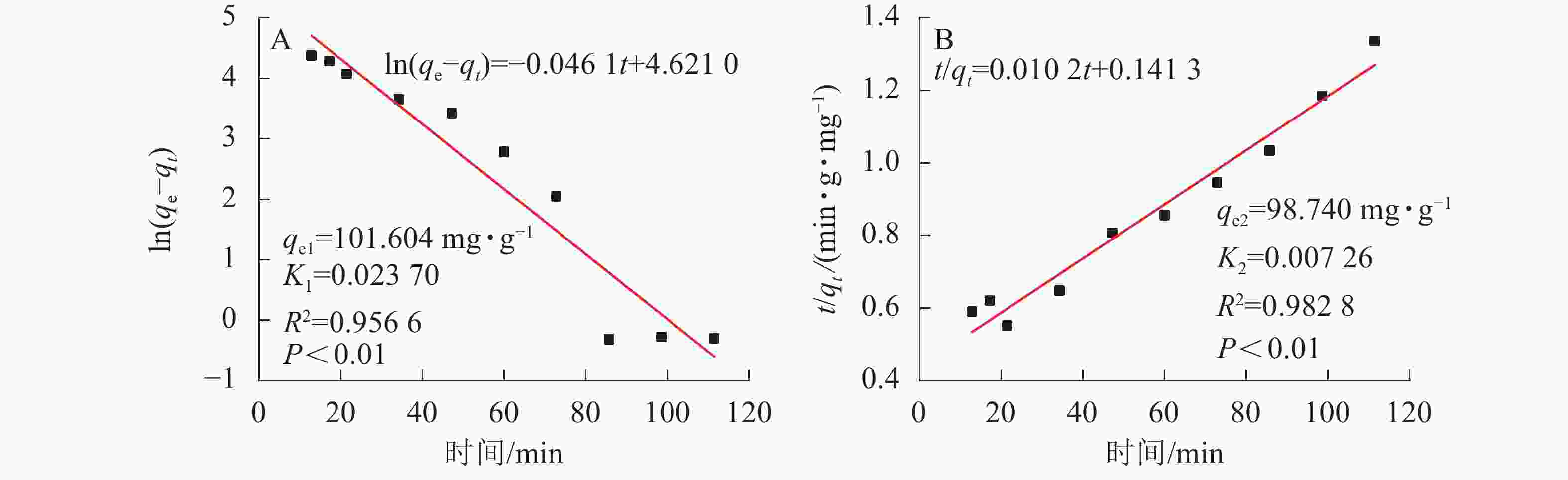
$\ln \left(q_{\mathrm{e}}-q_{\mathrm{t}}\right)=\ln q_{\mathrm{e}}-$ $K_{1} t $ 和准二级反应动力学模型[21]$\dfrac{t}{q_{t}}=\dfrac{1}{K_{2} q_{\rm{e}}^{2}}+\dfrac{t}{q_{\rm{e}}}$ 描述吸附反应动力学过程。其中:t是吸附时间;qe为吸附达平衡时吸附材料对重金属的吸附量(mg·g−1);qt为t时吸附材料对重金属的吸附量(mg·g−1);K1为一级吸附速率常数;K2为二级吸附速率常数。吸附剂动力学吸附特性分别采用准一级动力学模型和准二级动力学模型对其进行拟合,拟合方程及结果如图8所示。由图7可知:吸附量随时间增加而增大。在吸附反应的初始阶段(0~45 min),凝胶吸附速率大;在50~80 min时,吸附速率逐渐减慢。当时间达80 min时,水凝胶对Pb2+的吸附基本上达到了吸附动力学平衡。SA@AM对Pb2+的吸附过程是有规律性的,经显著性分析,呈极显著相关(P<0.01)。由图8可知:实验过程中,SA@AM凝胶对Pb2+的最大饱和吸附量为91.72 mg·g−1,从准一级吸附动力学模型线性拟合结果和准二级吸附动力学模型线性拟合结果可得:SA@AM凝胶对Pb2+最大吸附量分别为98.74和101.60 mg·g−1。由此可见:相比于实测值,准二级吸附动力学模型线性拟合的平衡吸附量比准一级动力学模型拟合的更接近。动力学吸附拟合方程的参数可以看出:准二级吸附动力学模型线性相关性(R2=0.982 8)较准一级动力学模型相关性(R2=0.956 6)高,表明极显著相关(P<0.01),故吸附材料对Pb2+的吸附动力学行为更符合准二级吸附动力学模型。LI等[22]关于海藻酸铁介孔碳微球对砷吸附性能的研究,以及CHEN等[23]关于海藻酸钠与纳米Fe3O4共混凝胶球对有机污染物甲基橙的吸附行为的研究均得到类似结果。
-
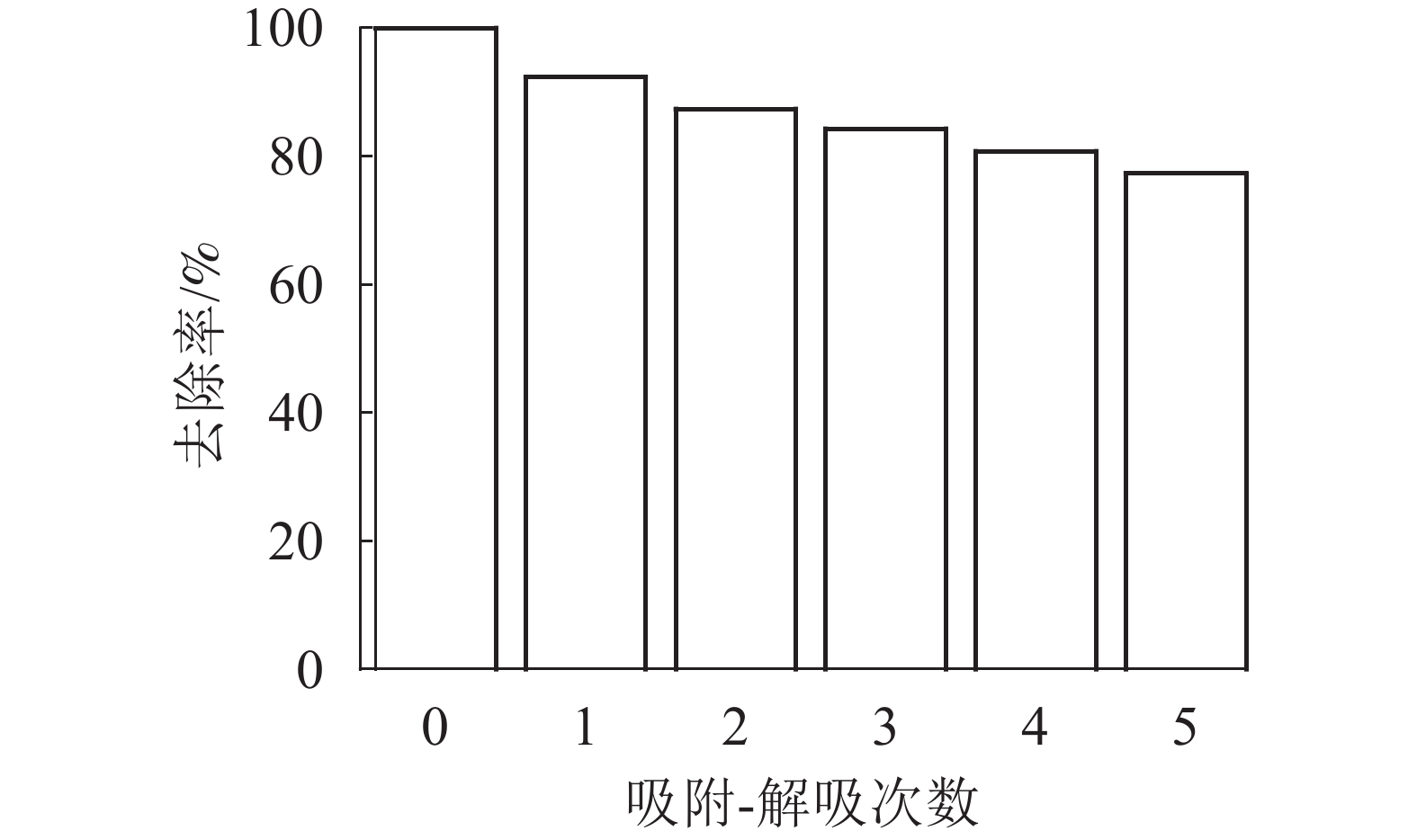
图9显示:磁性海藻酸钠凝胶球对Pb2+有良好的磁分离效果,这是由于海藻酸钠凝胶球中包覆有氨基末端的磁性粒子,使得凝胶球含有磁性。参考文献[24]方法,通过吸附-解吸过程来测试磁性海藻酸钠凝胶球的重复利用性。恢复率如图10所示。图10表明:SA@AM对Pb2+的去除率与重复循环解吸次数有关,而且吸附率逐渐减少。每次重复吸附-解吸后,凝胶对Pb2+的吸附量减少6%~7%。经过重复循环5次后,SA@AM对Pb2+的吸附去除率保持在76%以上,说明SA@AM循环性能良好,利于对Pb2+的重复吸附。
2.1. 原料及产物表征方法
2.1.1. 原料及产物的红外光谱(IR)分析
2.1.2. 产物的元素质量分数分析
2.1.3. 产物的表观形貌(SEM/TEM)测试
2.1.4. 原料及产物的XRD分析
2.1.5. 产物的磁化值(VSM)分析
2.2. 材料吸附性能研究
2.2.1. 等温静态吸附性研究
2.2.2. 吸附动力学研究
2.2.3. 重复使用性能测试
-
本研究通过滴定法,将表面接枝端氨基的磁性纳米颗粒包覆于海藻酸钠凝胶球中,制备得到1种新型磁性海藻酸钠复合凝胶球(SA@AM),并通过各种表征方法测试产物,得出结论如下:①红外光谱表明:已经成功获得磁性凝胶产物,通过分析元素质量分数及分布情况证实了产物的元素结构。XRD光谱分析证明端氨基磁性颗粒的非晶相峰的出现;磁化曲线分析可知:产物具备优异磁性能,同时磁化值的差异证实了磁性凝胶球的成功制备。②SA@AM具有独特的磁响应性能,对Pb2+具有较好的吸附能力,达102.12 mg·g−1,吸附能力相比于已有的磁性纳米材料(吸附能力65~90 mg·g−1)更优[25];二级动力学模型更符合凝胶球的吸附动力学性能,Langmuir模型更能充分描述凝胶球的等温吸附规律。循环脱附-吸附过程中证明了材料具有良好的重复利用性能。③本研究所制备出的SA@AM,相比化学吸附剂具有功能磁响应性,可以通过控制外加磁场实现对吸附剂分离回收,为生态环境中治理重金属污染提供了1种绿色、高效的新型应用型功能吸附剂。















 DownLoad:
DownLoad: