-
植物常因具有独特的叶片斑纹而观赏价值倍增。引种于日本的矢竹Pseudosasa japonica是园林绿化中的重要材料[1],变型有花叶矢竹P. japonica f. akebonosuji和曙筋矢竹P. japonica f. akebono。前者有3种叶色,白叶、白绿相间条纹叶和白色渐渐复绿的复绿叶[2],后者叶色多为放射状暗条纹的淡绿叶。3种矢竹叶色丰富、秆型挺拔,是研究叶色变异的理想材料。不同叶色会影响植物光合能力,主要由叶片中色素种类和含量决定。王振兴等[3]对狗枣猕猴桃Actinidia kolomikta彩叶和绿叶研究发现,色素含量是影响叶色的重要因素;吴雪霞等[4]揭示了遮光使得光化学反应的能量在茄子Solanum melongena叶片所吸收的光能中占比逐渐增加,天线色素耗散的能量逐渐减少;银丝竹Bambusa multiplex ‘Silverstripe’不同叶色间的光合色素含量存在显著差异,随绿色叶片面积的下降而下降[5]。叶色是各种色素含量的综合表现,通常表现为绿色是因为叶绿素含量较高,而其他的色素含量比较低[6]。研究发现:随着色素累积水平的改变,植物叶色也发生相应变化[7],这种叶色变异与色素类物质合成降解、叶绿体发育等因素密切相关。张向娜等[8]发现:叶色中叶绿素和类胡萝卜素对茶树Camellia sinensis光合作用、抗逆性、鲜叶适制性等有重要影响。在可见光波段,水稻Oryza sativa不同叶色的冠层光谱反射率随着植株生长而不断变化,叶绿素含量与一阶微分光谱的相关系数呈极显著正相关[9]。色素也反映了植物的光合能力和生理状态,光合色素在光能的吸收、传递和转换过程中起着关键的作用,能驱动光合作用把光能转变为化学能[10],载体为光系统Ⅰ(PSⅠ)和光系统Ⅱ(PSⅡ)。有研究发现:光胁迫下的杂种杨无性系光化学能量储存分配到PSⅡ的份额要比分配到PSⅠ的多[11]。本研究以3种矢竹不同叶色叶片为对象,解析不同叶色叶片反射光谱特性、PSⅡ和PSⅠ特性之间的差异,探索不同叶色竹种光合能力差异,为进一步探究叶色变异机制奠定基础。
HTML
-
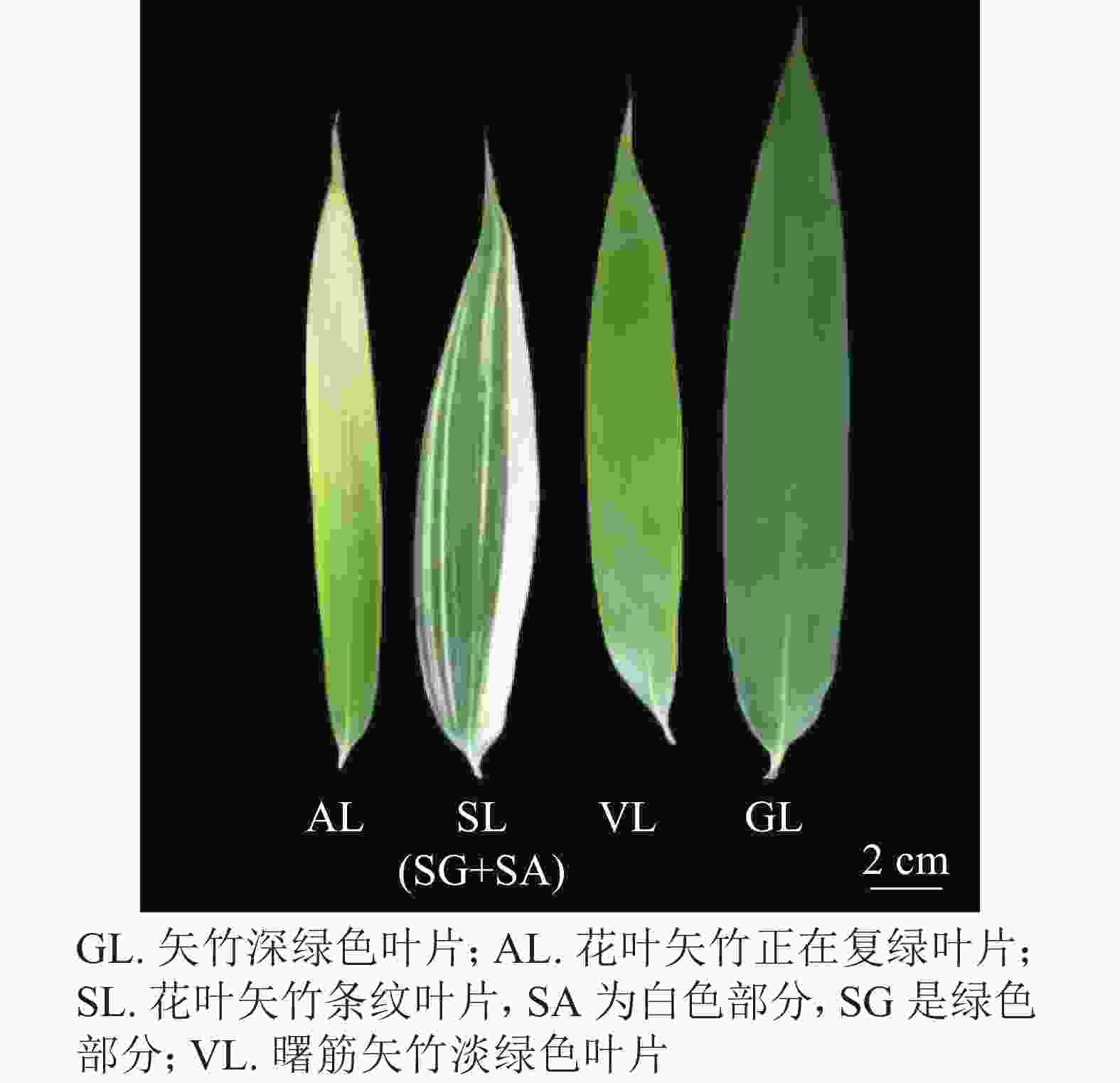
于2019年6月下旬,在浙江省杭州市浙江农林大学翠竹园进行采样测定。测量和取样位点均为中部。取竹高、叶位及长势一致,生长健壮的当年成竹,枝位第2档第2片完全展开叶。取样叶包括矢竹的深绿色叶片(green leaf,GL),花叶矢竹正在复绿叶片(alobino leaf,AL,相对矢竹复绿30%,SPAD值为12.5),花叶矢竹条纹叶片(trip leaf,SL。包括白色部分albino sector in leaf with strips,SA;绿色部分green sector in leaf with strips,SG),曙筋矢竹淡绿色叶片(virescent leaf,VL)。
-
取植株新鲜成熟功能叶片,去叶脉后剪碎混合均匀,称0.5 g,用体积分数为80%的丙酮溶液25 mL提取叶片叶绿素,静置48 h,重复3次。具体操作方法和步骤参照参考文献[12]。用紫外-可见分光光度计(UV-255)对350~1 000 nm波长范围内的光吸收值进行全光谱扫描。根据ARNON法[13]修正公式计算叶绿素a(Chl a)、叶绿素b(Chl b)和类胡萝卜素(Car)质量分数。
-
观测仪器为光谱分析仪(UniSpec-SC,美国),波段为310~1 150 nm,采样间隔1 nm。每个处理选取10个样株,每个样株选取3片叶片,取平均值作为该样品的光谱反射率。测量过程中用白板对其进行校正。用Multispec 5.1读取反射光谱原始数据,对原始光谱数据进行微分变换一阶求导,增强叶片反射光谱特征,减少背景噪声的影响和提高生化参数的监测效果。Dλi=Rλi+1−Rλi−1/(λi+1−λi−1),其中:Dλi为反射率光谱的一阶导数光谱;Rλi为波长λi处的光谱反射率值[14-15]。为了进一步研究不同叶色矢竹反射光谱与色素、光系统之间的关系,根据已有研究,选择与叶片色素关系密切的2个植被指数,即叶绿素归一化指数(ChlNDI)[16]和光化学植被指数(PRI)[17]。IChlND=(R750−R705)/(R750+R705);IPR=(R531−R570)/(R531+R570)。其中:IChlND为叶绿素归一化指数,IPR为光化学植被指数,Rλ为波长λ处的光谱反射率。
-
采用非调制式叶绿素荧光仪(Yaxin-1611)测定叶片快速叶绿素荧光动力学参数,方法参照STRASSER等[18]。测量前,叶片暗适应15 min,以二极管(465 nm,谱线半宽20 nm)为光源发出强度为3 000 μmol·m−2·s−1的饱和脉冲光照射叶片,以10 μs (2 ms之前)和1 ms (2 ms之后)的间隔记录荧光信号,并检测激发的叶绿素荧光。测定5 次·株−1,计算平均值及标准误。将得到的数据经Vt=(Ft−F0)/(FM−F0) 标准化之后得到快速叶绿素荧光诱导动力学曲线[19],快速叶绿素荧光诱导动力学得到的参数反应了PSⅡ供体侧、受体侧、反应中心等光合机构的特性。
-
以4种不同叶色矢竹叶片为材料,测量和取样位点均为中部。将叶片经过暗适应20 min后利用M-PEA植物效率仪(Hansatech,英国)测定820 nm的光吸收值,以820 nm相对吸收值(ΔI/I0)作为衡量PSⅠ最大氧化还原能力的指标[20]。计算公式参照SCHANSKER等[21]。4种矢竹不同叶色叶片重复测定5次,计算平均值及标准误。
-
采用Excel 2019整理、计算数据,用SPSS 22.0和Duncan法进行方差分析和多重比较,用Origin 9.0、SigmaPlot及Photoshop CS5作图。
1.1. 材料
1.2. 方法
1.2.1. 光合色素测定与计算
1.2.2. 叶片反射光谱测定
1.2.3. 快速叶绿素荧光诱导动力曲线测定
1.2.4. 820 nm光吸收值测定
1.3. 数据分析
-
对同一发展期的叶片调查发现:不同种矢竹叶片颜色差异较大(图1)。花叶矢竹的叶色会出现白叶(AL)和具绿色条纹的花叶(SL),其中白叶能逐渐复绿,但是绿度小且泛黄;曙筋矢竹叶片(VL)为淡绿色;矢竹叶片(GL)呈深绿色。
由表1可知:AL中叶绿素a、叶绿素b和类胡萝卜素质量分数最低,与其他叶片差异极显著(P<0.05)。GL的叶绿素a 、叶绿素b 质量分数为最高,叶绿素a较VL高29.8%,较SL高8.8%;叶绿素b较VL高25.1%,较SL高7.6%;类胡萝卜素较VL高20.1%,较SL低11.4%;而SG与VL的差异不显著(P>0.05)。AL的Chl a/b、Car/Chl均为最高,显著高于其他3种叶片;其中SA为叶片白色部分,未测出光合色素。
材料 Chla/(mg·g−1) Chlb/(mg·g−1) Car/(mg·g−1) Chl a+b/(mg·g−1) Chl a/b Car/Chl AL 0.34±0.015 a 0.26±0.010 a 0.32±0.015 c 0.60±0.035 a 1.3±0.007 a 0.53±0.022 b SG 20.39±0.170 b 18.27±0.120 b 5.52±0.020 a 38.66±0.250 c 1.1±0.020 b 0.14±0.010 a VL 17.09±0.080 b 15.72±0.070 b 4.07±0.184 b 33.81±0.140 b 1.0±0.013 b 0.12±0.007 a GL 22.19±0.120 c 19.66±0.150 b 4.89±0.020 a 41.85±0.220 c 1.14±0.024 b 0.11±0.005 a 说明:同列内不同小写字母表示材料间差异显著(P<0.05) Table 1. Difference of photosynthetic pigment content in different colors’ leaves of P. japonica
-
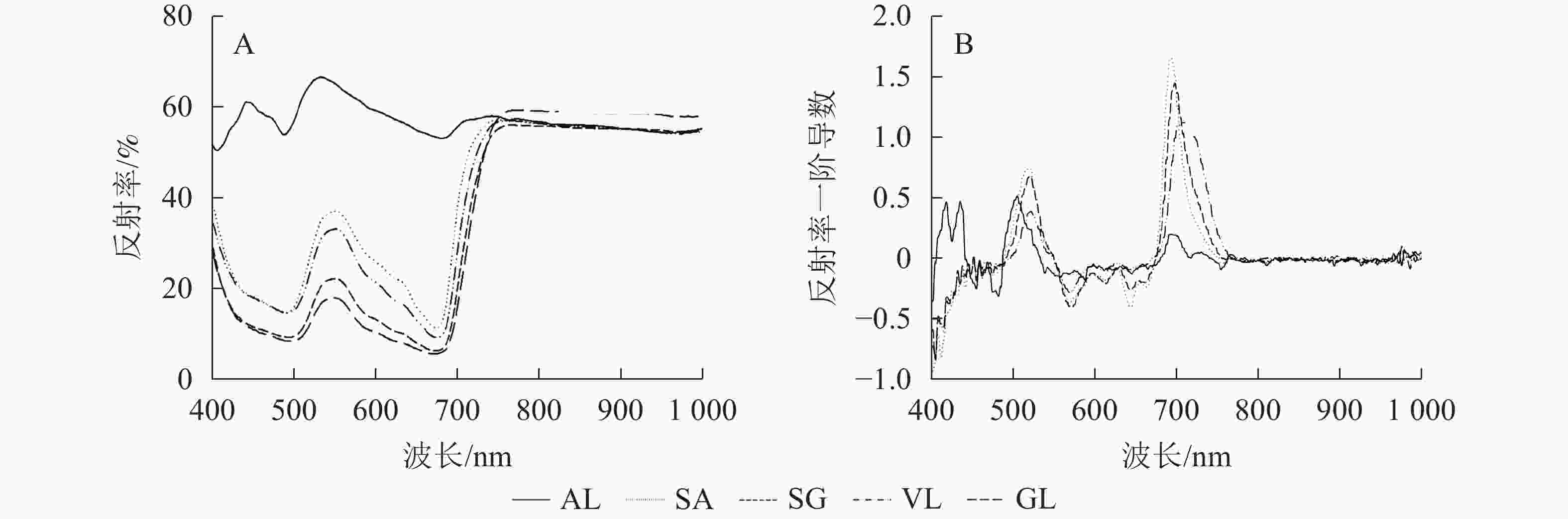
除AL外,其他的反射光谱曲线均具有典型的绿色植被反射光谱特征,在500与670 nm左右的位置有2个低反射区。不同叶片反射光谱的反射率在蓝光区(430~470 nm)、绿光区(500~560 nm)不同,SA在绿光区明显高于SG、VL、GL,在556 nm 处达最高值,为0.385,比VL、SG和GL分别高8.0%、46.7%和51.2%;GL在近红外区(780~1 000 nm)最高,达0.600,其他叶片趋于一致(图2A)。由一阶导数(图2B)可以看出,除AL外,其他叶片在绿光区(550~560 nm)和红光区(650~760 nm)均出现最大峰值,黄光区(560~600 nm)出现最小峰值。
-
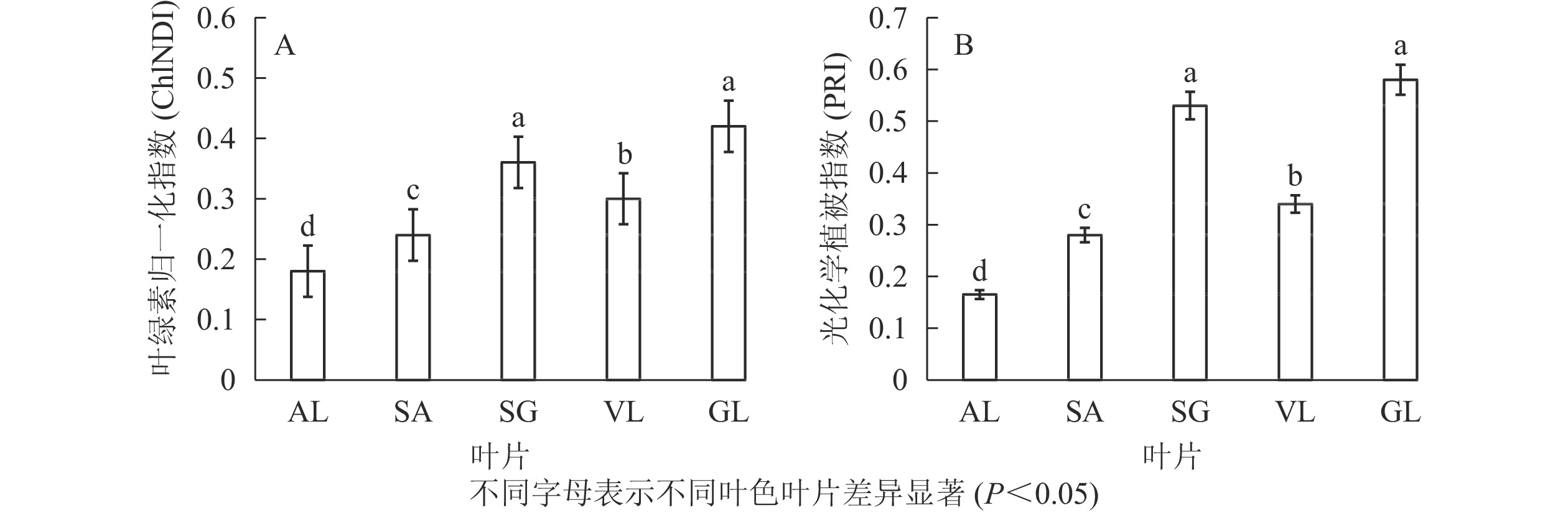
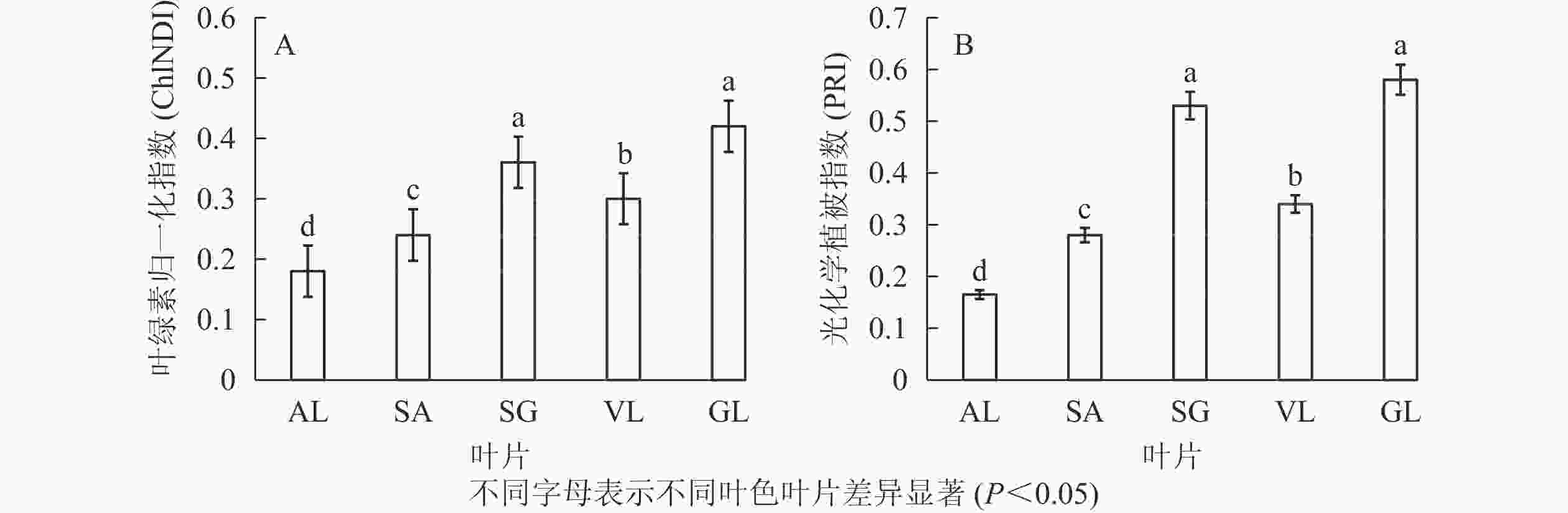
图3所示:不同叶色矢竹叶片的叶绿素归一化指数、光化学植被指数变化趋势一致,从大到小依次为GL、SG、VL、SA、AL。GL(0.420)与SG(0.350)叶绿素归一化指数无显著差异,但显著(P<0.05)高于其他叶片,AL的2种植被指数值均为最低,分别为0.180和0.165。
-
由图4可以看出:GL的Fv/Fm为0.80,SG和VL的Fv/Fm降低为0.72和0.75,说明它们反应中心的活性有所下降。而AL显著(P<0.05)低于其他叶片,仅为0.32。GL的Fv/Fm显著(P<0.05)高于AL和SG,VL的Fv/Fm占GL的94%。说明反应中心活性从大到小依次为GL、VL、SG、AL。叶片光化学性能指数PIABS可以更为准确地反映植物光合机构的状态[22]。其中:ψP0为暗适应后的最大光化学效率,ψ0为反应中心捕获的激子将电子传递到电子传递链中QA−下游电子受体的概率。SG与VL的PIABS差异不显著(P>0.05),但都与GL差异显著(P<0.05),分别为GL的46.2%与42.1%。AL的PIABS最低,为0.32。VL与GL的Fv/Fm无显著差异(P>0.05),PIABS存在显著差异(P<0.05),GL较VL高56.3%。
-
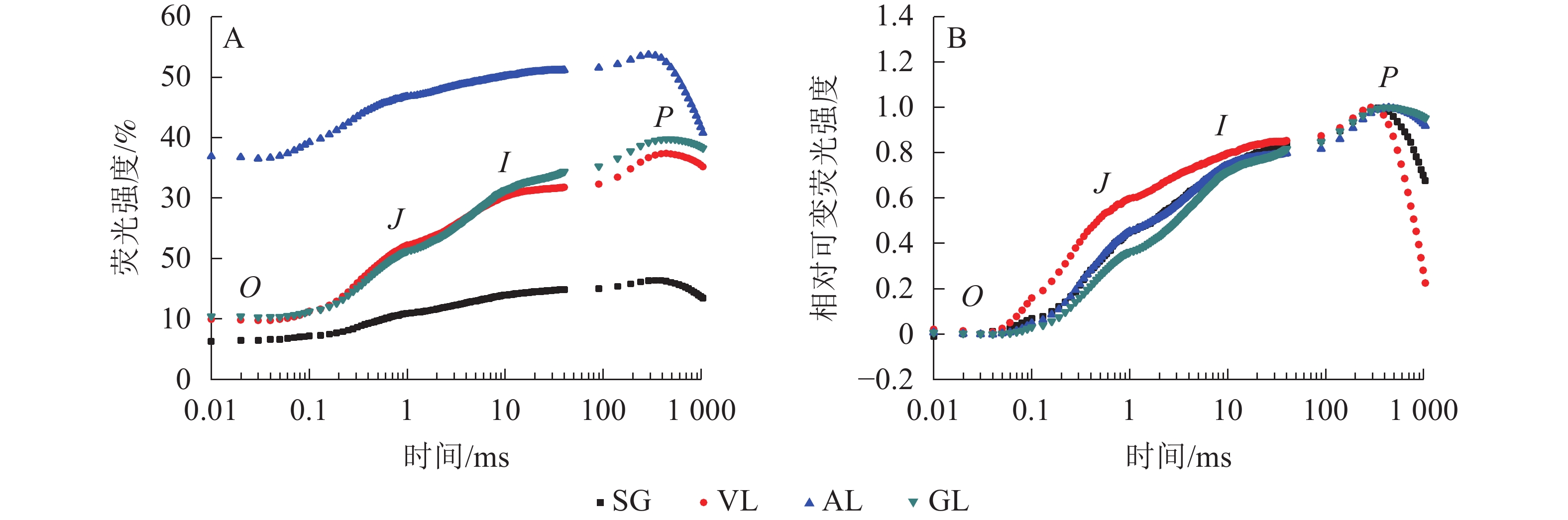
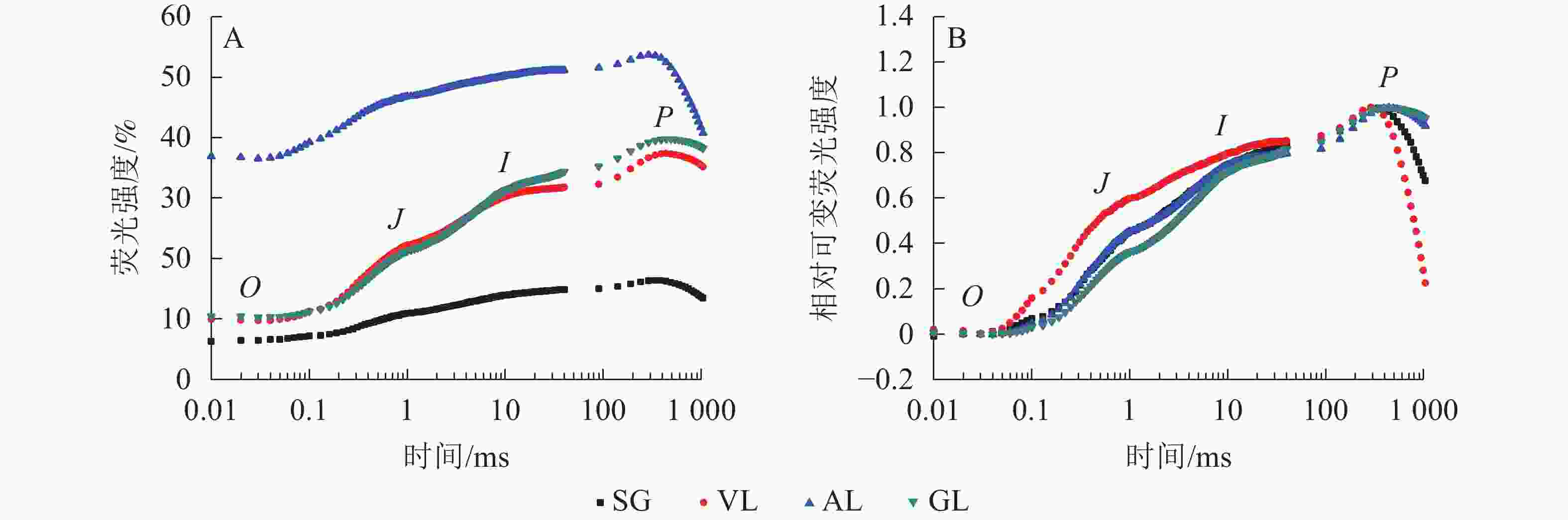
如图5A所示:AL的初始荧光(F0)最大,SG的F0最小,说明AL的反应中心发生可逆性失活,SG的反应中心活性或类囊体的完整性均大于其他叶片。2 ms时的荧光强度(FJ)和30 ms时的荧光强度(FI)、最大荧光产量(FP=Fm)均表现为AL大于其他叶片,SG均小于其他叶片。SA为花叶矢竹条纹叶(SL)中的白色部分,未检测出相关数据。GL和VL的荧光诱导动力学曲线(OJIP曲线)差异不大,在O、J、I点近乎相同,大于SG且小于AL。将OJIP曲线双重归一化后得到快速叶绿素荧光诱导动力学曲线(图5B),3个竹种的不同叶色叶片均存在O、J、I、P点,说明光合电子链仍然能够有效运转。J点和I点主要反映PSⅡ受体侧活性。VL的曲线与GL没有重合,在J点处明显分开。J点出现是由于电子传递时间差而还原态质体醌A(QA−)大量累积导致的荧光迅速上升,说明VL累积的QA−最多。I点与P点一致,从大到小依次为VL、AL和SG、GL,当达到P点时,说明PSⅡ完全关闭,荧光产量最高。
-
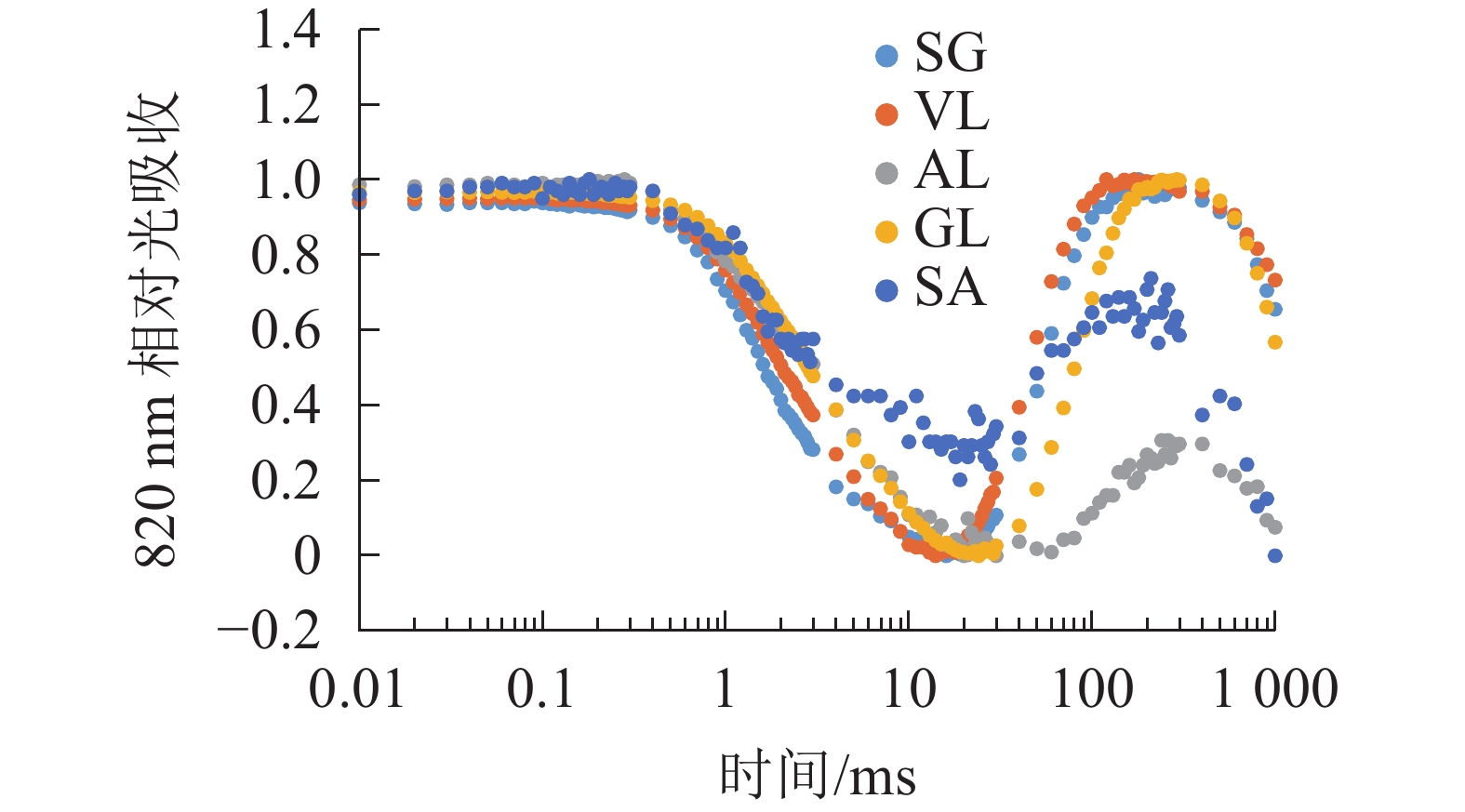
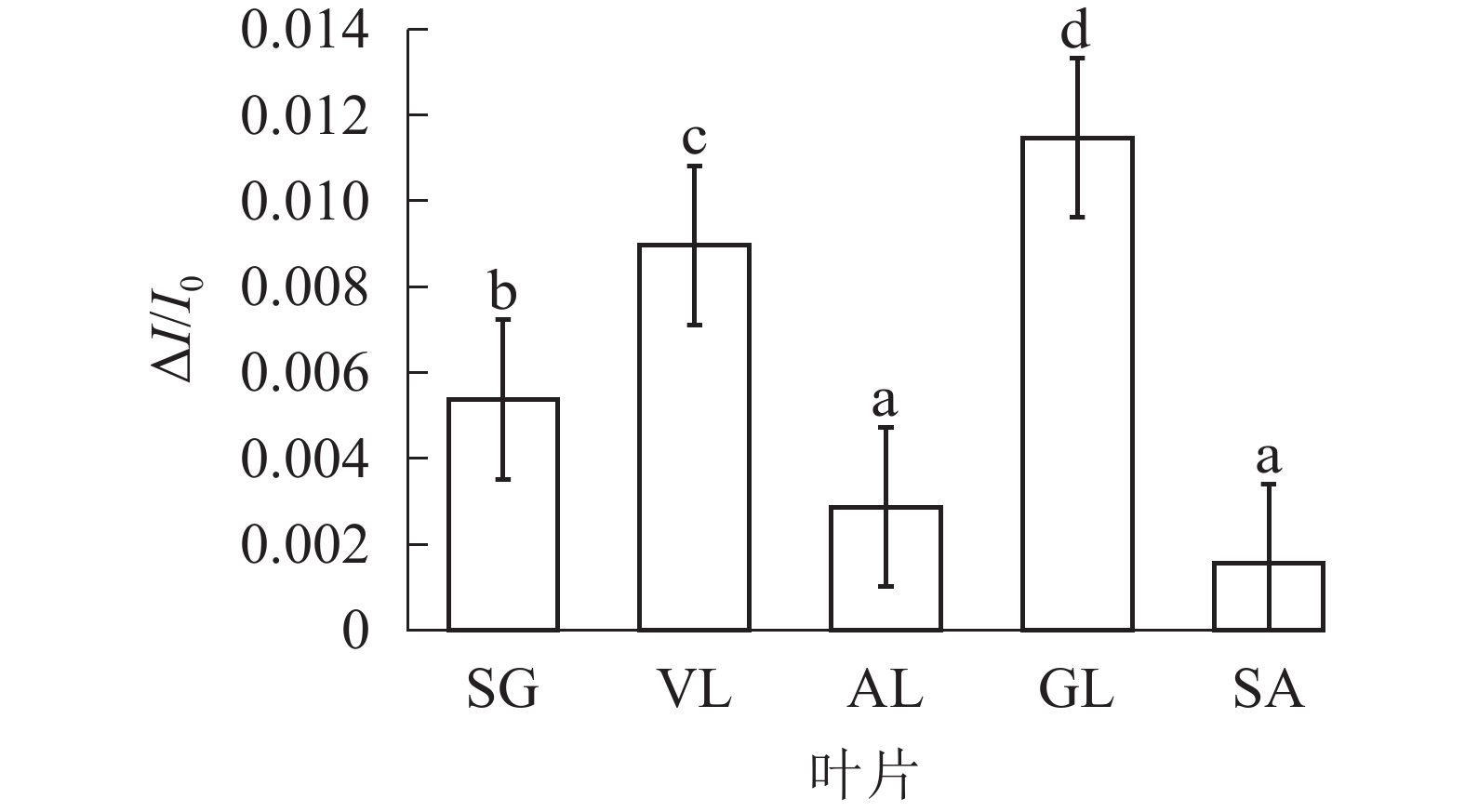
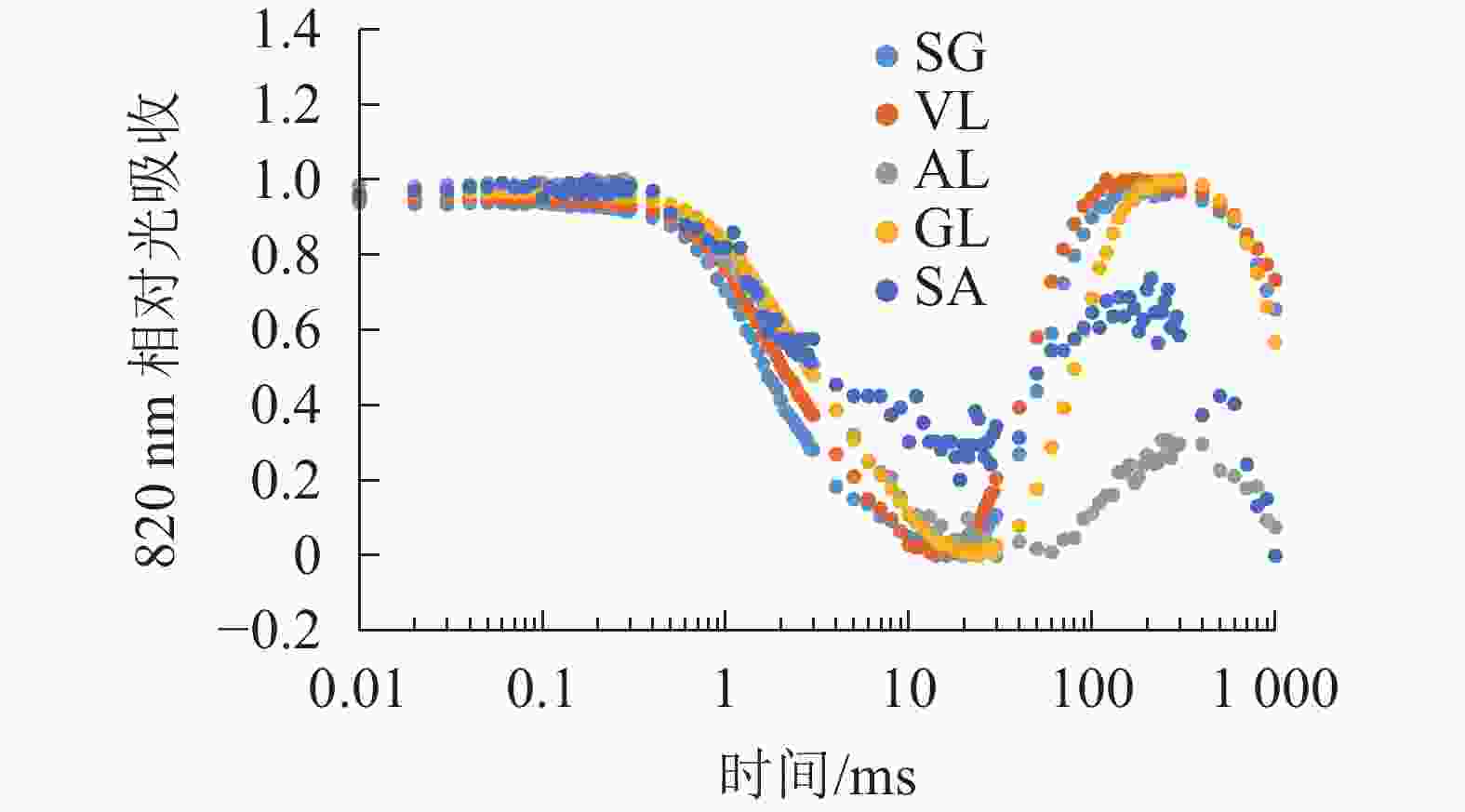
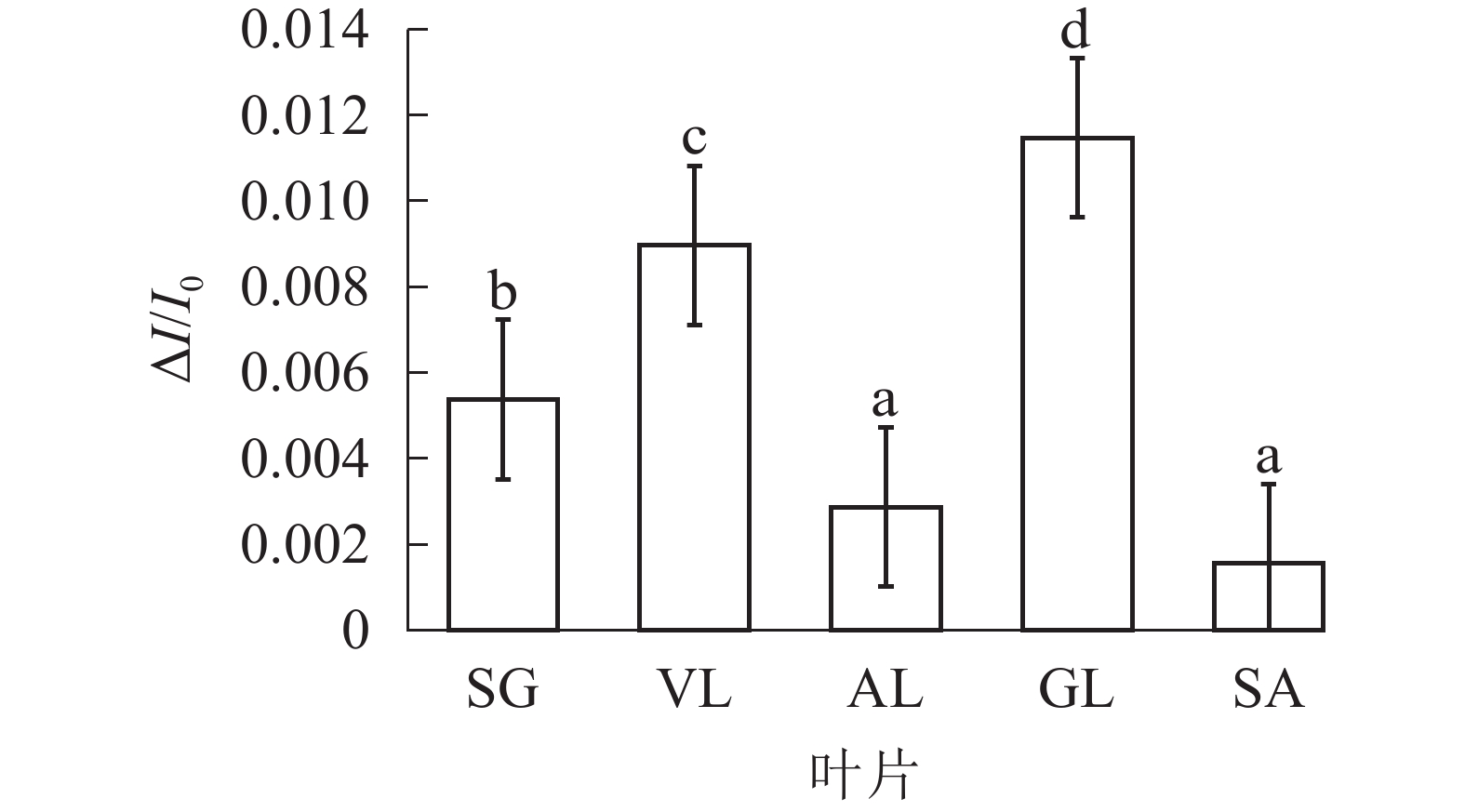
由图6可以看出:各叶片在t时的820 nm相对吸收值(It)呈“V”型变化,且SG、VL、GL变化趋势相同。在16 ms时,各叶片的820 nm相对光吸收值达到最低点,1~16 ms,各叶片的820 nm相对吸收值均呈现下降趋势,且下降速度从大到小依次为SG、VL、GL、AL、SA。180 ms时,820 nm相对光吸收值达到最大值,从大到小依次为SG、VL、GL、SA、AL。16~180 ms,各叶片的820 nm相对吸收值整体呈现上升趋势。由图7可以看出:GL的ΔI/I0最大,且与其他叶片差异显著(P<0.05),SA的ΔI/I0最低,AL与SA的ΔI/I0差异不显著(P>0.05),与其他叶片差异显著(P<0.05)。PSⅠ最大氧化还原能力从大到小依次为GL、VL、SG、AL、SA。花叶矢竹条纹叶白色部分(SA)复绿之后(AL)ΔI/I0上升,说明PSⅠ活性上升。
-
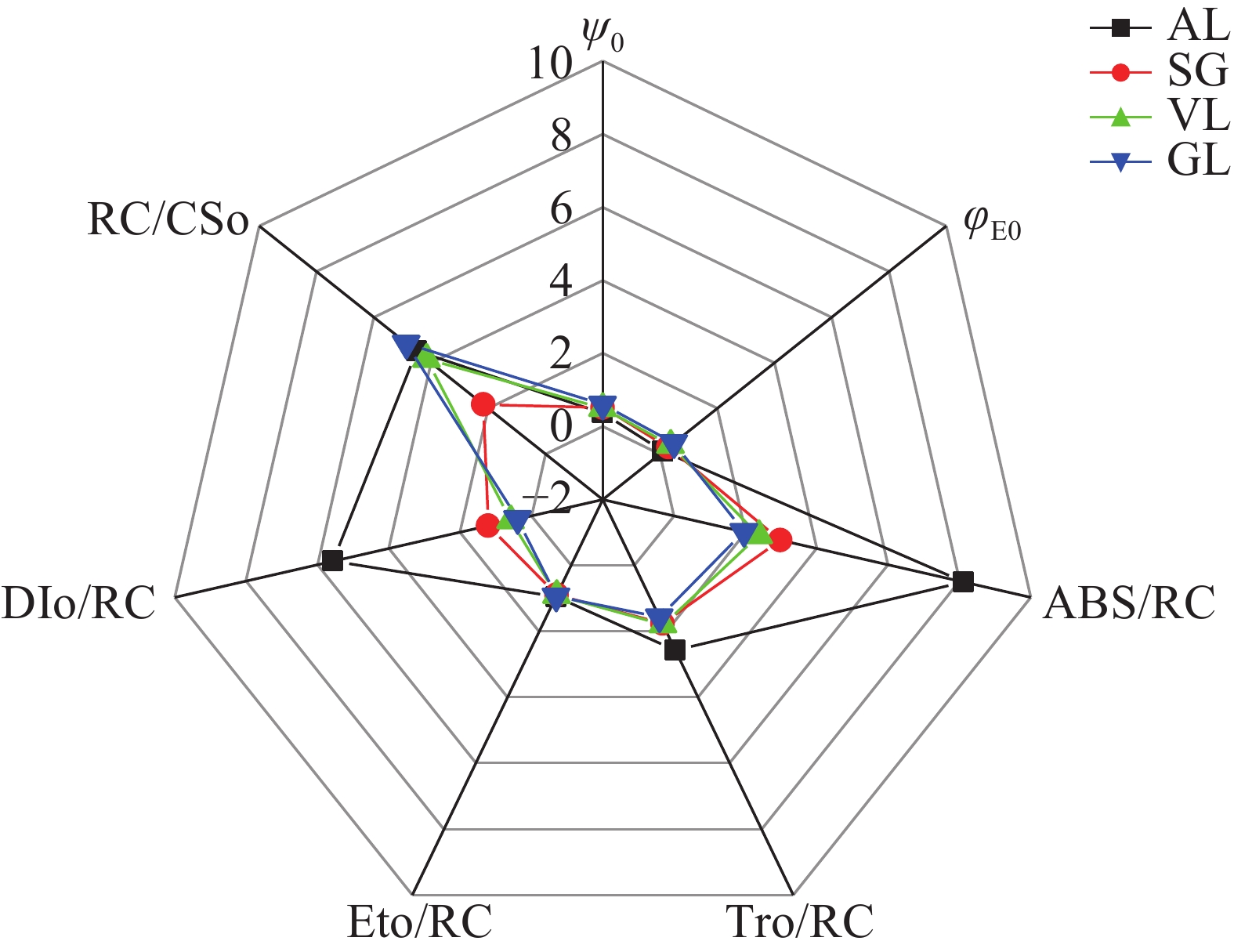
如图8所示:在能量传递效率方面,捕获的光量子进入电子传递的效率(Ψ0)和吸收的光能进入电子传递的效率(φE0)的趋势一致,均从大到小依次为AL、SG、VL、SG,且AL与其他叶片差异显著(P<0.05)。在单位反应中心的性能方面,不同叶片的反应中心吸收光能(ABS/RC)、PSⅡ的最大捕获量(TRO/RC)、用于电子传递的能量(ETO/RC)、单位反应中心的热耗散(DIO/RC)这4个代表单位反应中心的性能的参数趋势较为一致。ABS/RC从大到小依次为AL(8.13)、SG(2.97)、VL(2.38)、GL(1.96)。AL吸收的光能远远高于其他叶片,还原QA的能量及用于电子传递的能量最高。但是GL的单位面积反应中心(RC/CSO)的数量最多,SG的RC/CSO数量为最少。
-
相关分析表明(表2):矢竹类叶片的叶绿素a、叶绿素b、叶绿素a+b、类胡萝卜素质量分数与叶绿素归一化指数(ChlNDI)、光化学植被指数(PRI)都显著正相关(P<0.05)。且叶绿素a、叶绿素b与ChlNDI、PRI呈极显著正相关(P<0.01)。ChlNDI和吸收的光能进入电子传递的效率(φEo)呈极显著正相关(P<0.01),与Fv/Fm、PIABS显著正相关(P<0.05);但PRI与PIABS相关性不显著(P>0.05),PRI与Fv/Fm、φEo为显著(P<0.05)相关。
光谱参数 Chla Chlb Chl a+b Car Fv/Fm PIABS φEo ChlNDI 0.966** 0.961** 0.924** 0.898* 0.883* 0.802* 0.937** PRI 0.969** 0.977** 0.931** 0.929** 0.759* 0.648 0.823* 说明:*表示P<0.05,**表示P<0.01 Table 2. Correlation between reflectance spectrum parameters and pigment contents
2.1. 叶色及光合色素变化
2.2. 反射光谱特征
2.2.1. 反射光谱及其一阶导数
2.2.2. 植被指数
2.3. 快速荧光特性的差异分析
2.3.1. 最大光化学效率(Fv/Fm)和叶片性能指数(PIABS)的变化
2.3.2. 叶绿素荧光拐点的变化及诱导曲线
2.3.3. 不同叶色矢竹PSⅠ性能差异
2.3.4. 不同叶色矢竹能量传递效率及单位反应中心性能变化
2.4. 相关性分析
-
不同叶色变异现象与光合色素含量的差异有关。在高等植物中, 决定叶片颜色的色素主要有叶绿素、类胡萝卜素和花青素等[23]。季鹏章等[24]研究发现:茶树紫色变异品种‘紫娟’Camellia sinensis var. assamica叶绿素含量随着叶片的成熟度增加呈上升趋势。WANG等[25]发现:黄色变异茶树‘中黄2号’C. sinensis‘Zhonghuang 2’的叶绿素和类胡萝卜素含量显著低于正常绿色品种‘龙井43’C. sinensis ‘Longjing 43’。本研究中,不同叶色矢竹叶片的光合色素质量分数存在差异,导致叶色变异。3种矢竹不同叶色叶片的光合色素质量分数均不同,矢竹叶片GL叶绿素和类胡萝卜素质量分数最高,叶绿素a质量分数显著高于其他叶片;AL的叶绿素a、叶绿素b和类胡萝卜素质量分数不到0.5 mg·g−1,显著低于其他叶片,所以叶色主要呈白色。
植物色素是植物体内主要吸收光能的物质,且植物叶片色素质量分数与光谱参数也有着紧密的联系[26]。叶绿素参与吸收和传递光能、光化学反应,在光合作用中起着非常重要的作用。且叶绿素a是构成反应中心的重要组分,叶绿素的质量分数会影响反应中心的数量[27-29]。反射光谱特征能反映光合能力。本研究中,色素质量分数的差异导致矢竹不同叶色叶片光谱曲线也存在较大差异。不同绿度叶片的反射光谱曲线整体变化趋势一致,且相同波长下反射率差异较大,而花叶矢竹白叶(AL)反射光谱无典型的绿色植被光谱曲线特征,表明白叶复绿过程中光能吸收存在异常。矢竹叶片的叶绿素及类胡萝卜素质量分数与叶绿素归一化指数(ChlNDI)、光化学植被指数(PRI)都存在显著相关性,说明光合色素质量分数会影响反应中心数量,进而影响叶绿素归一化指数和光化学反射指数特征参数。
不同叶色叶片PSⅠ、PSⅡ之间的活性存在差异且相互作用,叶绿素缺乏会影响PSⅡ活性反应中心和PSⅡ天线的合成[30]。当吸收、转化的光能在PSⅠ和PSⅡ间达到平衡时,植物的光化学效率和光化学活性才能达到最大;光合作用电子传递是由PSⅠ和PSⅡ协调作用共同完成的,PSⅠ或PSⅡ受到伤害,都会导致光合能力下降[31]。不同叶色矢竹叶片均具有叶绿素荧光曲线动力学活性。但PSⅡ反应中心开放降低程度差异显著(P<0.05),花叶矢竹的复绿叶和淡绿叶显著(P<0.05)低于矢竹,且能量用于电子传递份额变小;ΔI/I0是作为衡量PSⅠ最大氧化还原能力的指标,ΔI/I0越高说明其最大氧化还原能力越强[20]。不同叶片间参数ΔI/I0的变化,表明了PSⅠ的氧化还原能力存在显著差异,PSⅠ 抑制会限制PSⅡ向受体侧的电子传递,导致PSⅡ的受体侧电子传递链受损,PSⅡ反应中心发生可逆性失活。叶绿素质量分数最低的AL叶片最大光化学效率Fv/Fm和叶片性能指数PIABS均为最低,PSⅡ反应中心活性最低,植物光合机构状态未恢复,充分表明PSⅡ活性最弱。这些结果表明:矢竹叶片随着相对叶绿素的减少会降低光能的吸收,而花叶矢竹白叶色素质量分数低可能与叶色变异有关。花叶矢竹条纹叶片反应中心较少,但仍具有较好的PSⅡ活性和叶绿素水平,这可能与叶色变异有密切关系。
综上所述,不同叶色矢竹叶片均具有典型的反射光谱特征,但是不同叶色叶片间光谱特征与光系统特性存在差异,这与叶绿素质量分数的变化有直接的关系。叶绿素减少会大大降低光能的吸收,而光抑制会限制PSⅡ向受体侧的电子传递,电子传递链受损,反应中心失活。花叶矢竹条纹叶片反应中心数量较少,但仍具有较好的PSⅡ活性和叶绿素水平,维持较好的光合能力。












 DownLoad:
DownLoad: