-
近年来,全球气候的持续变化加剧了干旱对森林的威胁[1]。以往研究表明:水力安全阈值(导水率损失50%时的水势与最小水势差)与年均降水量无关,这意味着干旱引发的森林衰退存在全球趋同现象[2]。在严重干旱后的数年内,植物普遍存在生长缓慢和恢复不完全的遗留效应,植物生长强弱根据水力安全阈值而异[2-3],生长在湿润地区的植物受遗留效应的影响,恢复能力较弱,难以在未来更为频繁的干旱威胁下正常生长,从而导致湿润区生态系统的永久损伤,并进一步造成森林碳汇的普遍退化[4]。通过对湿润区不同水分环境植物的研究,有助于理解水分有效性对湿润区植物生存策略的影响。
水力失效是干旱期间植物生产力下降和死亡的主要原因[5]。水分供需矛盾的加剧迫使更多空气进入木质部管道,由此产生的栓塞阻碍了植物的水分运输,最终导致水力失效。植物的输水效率常通过植物茎的比导率(Ks)来衡量[6],湿润生境下的植物倾向于最大化输水效率而非增加木质部对栓塞的抵抗力以满足生长需求[7]。栓塞抗性(植物导水率损失50%的水势,P50)常用来表征植物应对干旱的能力[6]。研究表明:植物的栓塞抗性与干旱胁迫强度呈正相关[2, 8]。当水势降至P50以下时,木质部栓塞加速,水力运输功能明显受阻。通过对水力性状的研究,有助于描述不同植物水力策略的范围,进而深入理解植物的驱动因素[9]。
裸子植物和被子植物的木质部结构差异较大[10-11],管胞在运输和支撑方面发挥作用,导管则仅具有运输功能。依赖纤维提供木材强度[12-13],厚度跨度比及纹孔形态作为管壁的重要特征,与栓塞抗性密切相关[14]。输水效率由管腔面积及管道密度决定。根据Hagen-Poiseuill定律[15],木质部管腔面积分数的减少可以通过增大管道尺寸弥补。相比由管胞构成的裸子植物木质部,被子植物复杂的木质部结构可独立改变导管结构以优化运输,为机械强度或储存功能提供更大的木质部空间[16]。
环境水分有效性对植物的水力策略具有选择性,从而驱动植物群落的分布[17]。本研究通过比较7种裸子植物和7种被子植物在不同生境下的栓塞抗性、输水效率和解剖结构的性状差异,探究植物水力性状与木质部解剖结构的关系,以期为研究湿润区亚热带植物在不同水分条件下的水力适应策略提供参考。
-
研究区位于浙江省杭州市临安区,该区地处中亚热带向北亚热带过渡区域,四季分明,区内年平均气温为8.8~14.8 ℃,年降水量为1 390~1 870 mm,无霜期为209~235 d,相对湿度为76%~81%。在研究区内选择自然和人工生境进行植物样本采集(表1)。自然生境位于西天目山景区,除自然降水外无额外灌溉。植被类型以常绿-落叶阔叶混交林为主,除研究树种外(表2),其他常见树种有北美香柏Thuja occidentalis、短尾柯Lithocarpus brevicaudatus、榧树Torreya grandis和榉树Zelkova serrata等。人工生境为浙江农林大学植物园,相比自然生境土壤水分有效性高,人工灌溉充分。除研究树种外(表2),其他常见树种有枫香Liquidambar formosana、桂花Osmanthus fragrans、冬青Ilex chinensis、垂柳Salix babylonica和竹柏Podocarpus nagi等。
生境 经纬度 海拔/m 坡向 坡度/(°) pH 生长季土壤含水量/% 自然生境 30°26′N, 119°73′E 400~450 西南 9~12 4.85±0.16 29.53±1.21 人工生境 30°15′N, 119°43′E 51~74 西南 15~20 5.23±0.12 35.98±1.22 说明:pH和生长季土壤含水量数值为平均值±标准误(n=3) Table 1. Basic characteristics for study sites in natural and artificial habitats
植物 科 植物 生长习性 生活型 裸子植物 杉科 Taxodiaceae 柳杉 Cryptomeria japonica 落叶 乔木 松科 Pinaceae 金钱松 Pseudolarix amabilis 落叶 乔木 杉科 Taxodiaceae 落羽杉 Taxodium distichum 落叶 乔木 杉科 Taxodiaceae 杉木 Cunninghamia lanceolata 常绿 乔木 松科 Pinaceae 雪松 Cedrus deodara 常绿 乔木 柏科 Cupressaceae 日本扁柏 Chamaecyparis obtusa 常绿 乔木 杉科 Taxodiaceae 北美红杉 Sequoia sempervirens 常绿 乔木 被子植物 槭树科 Aceraceae 三角槭 Acer buergerianum 落叶 乔木 大戟科 Euphorbiaceae 重阳木 Bischofia polycarpa 落叶 乔木 胡桃科 Juglandacea 青钱柳 Cyclocarya paliurus 落叶 乔木 壳斗科 Fagaceae 青冈 Cyclobalanopsis glauca 常绿 乔木 木犀科 Oleaceae 女贞 Ligustrum lucidum 常绿 乔木 木兰科 Magnoliaceae 广玉兰 Magnolia grandiflora 常绿 乔木 樟科 Lauraceae 樟树 Cinnamomum bodinieri 常绿 乔木 Table 2. Basic overview of 7 species of gymnosperms and 7 species of angiospermae
-
2021年4—6月,在不同生境下选取生长状况良好的7种裸子植物和7种被子植物作为目标物种(表2)。选择同一生境下立地条件基本一致,且胸径、树高、树龄、冠幅等相近的同一植物15~21株。在天气晴朗的8:00—11:00采集枝条,所选枝条均位于树体南部外层和树冠中部。每株随机截取3~5个枝条,所剪枝条基部直径为6~8 mm,长为15~30 cm。剪取后迅速放入装有水的黑色收纳箱中(防止水分散失和外界空气等进入被切开的导管内),立即带回实验室,进行水力功能性状和结构性状的测定。
-
采回样品在水中暗适应30~60 min后,在水下再次剪短样品,并修平切口。裸子植物剪取枝段平均长度为134.23 mm,平均直径为4.95 mm,被子植物剪取枝段平均长度为160.04 mm,平均直径为5.47 mm。本研究采用空气注入法构建枝段脆弱性曲线,具体如下:将枝段放入压力腔,并将枝段近轴端连接到木质部导水率与栓塞测量系统XYL’EM-Plus (Bronkhorst, Montigny-les-Cormeilles, 法国)。使用测量溶液20 mmol·L−1 氯化钾+1 mmol·L−1 氯化钙[18],在高压(120 kPa)下反复冲刷枝段10 min至最大导水率不再变化,以确保潜在栓塞被去除。在低压(6 kPa)下测量枝段的最大导水率(Kmax,kg·m·s−1·MPa−1)[19]。在确定Kmax后,对枝段施加压力2 min,用测量Kmax的方法测量相应的导水率(Kh,kg·m·s−1·MPa−1)。该过程以0.2~0.3 MPa的增量重复进行(取决于植物),直到导水率损失(PLC)至少达到90%[20]。采用XylWin 3.2软件(Bronkhorst, Montigny-les-Cormeilles, 法国)对其导水数据进行分析。比导率(Ks,kg·s−1·m−1·MPa−1)作为输水效率指标,通过Kmax除以无髓和树皮的基部边材横截面积得到[19]。导水率损失百分比计算如下:PLC=(1−Kh/Kmax)×100%[21]。导水率损失50%时的水势(P50)作为本研究的栓塞抗性指标。
-
从测定导水率的枝段上截取0.5 cm长小段样品,固定软化后,采用石蜡切片法制成永久切片,所用样品的横切面用Leica DM 3000显微镜在50和400倍镜下摄像,所有样品的纵切面在400倍镜下摄像。用Image J图像处理软件分析照片,测量管胞壁厚度(μm)及导管壁厚度(μm),并计算管胞水力直径(μm)、导管水力直径(μm)、管胞密度(个·mm−2)、导管密度(个·mm−2)、厚度跨度比。根据文献[22]计算管胞水力直径和导管水力直径。管胞密度和导管密度分别通过横截面管胞数量和导管数量除以横截面面积得到。管胞壁和导管壁厚度跨度比具体测量方法可参考相关文献[14, 23]。
-
从测定导水率的枝段上截取3根3~5 cm的小枝段,用于木质部密度(WD, g·cm−3)测量。参考文献[24]方法:用刀片除去枝段样品树皮,利用阿基米德原理确定样品的新鲜体积(V,cm3);测得体积后,将样品置于75 ℃烘箱烘48 h,测得干质量(W,g),则WD=W/V。
-
图表制作与统计分析利用R软件(version 3.5.3)。同一植物在不同生境下各水力性状和解剖结构性状的差异,在除去异常值后,在种内进行t检验。植物枝水力性状与解剖结构性状间的关系采用Pearson相关性分析。文中所有数值为平均值±标准误。
-
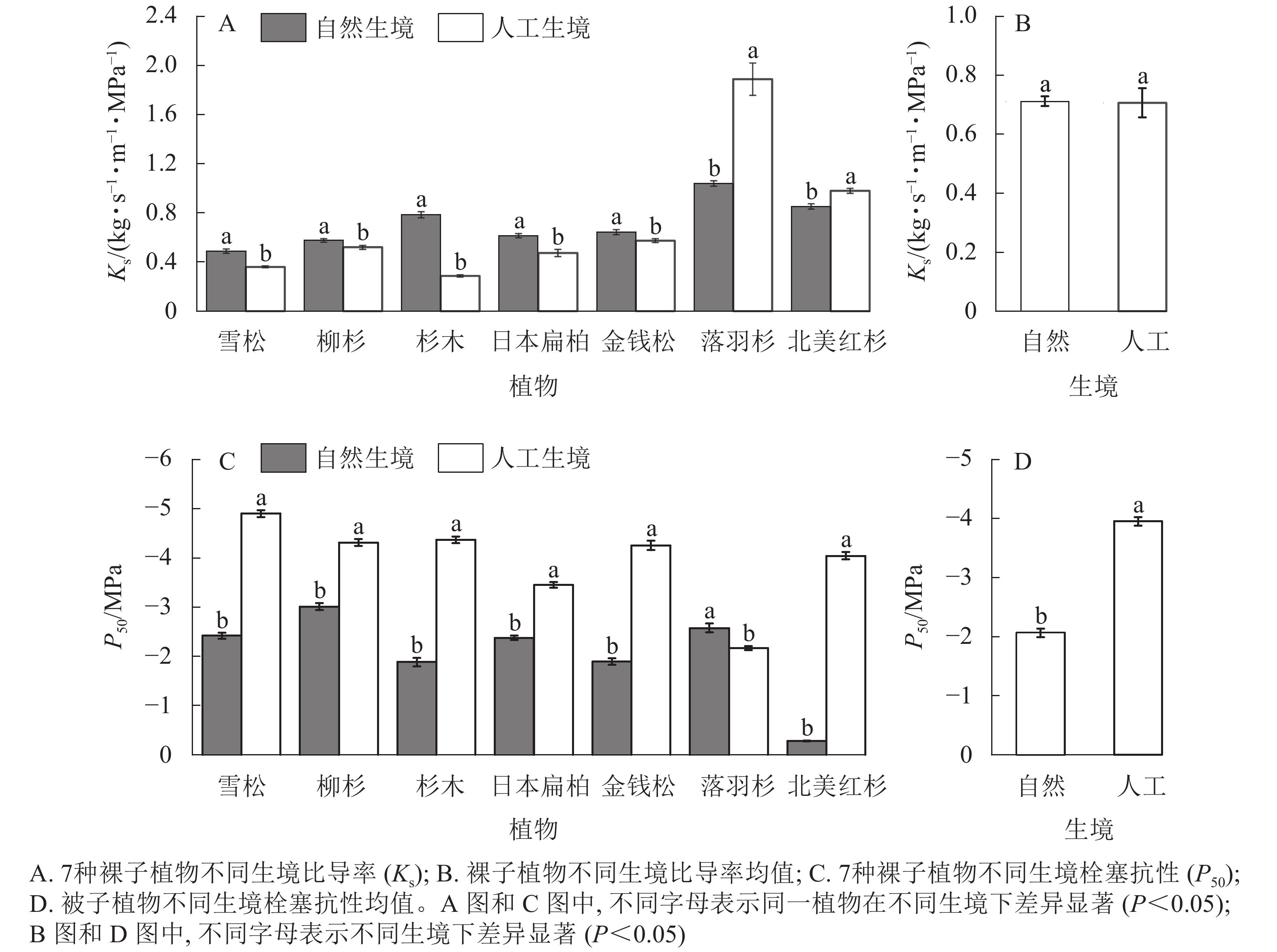
在植物输水效率方面,自然生境下6种被子植物的Ks显著大于人工生境(P<0.05,图1A),且被子植物Ks的均值显著大于人工生境(P<0.05,图1B)。在栓塞抗性方面,自然生境下7种被子植物的P50均显著小于人工生境(P<0.05,图1C),且被子植物P50的均值显著小于人工生境(P<0.05,图1D)。
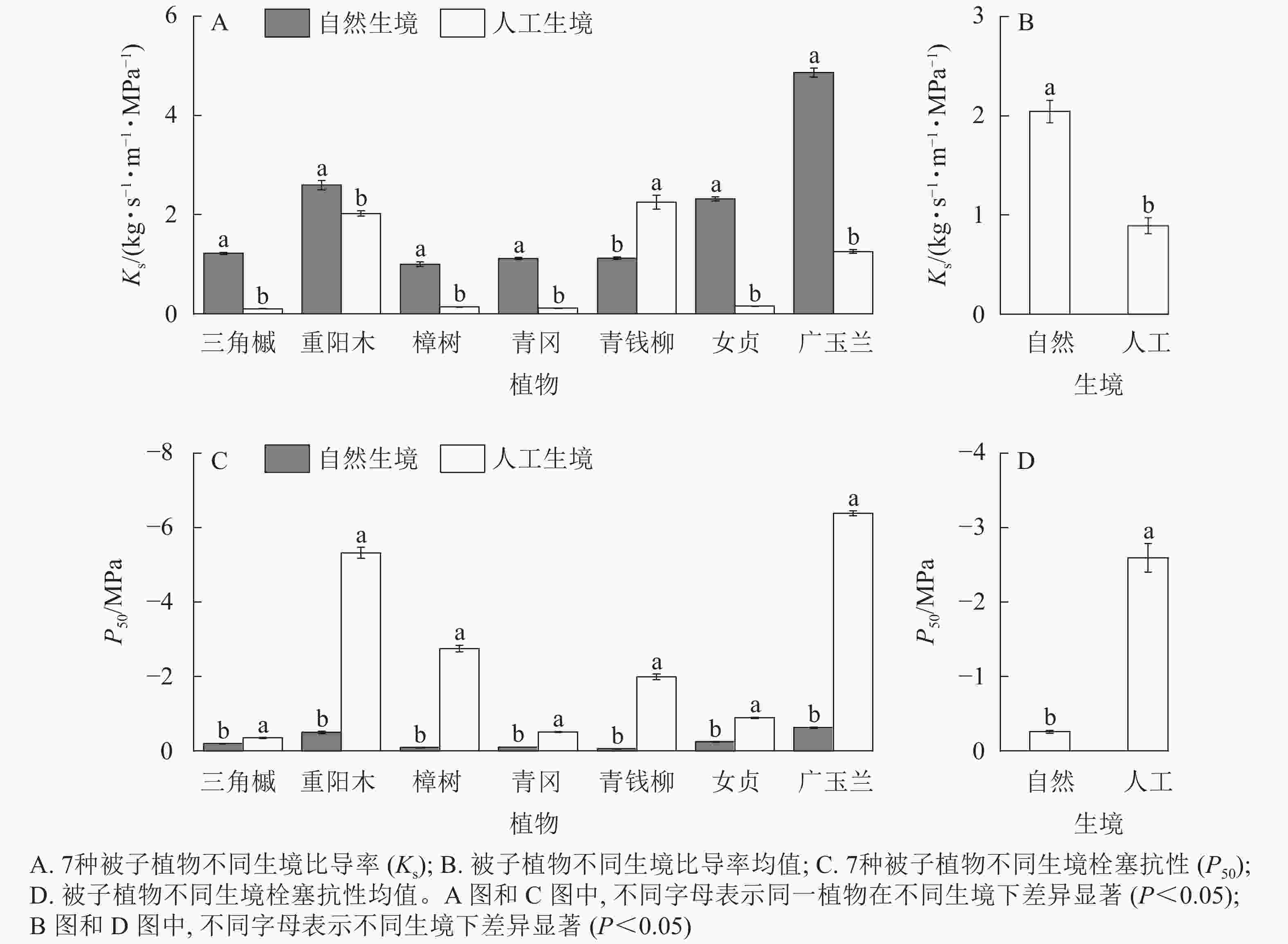
Figure 1. Hydraulic functional characteristics of 7 species of angiosperms in natural and artificial habitats
在植物输水效率方面,自然生境下5种裸子植物的Ks显著大于人工生境(P<0.05,图2A),但裸子植物Ks的均值在不同生境间差异不显著(P>0.05,图2B)。在栓塞抗性方面,自然生境下6种裸子植物的P50显著小于人工生境(P<0.05,图2C),且裸子植物P50的均值显著小于人工生境(P<0.05,图2D)。

Figure 2. Hydraulic functional characteristics of 7 species of gymnosperms in natural and artificial habitats
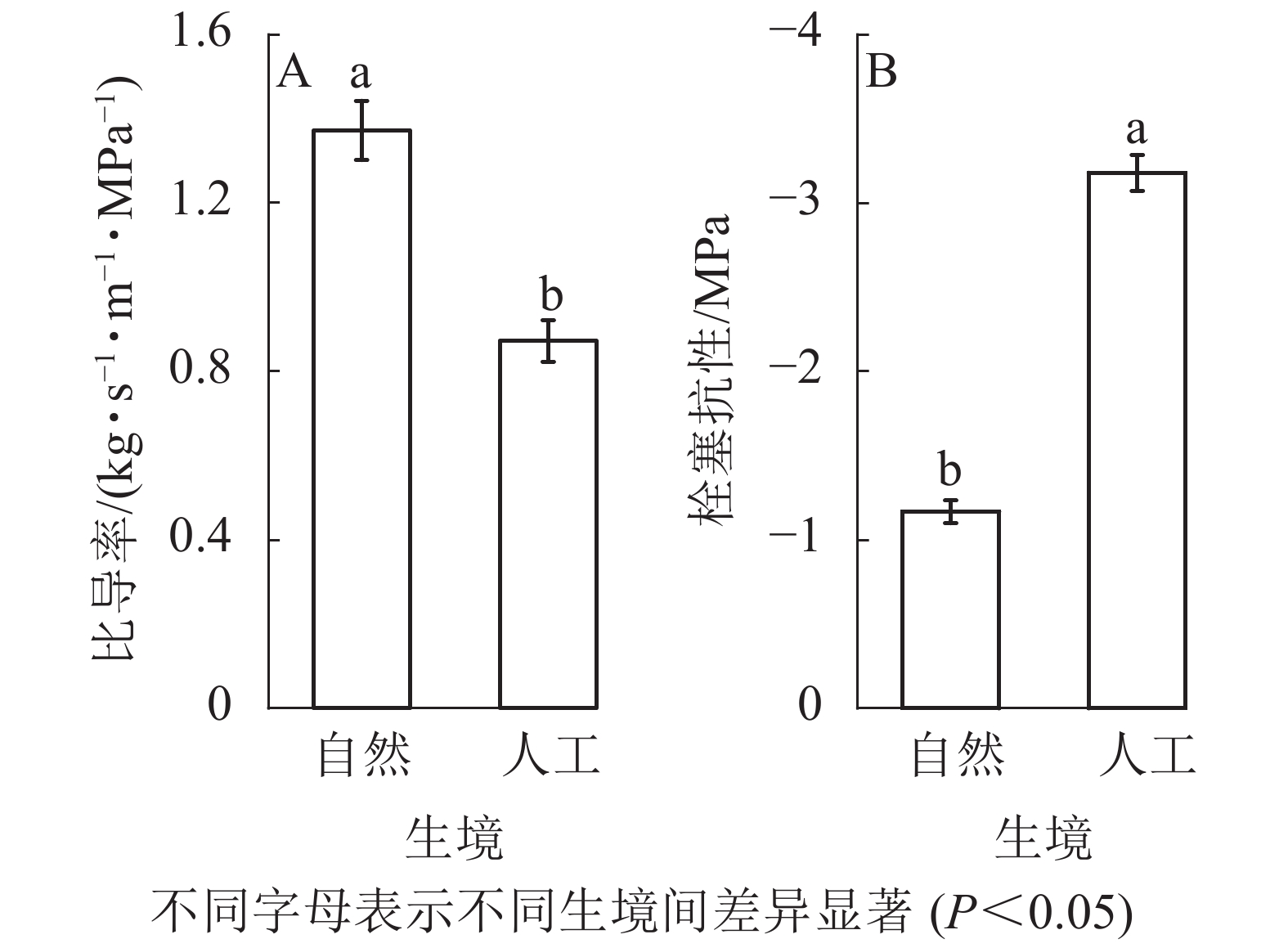
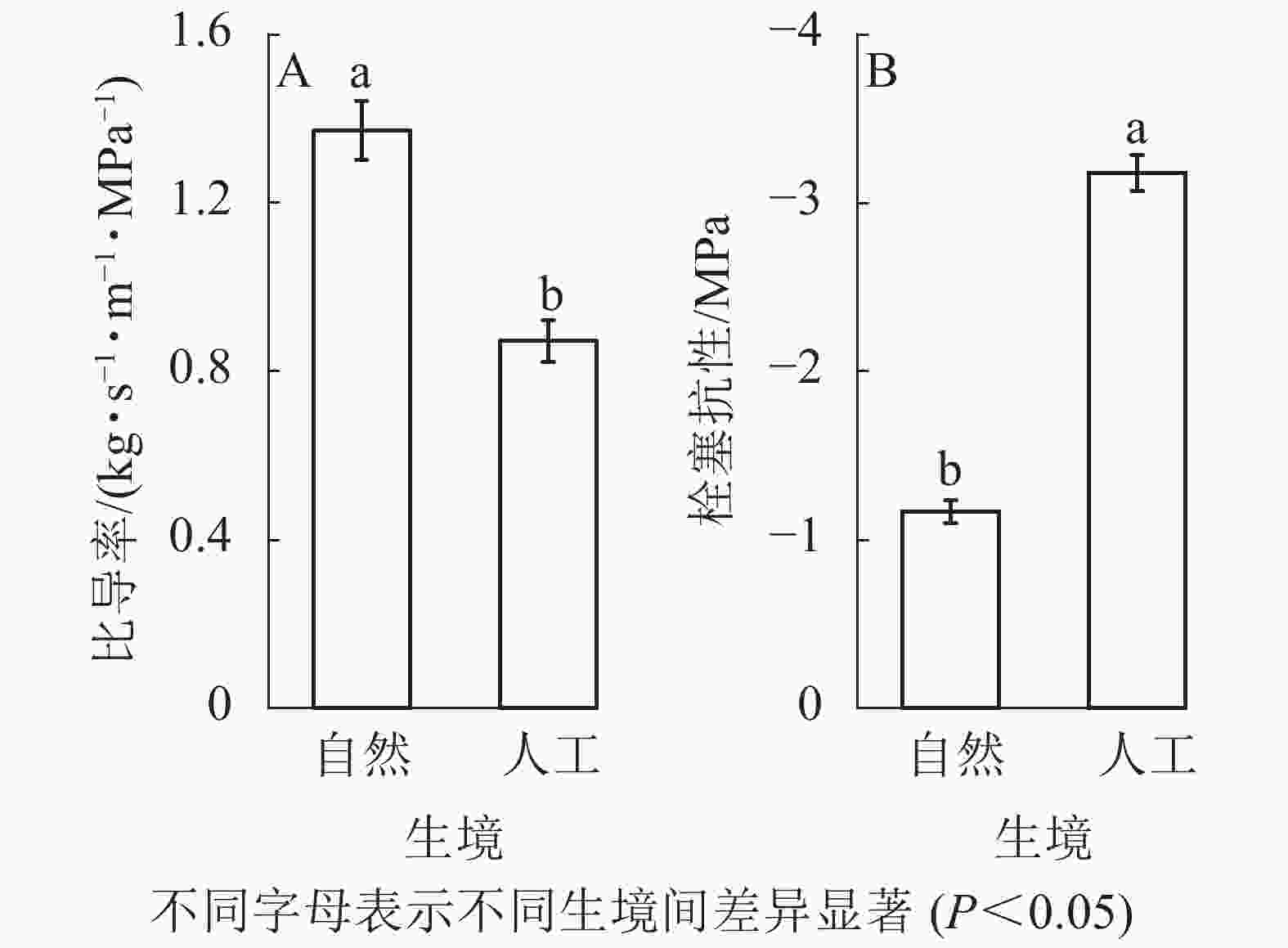
对14种被子植物和裸子植物的输水效率和栓塞抗性比较发现:自然生境植物的Ks均值显著大于人工生境(P<0.05,图3A),自然生境植物的P50显著小于人工生境(P<0.05,图3B)。
-
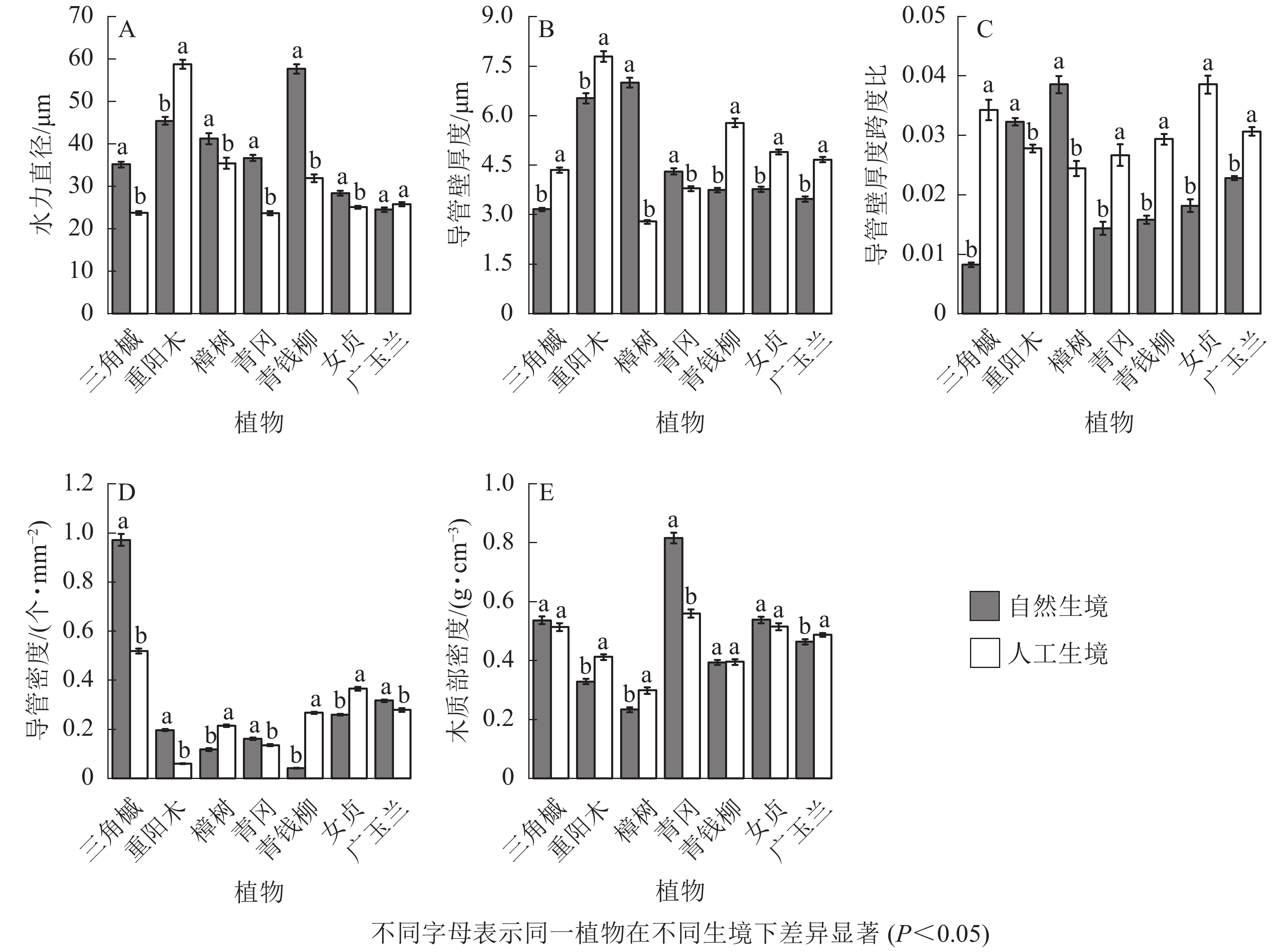
从被子植物的解剖结构可以看出:自然生境下5种被子植物的水力直径显著大于人工生境(P<0.05,图4A);5种被子植物的导管壁厚度显著小于人工生境(P<0.05,图4B);5种被子植物的厚度跨度比显著小于人工生境(P<0.05,图4C);樟树、青钱柳、女贞的导管密度显著小于人工生境,其余4种被子植物则相反(P<0.05,图4D);不同生境下,三角槭、青钱柳、女贞的木质部密度无显著差异,重阳木、樟树、广玉兰的木质部密度显著小于人工生境,青冈的木质部密度显著大于人工生境(P<0.05,图4E)。
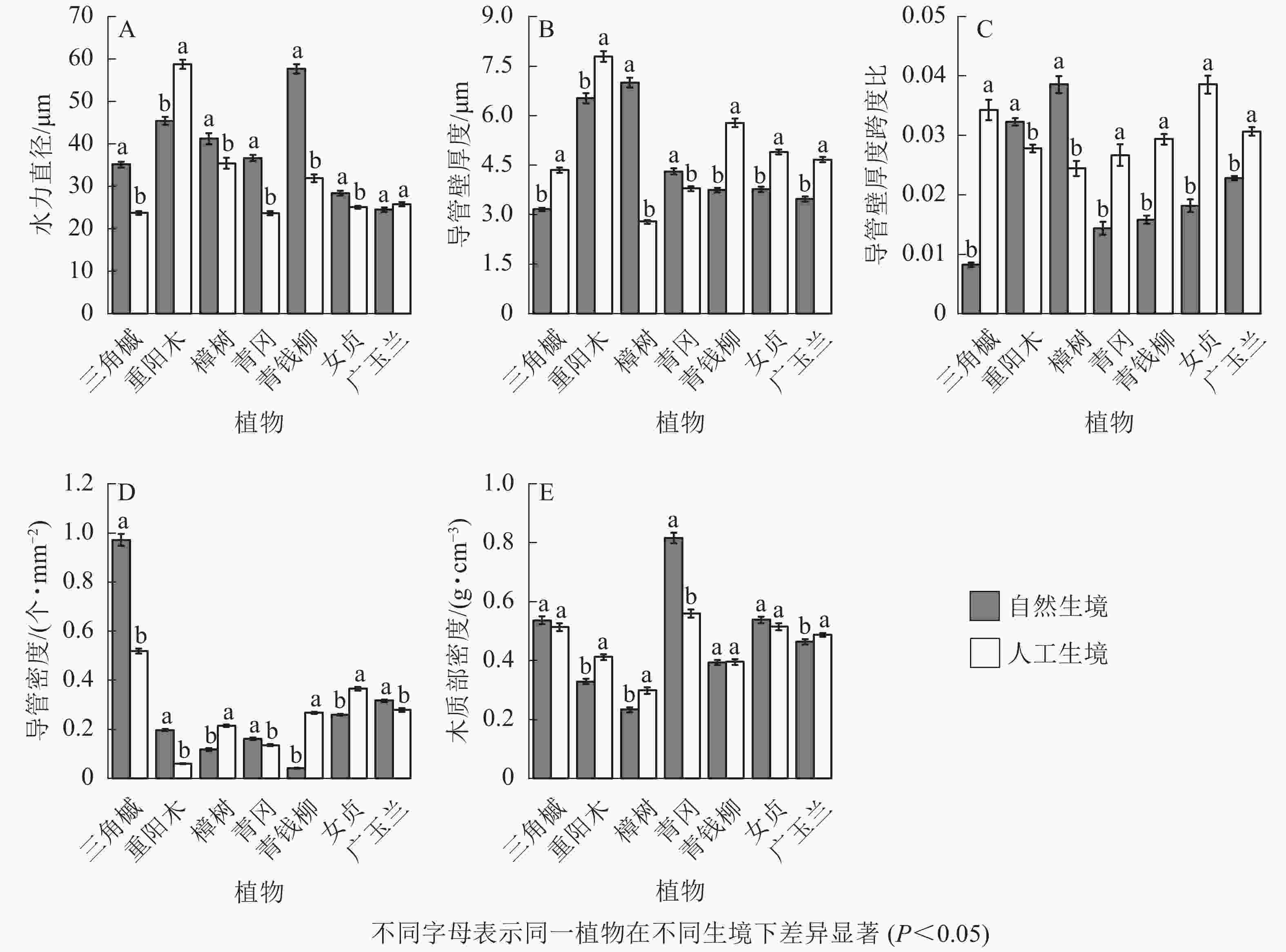
Figure 4. Characteristics of xylem anatomical structure of 7 species of angiosperms in natural and artificial habitats
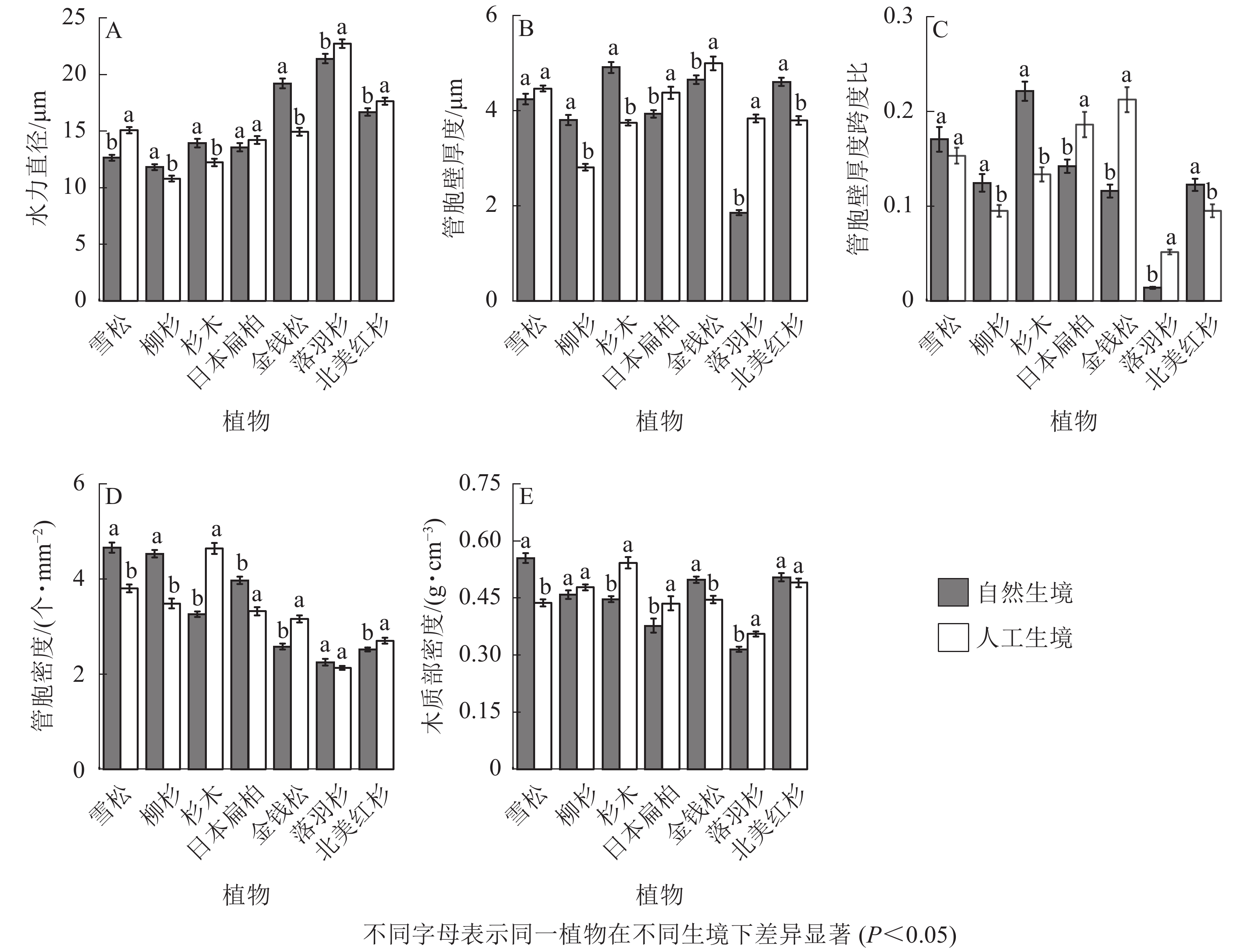
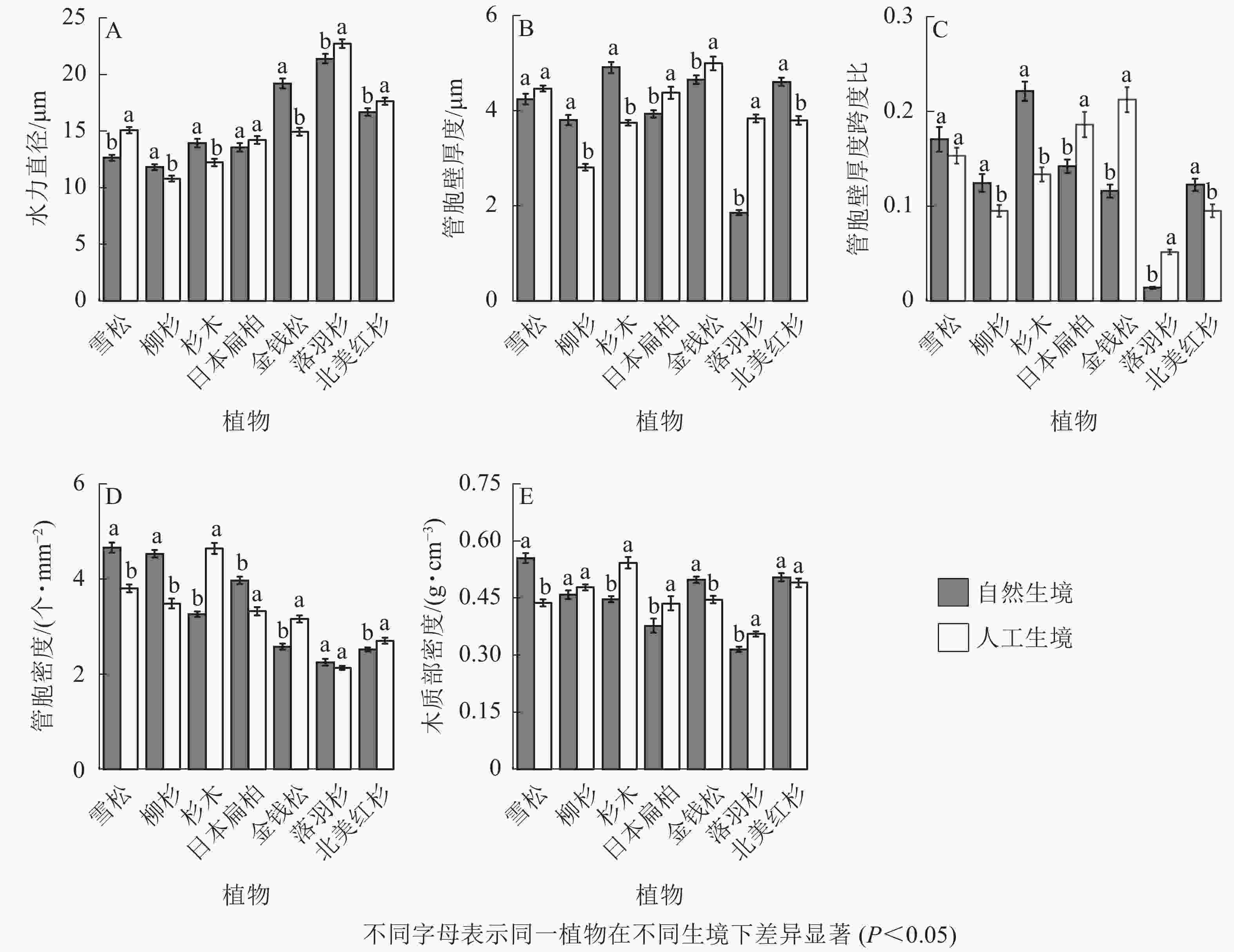
从裸子植物的解剖结构可以看出:自然生境雪松、落羽杉、北美红杉的水力直径显著小于人工生境,柳杉、杉木、金钱松的水力直径显著大于人工生境(P<0.05,图5A);柳杉、杉木、北美红杉的管胞壁厚度显著大于人工生境,日本扁柏、金钱松、落羽杉的管胞壁厚度显著小于人工生境(P<0.05,图5B);柳杉、杉木、北美红杉的厚度跨度比显著大于人工生境,日本扁柏、金钱松、落羽杉的厚度跨度比显著小于人工生境(P<0.05,图5C);雪松、柳杉、日本扁柏的导管密度显著大于人工生境,杉木、金钱柳、北美红杉的导管密度显著小于人工生境(P<0.05,图5D);雪松、金钱松的木质部密度均显著大于人工生境,杉木、日本扁柏、落羽杉的木质部密度均显著小于人工生境(P<0.05,图5E)。
-
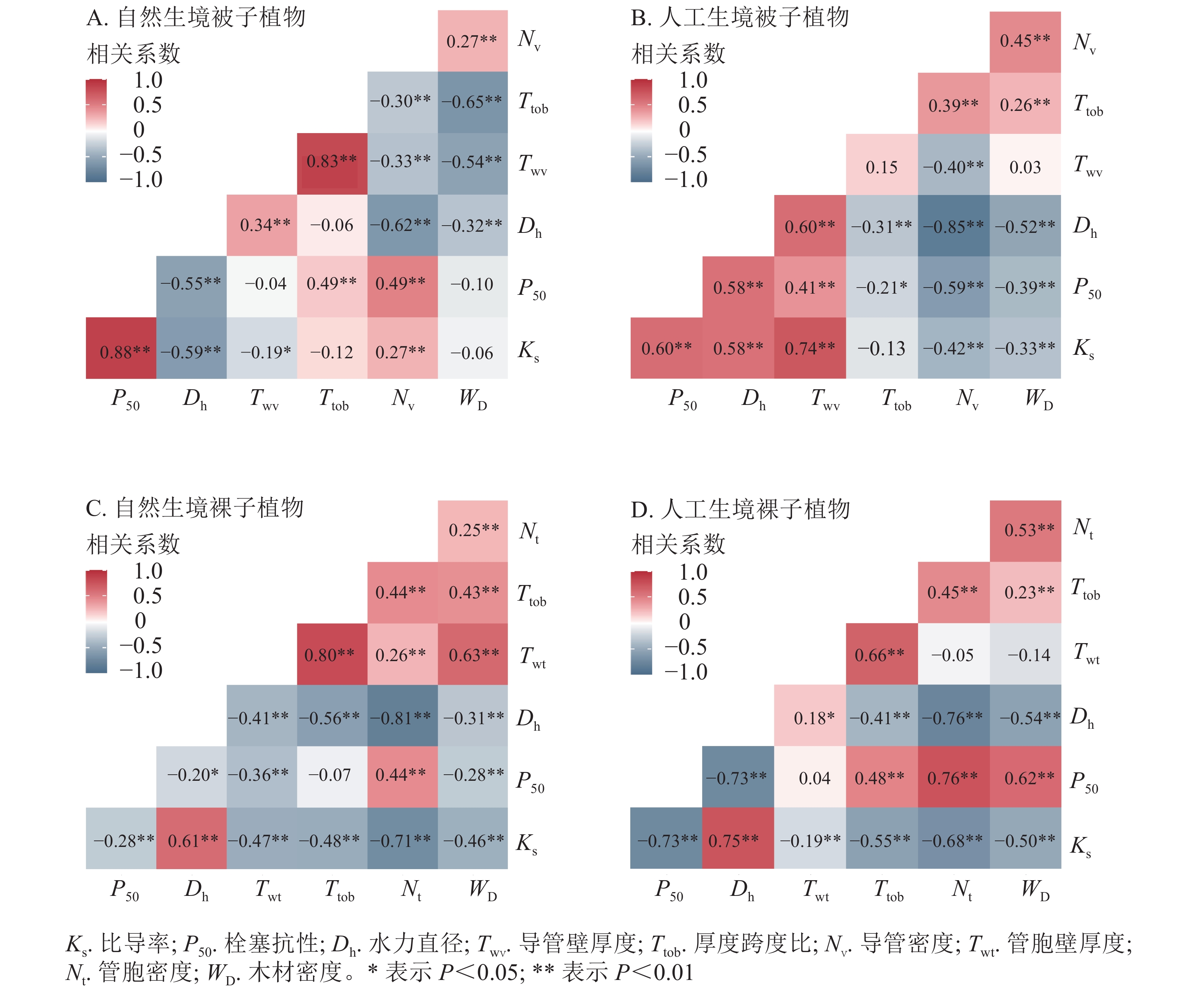
被子植物水力功能性状与木质部解剖结构性状相关性分析表明(图6A和图6B):自然生境下,导管密度、厚度跨度比与Ks和P50均呈正相关,其中导管密度与Ks、P50相关极显著(P<0.01)。水力直径、导管壁厚度、木质部密度与Ks和P50均呈负相关,其中水力直径与Ks、P50相关极显著(P<0.01);人工生境下,水力直径、导管壁厚度与Ks和P50均呈正相关关系,且相关极显著(P<0.01),厚度跨度比、导管密度、木质部密度与Ks及P50均呈负相关,除厚度跨度比外,其他指标间相关性均极显著(P<0.01)。此外,被子植物的水力直径与Ks和P50在自然生境均为负相关,在人工生境则均为正相关,导管密度与Ks和P50在自然生境均为正相关,在人工生境则均为负相关。
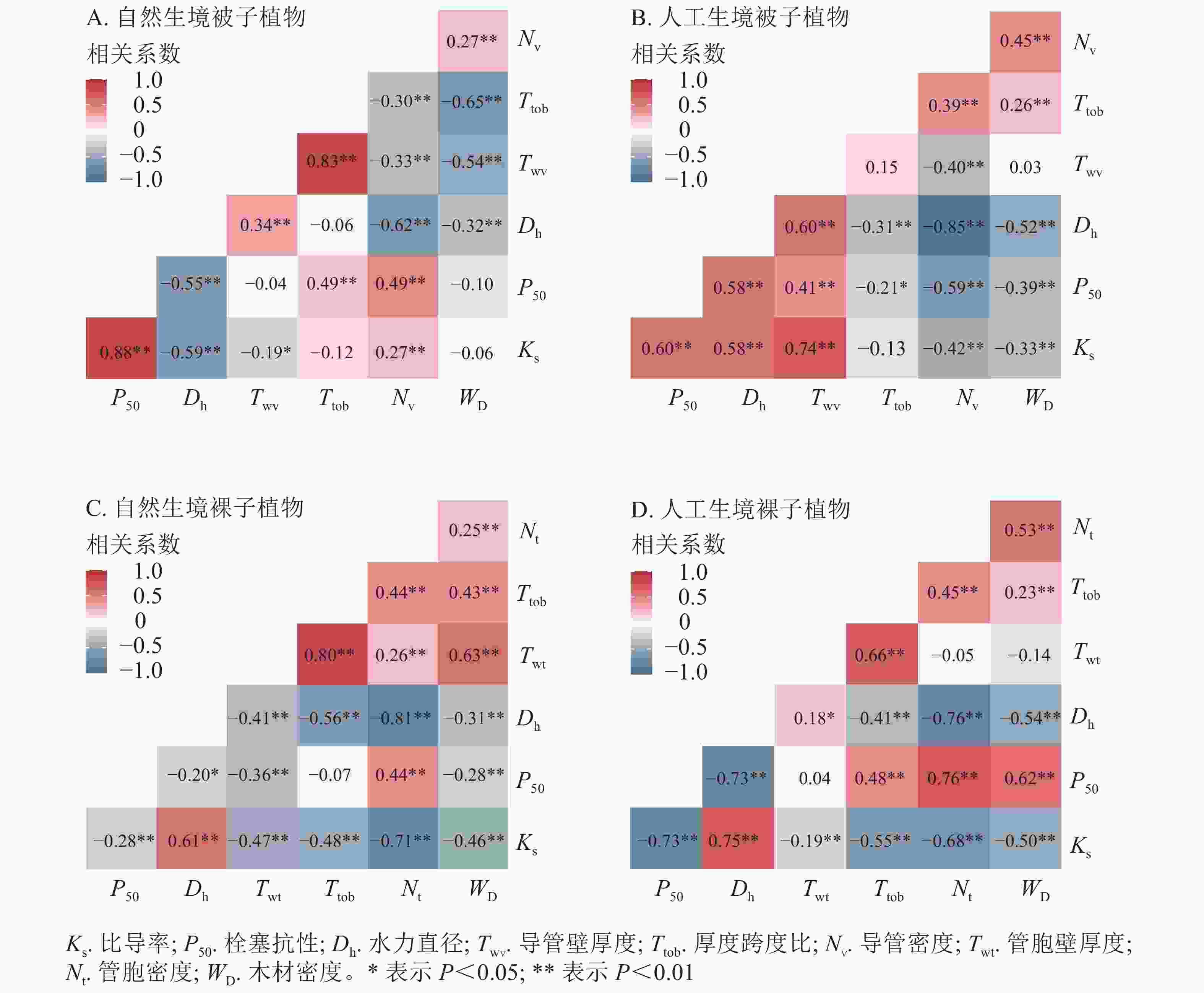
Figure 6. Correlation analysis between hydraulic function and anatomical structure traits of angiosperms and gymnosperms.
裸子植物水力功能性状与木质部解剖结构性状相关性分析表明(图6C和图6D):自然生境下,水力直径与Ks呈显著正相关(P<0.01),与P50呈显著负相关(P<0.01)。管胞密度与Ks呈显著负相关(P<0.01),与P50则呈显著正相关(P<0.01);人工生境下,水力直径与Ks正相关,与P50负相关,且与Ks、P50相关性均极显著(P<0.01),其余性状与Ks均呈负相关(P<0.05),与P50均正相关,其中厚度跨度比、导管密度、木质部密度与P50相关显著(P<0.05)。此外,裸子植物水力直径、管胞密度在不同生境下均与Ks、P50保持一致相关性,其中水力直径与Ks呈显著正相关(P<0.05),与P50呈显著负相关(P<0.05)。管胞密度与Ks呈极显著负相关(P<0.01),与P50呈极显著正相关(P<0.01)。
-
以往研究表明:相比于干燥环境,水分充足环境下的同一植物的Ks通常较高[2, 7]。本研究中,除被子植物青钱柳及裸子植物落羽杉、北美红杉外,自然生境中植物的Ks显著大于人工生境,表明自然生境的植物拥有更高的输水效率,这与上述研究结果一致。MAHERALI等[25]指出:相比于高山环境,西黄松Pinus ponderosa在沙漠环境的Ks较高;MAHERALI等[26]研究表明:落叶被子植物Ks随生境降水量的降低而增加,因此植物通过增加输水效率以适应相对干旱环境的生存策略可能较为普遍。Hagen-Poiseuill定律表明:更高的输水效率需要较大的导管直径以满足功能需求[15],然而本研究植物较大的水力直径并未与更高的输水效率一一对应,与上述定律存在一定的偏差[27]。
植物的栓塞抗性受环境控制的假设已被证实[20, 28]。通常认为,植物的栓塞抗性随栖息地干旱程度的加剧而增加[2, 8]。本研究中除裸子植物落羽杉外,植物的栓塞抗性在自然生境显著较低,表明人工生境植物的栓塞抗性更强,这与HAJEK等[29]研究结果一致。不同生境同一植物的栓塞抗性差异显著[2],这与先前报道植物的栓塞抗性可塑性较低不符[30-31],生境干旱水平对植物栓塞抗性的预测亦存在偏差[32],栓塞抗性可能与生境水分差异无关[33]。通常植物栓塞抗性的强弱与木质部机械强度息息相关,厚度跨度比可独立于导管直径而变化,因此相比于木质部密度,厚度跨度比对植物栓塞抗性的预测效果更好[14, 23]。本研究结果与上述观点一致,厚度跨度比能更好指示栓塞抗性在不同生境的强弱。
水分条件的差异促使植物采取不同的水力策略。本研究中,自然生境下的植物通过提高输水效率以满足蒸发需求的加剧[25-26],减轻对栓塞抗性的依赖[34]。表明栓塞抗性并非唯一的抗旱手段,植物亦可通过有效的性状组合应对干旱胁迫[33, 35],尽管这将迫使植物更接近其自身的功能极限[2]。落羽杉、北美红杉均为杉科植物,两者在应对持续水分胁迫后脱落酸(ABA)含量较低[36]。先前研究表明[37]:当植物处于轻度水分胁迫且脱落酸水平较低时,导水效率会相应提升,这可能意味着湿润区人工生境同样面临着干旱威胁。
-
人工生境被子植物的水力直径、导管密度与Ks、P50间存在显著相关性,这可能是纤维对木质部机械强度的驱动所造成[38],纤维与导管的功能差异可能导致输水效率与机械支持解隅[39],使植物在提升输水效率的同时最大化木质部安全投资。裸子植物木质部多达90%由管胞构成,管胞具有水力运输和机械支持的功能[12, 16],本研究中裸子植物的水力直径、管胞密度在不同生境下的相关性均与Ks、P50保持一致,可能与裸子植物的稳定组织结构有关。其中管胞水力直径与Ks的关系符合Hagen-Poiseuill定律[15],且更宽的管胞往往更长,管胞的输水效率随管胞直径和长度的增加而增加[40]。此外,不同生境下裸子植物的厚度跨度比与管胞水力直径呈显著负相关,与管胞壁厚度呈显著正相关,表明裸子植物是通过缩小管胞直径而非管胞壁厚度以实现机械强度的增加[41]。本研究中裸子植物的管胞密度均与不同生境Ks呈显著负相关,与P50呈显著正相关,这与上述观点一致。拥有较小管胞直径、较大管胞密度的裸子植物,其栓塞抗性可能更强[28]。具有较高机械强度的植物其栓塞抗性通常较强[14]。本研究中自然生境下植物的P50变异范围较小,这可能导致P50变异与解剖特征无关[31]。此外,被子植物木材结构属性在一定程度决定了P50的变异[42],针对木质部不同组织结构的投资亦会对P50的变异产生影响[14, 35],这些因素在一定程度上解释了人工生境下被子植物的厚度跨度比与P50的负相关关系。本研究中除自然生境被子植物外,不同物种、生境下的水力直径均与Ks呈显著正相关。尽管植物平均导管直径和导管密度存在显著差异,但导管横截面积差异可能较小[38],自然生境下被子植物导管腔面积分数的差异可能导致输水效率并未与水力直径相对应[43]。
-
湿润区裸子植物和被子植物在同一生境的水分利用策略相似,自然生境水分有效性较低,植物通过提高输水效率以避免水势的下降,从而降低潜在栓塞风险。裸子植物与被子植物木质部结构与功能的差异可能是同一生境下植物水分策略存在差异的主要原因,导致不同水分环境对植物的驱动差异。植物性状对植物水分策略具有一定指示作用,厚度跨度比在本研究中能较好预测植物栓塞抗性在不同生境的强弱,对植物性状更为深入的研究将有助于提升对植物群落分布的理解。
Relationship between hydraulic properties and xylem anatomical structure of subtropical plants
doi: 10.11833/j.issn.2095-0756.20210813
- Received Date: 2021-12-22
- Accepted Date: 2022-02-16
- Rev Recd Date: 2022-01-20
- Available Online: 2022-03-25
- Publish Date: 2022-03-25
Abstract:
| Citation: | SHANGGUAN Fangjing, ZHAO Mingshui, ZHANG Bona, et al. Relationship between hydraulic properties and xylem anatomical structure of subtropical plants[J]. Journal of Zhejiang A&F University, 2022, 39(2): 252-261. DOI: 10.11833/j.issn.2095-0756.20210813 |










 DownLoad:
DownLoad: