-
三叶青Tetrastigma hemsleyanum为葡萄科Vitaceae崖爬藤属Tetrastigma的药用植物,主要以地下块根入药。在临床中,三叶青内服可治疗咽喉炎、肝炎、肺炎、小儿高热惊厥及病毒性脑膜炎等炎症,外用可治毒蛇咬伤、跌打损伤、痈疽等疾病,被誉为“植物抗生素”,是中国独有的珍贵药材,主要分布于浙江、湖南、广东、广西、四川等地[1]。由于三叶青具有良好的抗肿瘤功效且无毒副作用[2],导致三叶青需求量大量增加,人工栽培面积不断扩大。人工种植三叶青多采取遮光大棚雾喷管理,藤叶生长茂盛,但块根品质却远不如生长环境苛刻的野生三叶青[3-5]。逆境胁迫虽抑制植物的生长发育,特别是营养生长,但同时也会促进或者抑制植物中黄酮等一些次生代谢产物的累积[6-9]。三叶青喜阴湿环境,野生三叶青多生长于山坡或山沟、溪谷两旁的针阔混交林或杂木林林下背阴处[10-11],可见水分条件对三叶青的生长影响较大。目前,温度[12-13]、光照[14-15]、土壤肥料[16-17]等环境因子对三叶青生长的影响已有研究,但关于水分胁迫对三叶青特别是三叶青黄酮含量的影响还未见报道。因此,本研究分析了水分胁迫对三叶青生长、黄酮含量及其合成途径中关键酶的影响,为人工种植高品质三叶青提供理论依据。
HTML
-
三叶青由浙江五养堂药业有限公司遂昌县金竹药王谷三叶青基地提供。为了保持试验材料的一致性,以专用袋标准化繁育的2年生三叶青植株作为供试材料。
-
实验地位于浙江农林大学东湖校区(30°15′28″N,119°43′35″E),该区全年降水量1 628.6 mm,全年平均气温16.4 ℃,年日照时数1 847.3 h,无霜期237.0 d。取长势良好的2年生的三叶青植株,种植在上口径20 cm、下口径15 cm、高20 cm的塑料花盆中,栽培基质为过筛土∶营养基质=2∶1(质量比),每盆1株,缓苗5周,缓苗期间定时定量浇水。5周后,选取长势基本一致的2年生植株57株,随机分为3组,每组19盆,设置3个处理组,分别为干旱、水涝和对照。干旱组在试验期间一直不浇水,水涝组保证地下部分在试验期间一直处于淹水状态,对照组在试验期间正常浇水。
-
干旱组在胁迫处理的第1、8、16、24、32、40天,于相同叶位随机取样3盆(预试验发现三叶青在不浇水40 d左右出现死亡现象),水涝组在胁迫处理的第1、4、8、12、16、20天,于相同叶位随机取样3盆(预试验发现三叶青在水淹20 d左右出现死亡现象),对照组在胁迫处理的第1、4、8、12、16、20、24、32、40天,于相同叶位处随机取样3盆。每个试验组设置3个重复,所有样品取样后立即用清水洗净擦干后再立即用液氮预冷,于−80 ℃冰箱保存待测。
电镜样品取样,在胁迫处理最后1 d,取植株的中部成熟叶片,中间靠近大叶脉处部位切成长0.1 cm、宽0.2 cm左右的小片(避开大叶脉)后迅速放入体积分数为2.5%的戊二醛溶液中,抽真空至材料沉入固定液底部,4 ℃固定过夜,每个试验组重复3次。
-
随机取实验三叶青进行叶片表型拍摄,再取块根,洗净后擦干进行块根表型拍摄。
-
倒掉固定液,用0.1 mol·L−1 pH 7.0的磷酸缓冲液漂洗样品3次,每次15 min;用质量分数为1%的锇酸固定样品1~2 h后漂洗3次。然后用不同体积分数的乙醇溶液(30%、50%、70%、80%、90%和95%)对样品进行脱水处理,每种体积分数处理15 min,再用纯乙醇处理20 min,最后用纯丙酮处理20 min。接着用Spurr包埋剂与丙酮的混合液(V/V=1/1)处理样品1 h;再用Spurr包埋剂与丙酮的混合液(V/V=3/1)处理样品3 h;最后用纯包埋剂处理样品过夜。将经过渗透处理的样品包埋起来,70 ℃加热过夜,即得到包埋好的样品;样品在LEICA EM UC7型超薄切片机中切片,获得70~90 nm的切片;切片经柠檬酸铅溶液和醋酸双氧铀50%乙醇饱和溶液(体积分数)各染色5~10 min。最后在Hitachi H-7650型透射电镜中观察叶肉细胞中的叶绿体。
-
黄酮质量分数的测定方法参照文献[18-19]。以芦丁不同质量分数(mg)为纵坐标(y),以波长500 nm下的吸光度D(500)为横坐标(x)绘制标准曲线,得到回归方程y=0.245 9x+0.001 6(R2=0.999 9)。测定样品在500 nm波长下的吸光度D(500),计算三叶青总黄酮质量分数,每个样品3个重复。
-
酶液制备及苯丙氨酸解氨酶(PAL)活性的测定参照徐琳煜[20]的方法,以每克鲜质量叶片每分钟波长290 nm下的吸光度D(290)变化0.01为1个酶活力单位(16.67 nkat·g−1·min−1)。查尔酮合成酶(CHS)活性测定参照试剂盒法(上海晶抗生物工程):以标准物的浓度(×16.67 nkat·L−1)为纵坐标(y),波长450 nm下的吸光度D(450)为横坐标(x),得到标准曲线直线回归方程y=92.238x+1.193 8(R2=0.999),然后根据样品的D(450)计算样品相应的浓度。查尔酮异构酶(CHI)活性测定参照试剂盒法(上海晶抗生物工程):以标准物的浓度(×16.67 nkat·L−1)为纵坐标(y),波长450 nm下的吸光度D(450)为横坐标(x),得到标准曲线直线回归方程y=1 165.3x+50.552(R2=0.999),然后根据样品的D(450)计算出样品相应的浓度。
-
用Excel进行数据处理与制图;运用单因素方差分析和最小显著极差法LSR进行方差分析和多重比较(α=0.05);采用Pearson’s进行相关性分析。
1.1. 供试材料
1.2. 材料处理
1.3. 样品获取
1.4. 研究方法
1.4.1. 三叶青叶片及块根表型拍摄
1.4.2. 叶绿体超微结构电镜观察预处理
1.4.3. 黄酮质量分数测定
1.4.4. 黄酮合成关键酶活性测定
1.5. 数据处理
-
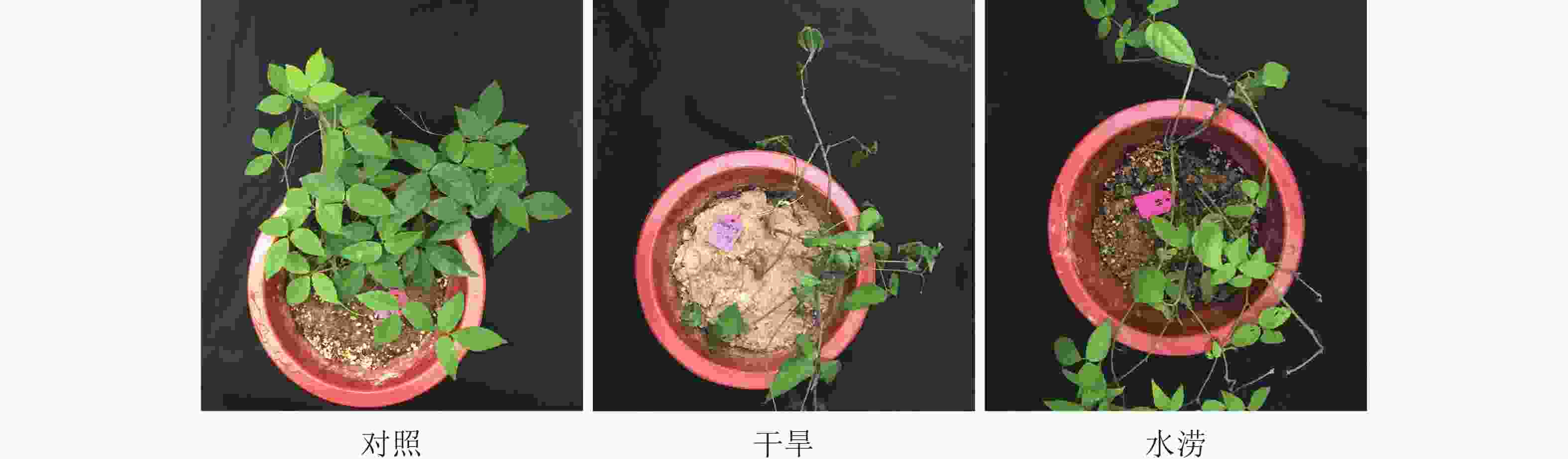
水分胁迫严重影响三叶青的营养生长。从叶片表型来看,未遭受水分胁迫的三叶青(对照)叶片饱满,色泽鲜艳,叶脉清晰;干旱胁迫40 d后,三叶青叶片失水,萎蔫,逐渐发黄枯萎;水涝胁迫20 d后,三叶青叶片失绿变黄(图1)。从块根的表观形态上来看,对照组的块根饱满,形状完好,而干旱组的块根失水皱缩,表皮布满沟壑,水涝组的块根绵软,表皮极易脱落,块根几乎腐烂(图2)。
-
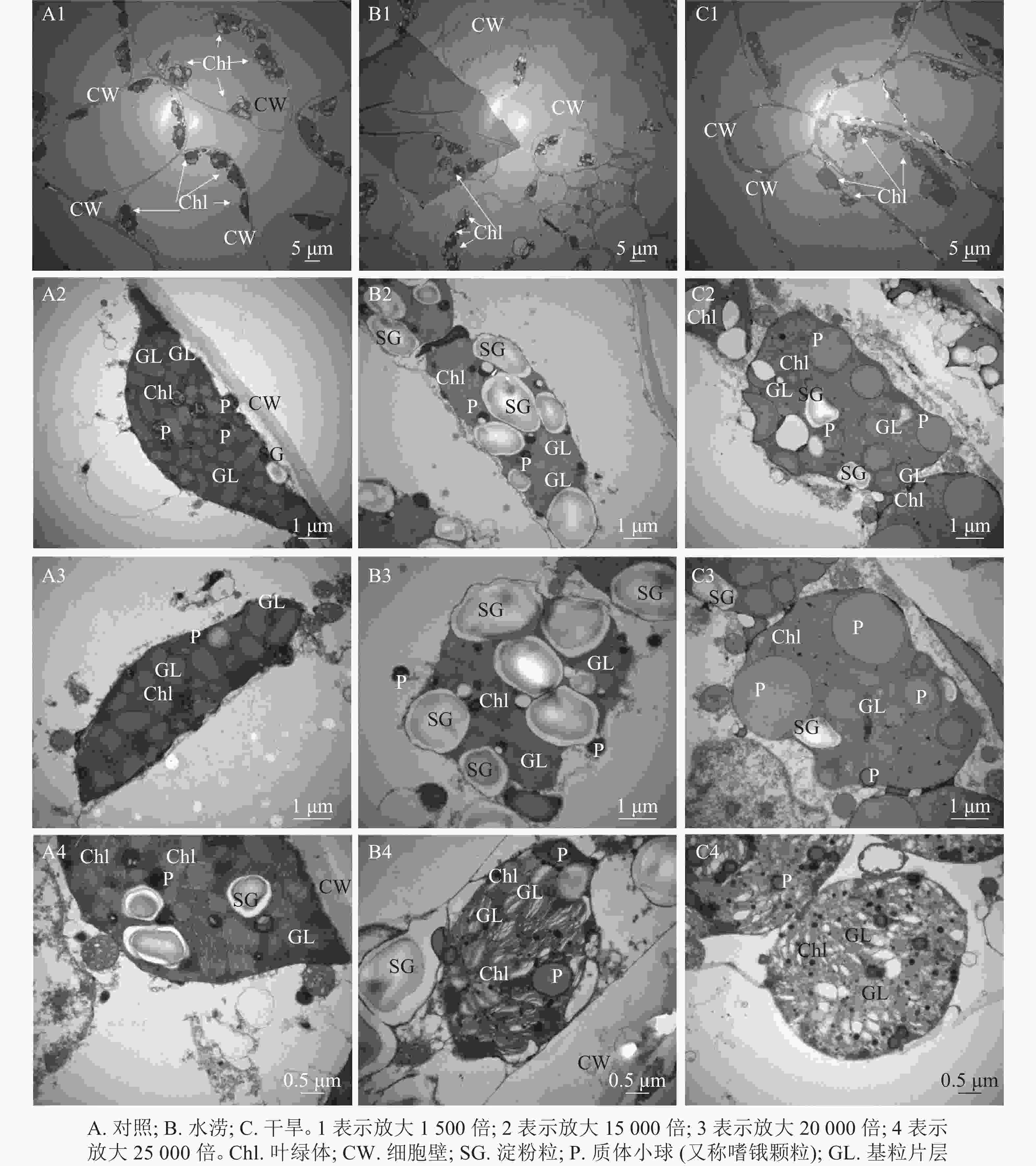
叶绿体结构的完整性及其内部结构的有序性对光合作用的光能吸收和传递有着重要作用。与对照相比,水涝和干旱处理下叶绿体数目明显减少(图3A1,图3B1,图3C1)。水分胁迫下,三叶青叶片中的叶绿体在细胞内的排列方式发生变化,与对照的紧贴细胞壁并且整齐分布相比,水涝和干旱处理后,叶肉细胞中的叶绿体与细胞壁分离,挤向液泡,在细胞中呈现拥挤混乱的现象,叶绿体向细胞中间靠拢,完全随机分布在细胞中(图3A1,图3B1,图3C1)。水涝和干旱处理后,叶肉细胞叶绿体膨胀,失去原本形态,叶绿体中淀粉粒数量增多,可能是代谢不正常导致淀粉粒无法运出造成的(图3A2,图3B2,图3B3)。与对照相比,水涝和干旱处理后,叶绿体基质颜色变浅,叶绿体膨大,质体小球颜色变浅,数量增多,体积变大(图3A3,图3B3,图3C3)。对照基粒片层结构整齐,紧密排列,而干旱和水涝处理后,基粒片层失去整齐紧密结构,与细胞质之间不再有完整界限,结构被破坏(图3A4,图3B4,图3C4)。
-
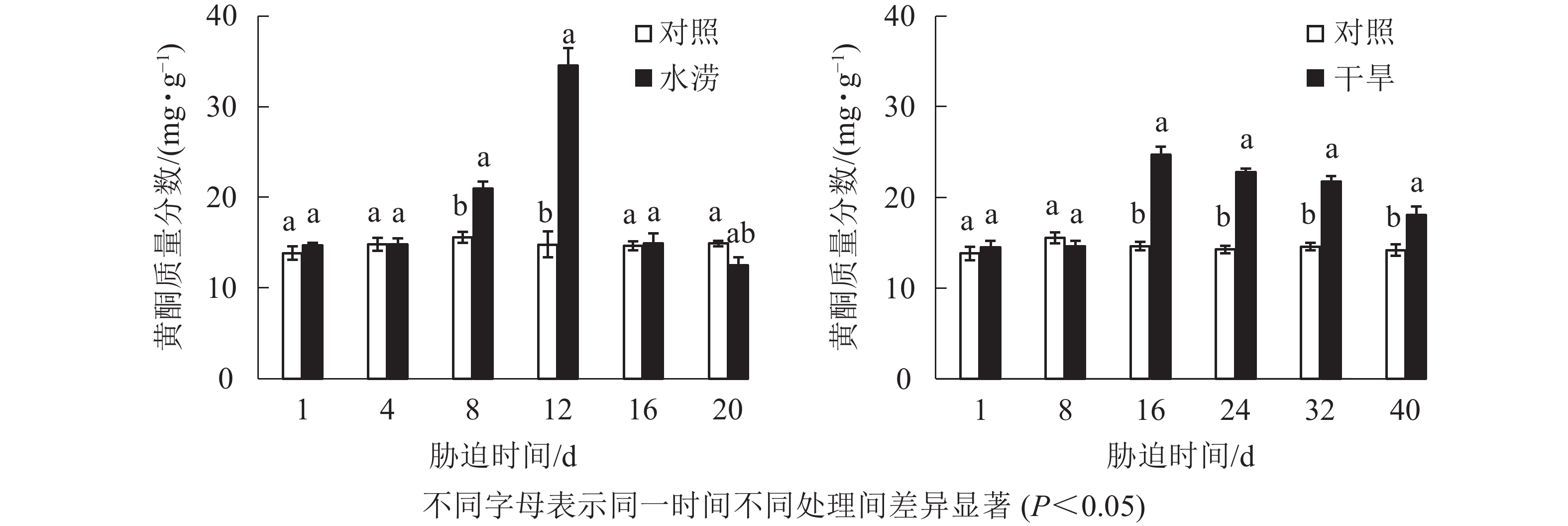
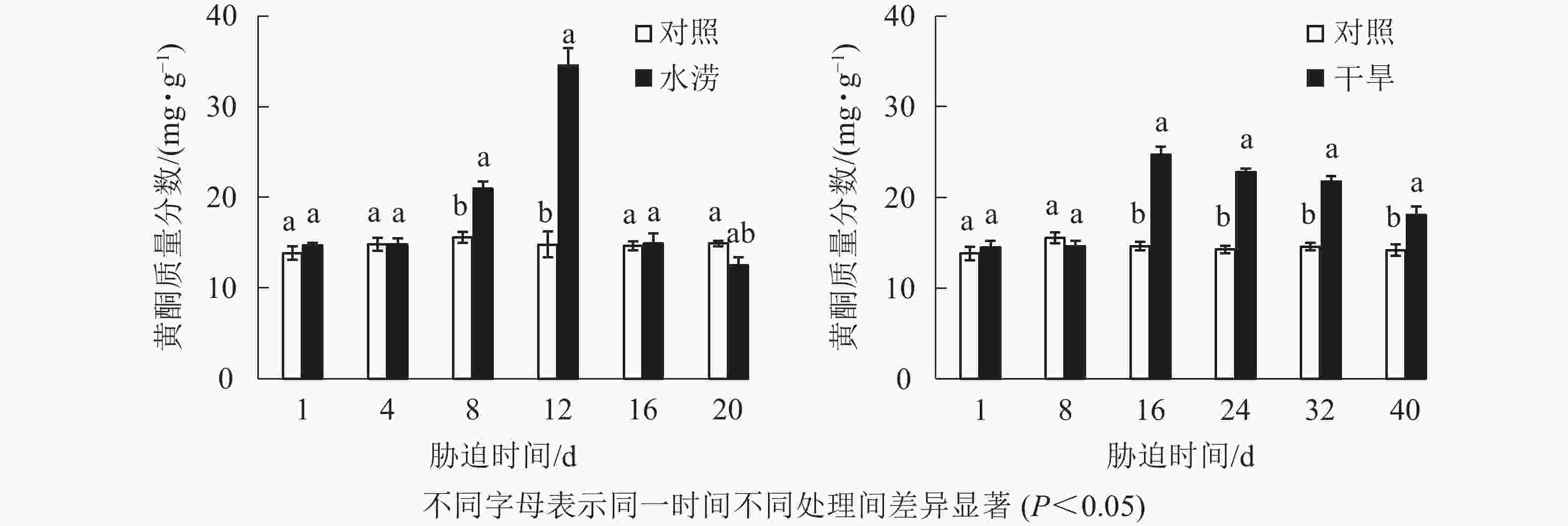
水分胁迫影响三叶青块根的黄酮质量分数。水涝胁迫8 d后,总黄酮质量分数显著高于对照(P<0.05),在胁迫12 d时达到最大值。干旱胁迫16 d后,总黄酮质量分数显著高于对照(P<0.05),在胁迫16 d时达到峰值(图4)。对照总黄酮质量分数保持在比较稳定的范围内,而水涝和干旱处理后,总黄酮质量分数呈先低后高再回落的变化趋势。
-
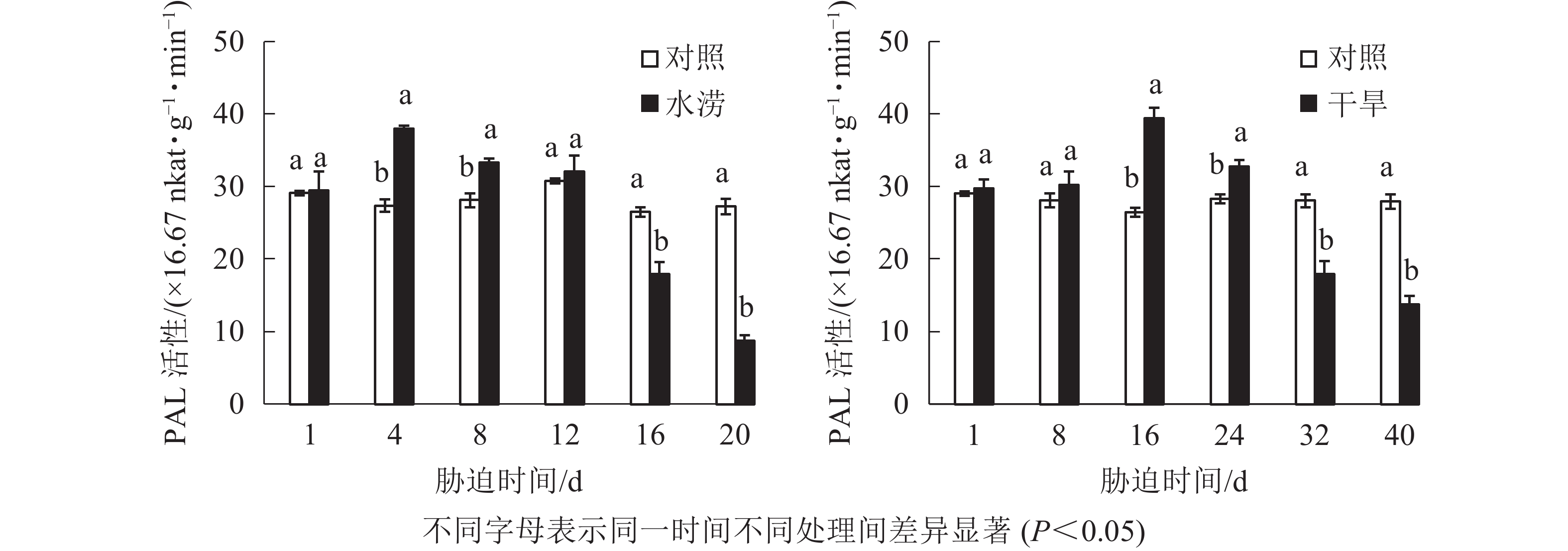
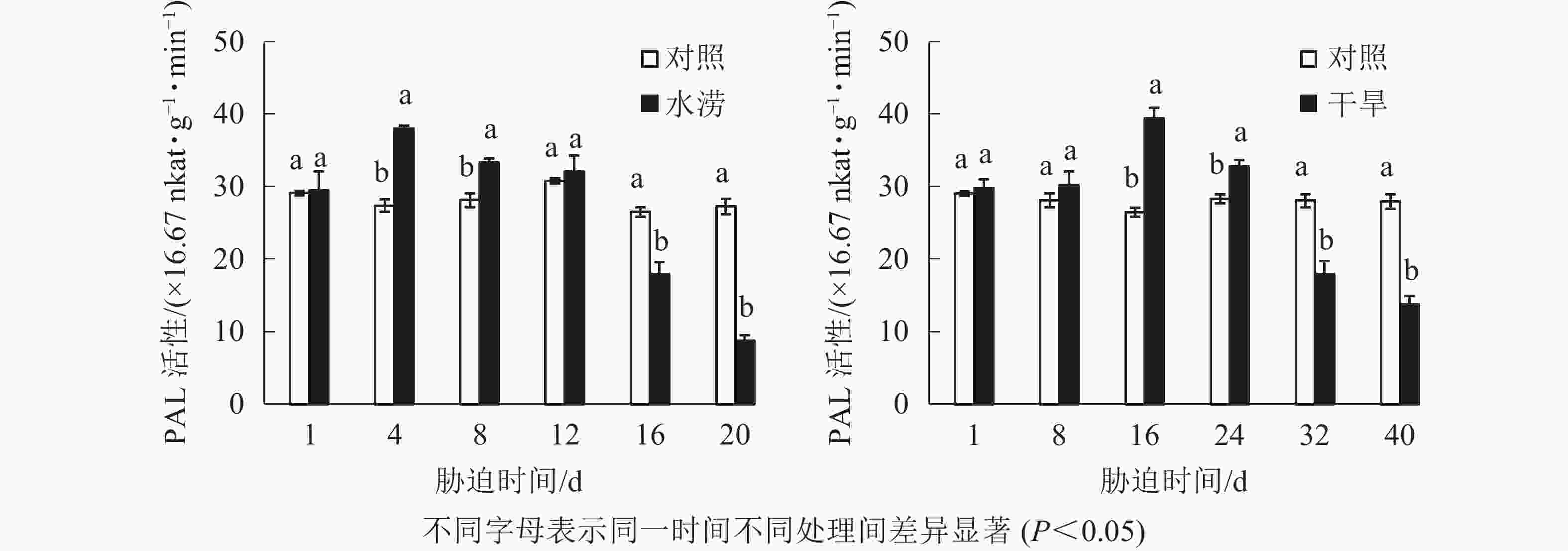
水分胁迫对三叶青块根黄酮合成关键酶活性的影响较大。水涝胁迫4 d后,PAL活性显著提高(P<0.05),并且在胁迫4 d时达到峰值,之后下降且在胁迫16 d时显著低于对照(P<0.05)。干旱胁迫16 d后,PAL活性显著高于对照(P<0.05),并且在胁迫16 d时达到峰值,之后下降且在胁迫32 d时显著低于对照(P<0.05)。即PAL活性呈先上升后下降的趋势,胁迫处理前期大于对照,胁迫后期则低于对照(图5)。
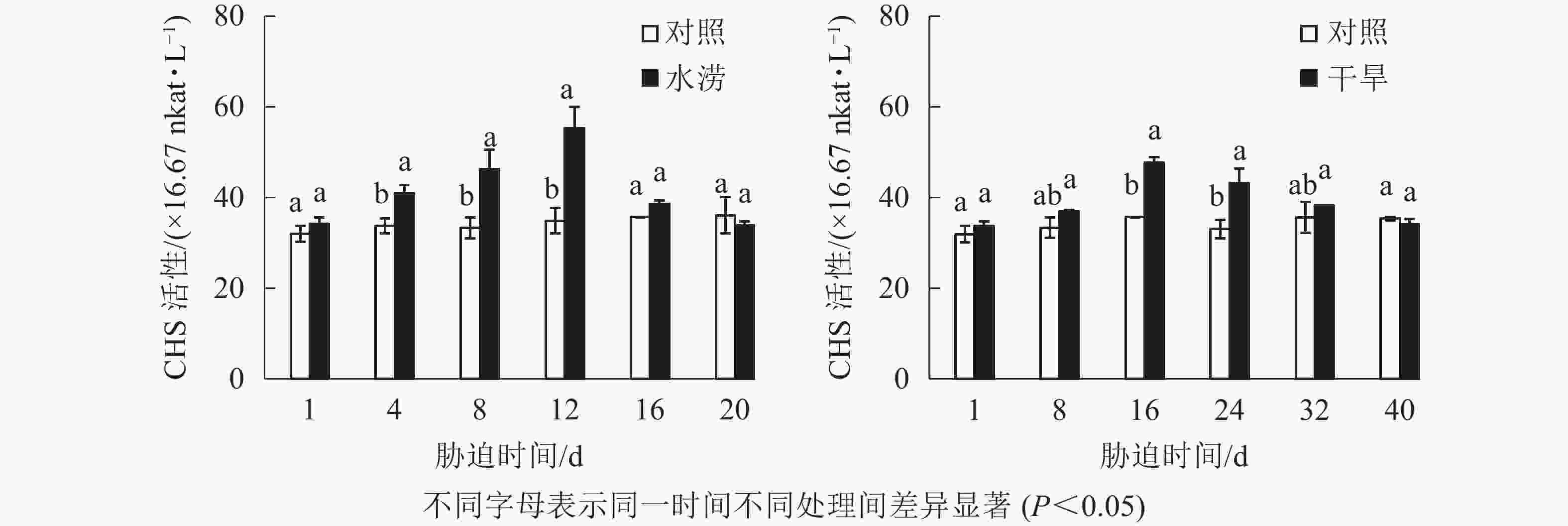
水分胁迫影响三叶青CHS活性。水涝胁迫4 d后,CHS活性显著高于对照(P<0.05),且在胁迫处理的12 d达到最大值,之后回落到与对照近似水平。干旱胁迫16 d后,干旱处理的CHS活性显著增强(P<0.05),并且在胁迫16 d达到峰值,之后下降至与对照近似水平。整个研究过程中,水涝和干旱处理的CHS活性呈先上升后下降的趋势,且酶活性大于对照组,对照组基本不变(图6)。
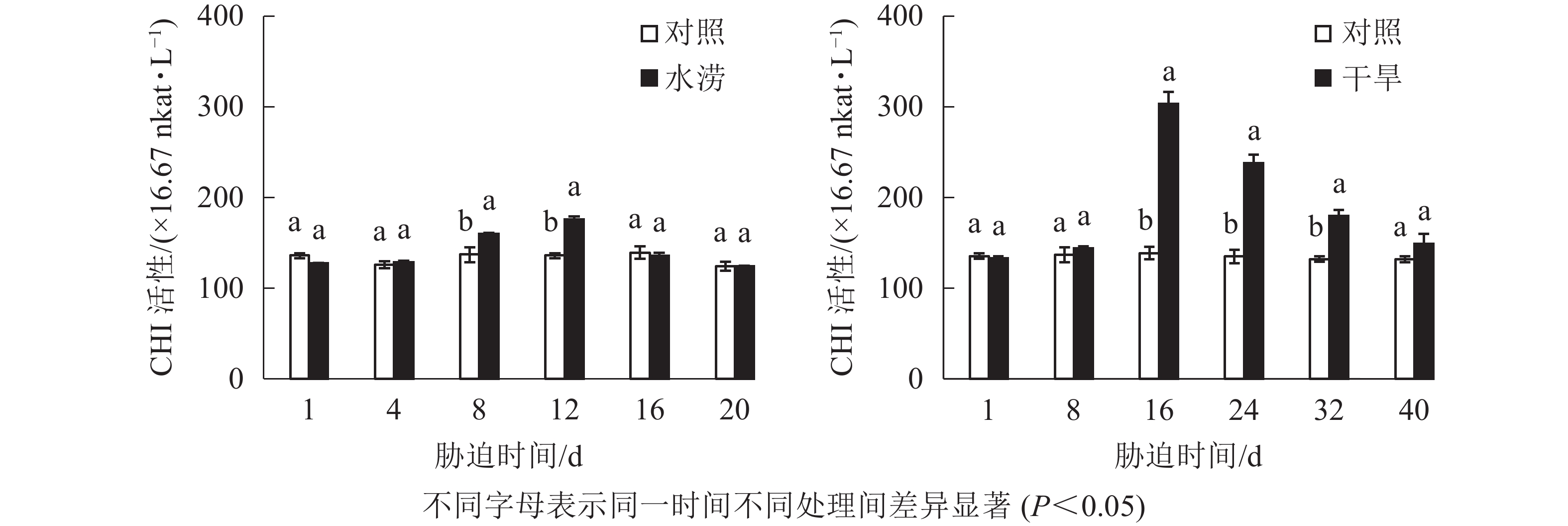
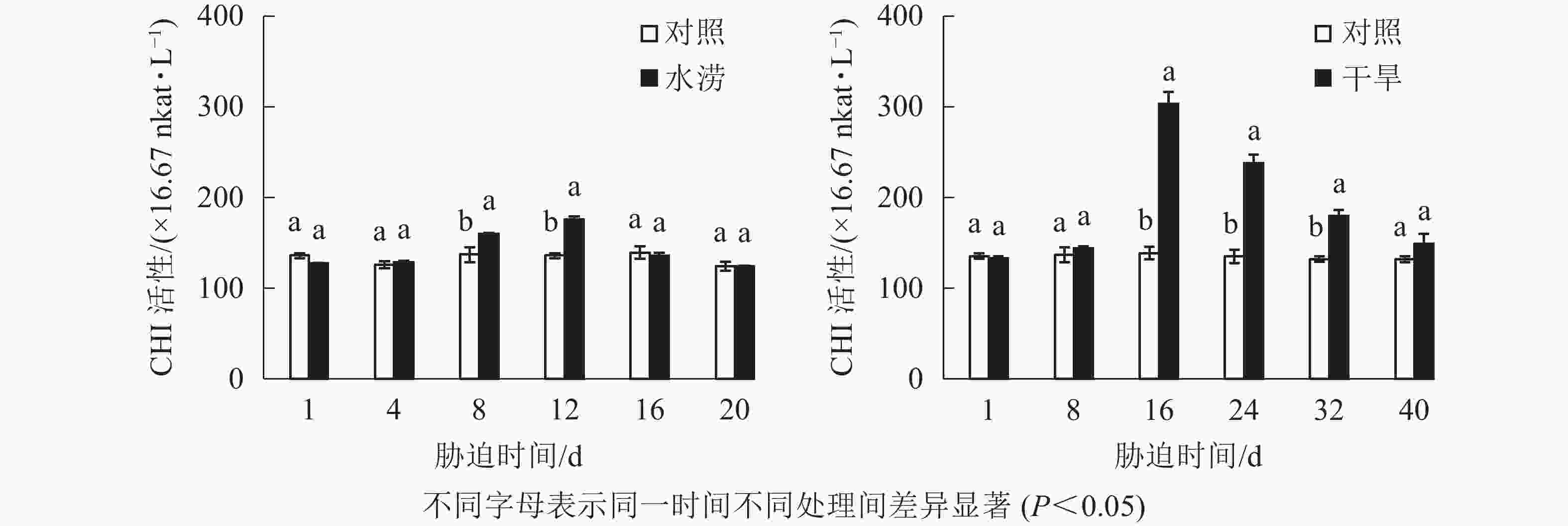
与对照相比,水涝胁迫12 d后,CHI活性显著增强(P<0.05),并且在胁迫处理的12 d达到峰值,之后开始下降。干旱胁迫下,CHI活性在8~16 d迅速增加,且在胁迫16 d显著高于对照(P<0.05),之后开始下降(图7)。总体来说,水涝和干旱的CHS活性呈先上升后下降的趋势,且酶活性大于对照组,而对照组的CHI活性一直比较稳定。此外,相比于CHS活性,CHI对胁迫的响应略有延缓,说明CHI可能在CHS之后起作用。
-
从表1可以看出:干旱和水涝胁迫下,三叶青黄酮质量分数、PAL、CHS、CHI活性之间均表现为正相关关系,个别呈显著相关(P<0.05),表明黄酮质量分数受酶活性的调控。胁迫处理组的PAL与CHS、CHI之间呈正相关,但相关不显著(P>0.05);CHS和CHI之间呈极显著相关(P<0.01),表明CHS和CHI共同促进黄酮质量分数增加。
处理 黄酮 PAL CHS CHI 水涝 黄酮 1 PAL 0.143 1 CHS 0.830* 0.614 1 CHI 0.854* 0.413 0.950** 1 干旱 黄酮 1 PAL 0.256 1 CHS 0.846* 0.699 1 CHI 0.895* 0.625 0.980** 1 对照 黄酮 1 PAL 0.565 1 CHS −0.014 0.464 1 CHI 0.336 0.553 0.920** 1 说明:*表示显著相关(P<0.05);**表示极显著相关(P< 0.01) Table 1. Correlation analysis between flavonoid content, PAL, CHS and CHI under water stress
2.1. 水分胁迫下三叶青叶片和块根表型特征
2.2. 水分胁迫下三叶青叶绿体超微结构特征
2.3. 水分胁迫对三叶青块根黄酮质量分数的影响
2.4. 水分胁迫对三叶青块根黄酮合成关键酶活性的影响
2.5. 水分胁迫下黄酮质量分数与黄酮合成关键酶的相关性分析
-
水分影响三叶青的光合作用,而叶绿体作为光合作用的反应场所,其结构的完整性是影响光合作用正常运行的关键。本研究在三叶青叶绿体超微结构中发现:胁迫使三叶青叶绿体数量减少且不断向细胞中央靠拢,可能是三叶青叶片发黄的原因。王顺才等[21]在研究干旱胁迫对3种苹果属Malus植物的叶绿体超微结构的影响时也证实了这一点。本研究发现:干旱和水涝下,叶绿体形态结构发生改变,叶绿体膨大变圆,淀粉粒大量积累无法运出,质体小球的数量增多、体积变大、颜色变浅。杨凤军等[22]研究了干旱胁迫对番茄Lycopersicon esculentum叶面叶绿体超微结构后表明:逆境导致质体小球数量增加;因为质体小球是类囊体降解脂类聚集的结果,其数量的变化可作为叶细胞受损的标志[23]。本研究还观察到水涝和干旱的三叶青叶绿体基粒片层失去整齐紧密排列结构,变得松散模糊,没有界限,这与李冬林等[24]的研究结果一致;表明水涝和干旱使三叶青叶片叶绿体膜结构严重受损,失去生理活性,从而影响三叶青的光合作用和营养生长。
次生代谢过程是连接生态环境与药效成分含量的中间环节,产生的次生代谢产物在植物自我保护、生理调节等生命活动方面起着重要作用[25],并且是药用植物的主要药效成分。环境影响次生代谢产物的形成和积累[26]。目前,关于非生物因子影响药用植物次生代谢产物的研究已有很多,ZHU等[27]研究认为:柴胡Bupleurum chinense在水分胁迫下通过增加次生代谢产物的含量来提高抗氧化能力,进而抵制因水分胁迫产生的自由基;有研究发现:低温培养的黄豆Glycine max,其大豆黄素和染料木苷的代谢水平显著增高[28];LI等[29]在研究拟南芥Arabidopsis thaliana应对盐胁迫的响应时发现:黄酮类化合物含量上升。三叶青作为中药材,其药用活性成分主要有黄酮类化合物、酚酸类化合物、萜类化合物等次生代谢产物,本研究发现:水分胁迫促进了三叶青黄酮的积累,导致总黄酮质量分数高于对照,说明水涝和干旱使三叶青黄酮代谢增强,增加了三叶青块根单位干质量的黄酮质量分数。小麦Triticum aestivum、丹参Salvia miltiorrhiza等在水分胁迫下同样出现了黄酮类化合物质量分数上升的情况[30-31]。
PAL是连接初级代谢和苯丙烷类代谢途径中的关键酶和限速酶,可催化苯丙氨酸生成香豆酸、肉桂酸等中间产物,并且可以进一步转化为绿原酸、香豆素,也能够形成酯,再经历多条途径,进一步转化为木质素、类黄酮等物质。研究发现:药用植物在遭遇逆境胁迫时,PAL活性提高,当胁迫过严重时,PAL活性变弱[32],本研究也验证了这一点。胁迫前期,PAL活性增强,催化苯丙氨酸代谢,促进黄酮代谢的进行;随着胁迫时间加长,PAL活性降低。CHS和CHI分别是黄酮类化合物合成途径中的第1个和第2个关键酶,胁迫会上调CHS、CHI活性[33]。本研究中,CHS和CHI活性在PAL之后增强,而黄酮质量分数也在这时达到峰值,这与酶作用途径中的顺序一致;而在胁迫后期,PAL、CHS、CHI活性不断下降,三叶青黄酮质量分数也有所回落,这可能是由于胁迫使三叶青生长受到威胁,严重抑制各种生命活动,各种酶的活性也不断下降。
郭肖等[34]对水芹Oenanthe javanica黄酮质量分数和PAL活性的相关性分析发现:黄酮含量与PAL活性的变化趋势一致。本研究发现:三叶青块根中PAL、CHS、CHI活性与黄酮质量分数呈正相关,且部分指标显著相关(P<0.05),表明三叶青黄酮质量分数与关键酶活性相关,关键酶活性增强可能是引起三叶青黄酮高积累的关键因素。许多研究也证明了这一点,如LI等[35]研究发现:转基因烟草Nicotiana tabacum的总黄酮质量分数与CHI活性呈正相关;在温度与水分胁迫下,参与银杏Ginkgo biloba黄酮类代谢的PAL、CHS基因表达量的变化趋势与类黄酮质量分数变化趋势基本一致[36]。
水分胁迫影响三叶青的生长,表现为叶片块根受损、叶绿体超微结构损伤、黄酮质量分数以及黄酮合成途径相关酶活性变化等。三叶青通过产生一系列应激反应以应对水分胁迫,适度的水分胁迫可通过增强黄酮合成途径中的关键酶活性以增强抗逆性,同时增加黄酮的产量,提高三叶青的药材品质。











 DownLoad:
DownLoad: