-
扦插繁殖是无性繁殖的重要技术手段之一,操作简单,应用广泛,后代很好地保持了亲本的优良遗传性状,但受母树年龄影响较大。对双色栎Quercus bicolor、使君子Quisqualis indiaca及水黄皮Pongamia pinnata 等树种的研究发现[1-3]:诱导插穗产生不定根是植物扦插成功的关键,随着母树年龄的增加,林木插穗生根能力出现下降,插穗生根率随母树年龄的增加逐渐降低[4-5]。解剖结构观察发现:不同年龄母树的插穗组织结构不同;植物开花度过幼年期后进入到成年期,是生理老化的开始,植物内部生根抑制物质增加,生根促进物质减少,再生作用降低、生活力下降[6]。一般来说,幼年期是无性繁殖采条的最佳时期。扦插生根过程中,吲哚乙酸氧化酶(IAAO)通过影响内源生长素(IAA)氧化及含量的变化来影响生根[7],过氧化物酶(POD)活性升高被认为是诱导生根启动的信号,多酚氧化酶(PPO)在根起始阶段与酚类的代谢有关[8]。栓皮栎Quercus variabilis 是壳斗科Fagaceae栎属Quercus 植物,是中国重要的珍贵用材树种,经济和生态价值极高[9-10]。近年来,学者利用扦插繁殖对栓皮栎嫩枝扦插进行了相关研究[11-12],但对不同母树年龄的栓皮栎扦插生根研究报道并不多。本研究以1年生、5年生及10年生栓皮栎母树的半木质化嫩枝为插穗材料,测定插穗的生根率、生根数量、根直径和根长等特征变化,观察不同时期插穗茎段的组织结构和酶活性变化,旨在揭示不同母树年龄栓皮栎不定根诱导形成的相关机制,为不同年龄栓皮栎的扦插繁殖提供参考。
-
栓皮栎扦插繁殖试验在中国林业科学研究院科研温室进行。该温室顶部设有天窗和遮阳网,室内有风扇和湿帘。扦插期间室温为25~31 ℃,相对空气湿度≥85%,晴天9:00—11:00的光合有效辐射为800 μmol·m−2·s−1,二氧化碳(CO2)摩尔分数约380 μmol·mol−1[13]。试验苗床为南北走向,规格21.0 m×1.5 m,试验时间为2019年7—9月。
-
2019年7月16日分别在中国林业科学研究荒漠化研究所1年生、5年生和10年生的栓皮栎母树上采集树冠中下部生长势良好、无病虫害的当年生嫩枝。将嫩枝修剪为8~10 cm的插穗,每个插穗顶端保留1~2个叶片,每个叶片保留1/3面积;每个插穗预留2~3个腋芽,插穗枝的顶端在距上端芽1 cm处平切,底端斜切。
-
珍珠岩和泥炭按体积比7∶3配制成基质,用质量分数0.1%高锰酸钾灭菌后装入轻基质无纺布育苗袋中,育苗袋高9.0 cm,上下内径均为3.8 cm。
-
参照ABT生根粉使用说明,以200 mg·L−1植物生长调节剂ABT-1号(北京艾比蒂生物科技有限公司)浸泡2 h处理插穗。①解剖结构观察试验。以1年生、5年生和10年生插穗为试验材料,各年龄母树的插穗设置3次重复,各重复45个插穗,共135个。②酶活性测定试验。各年龄母树的插穗设置3次重复,各重复150个插穗,共450个。③生根率统计试验。各年龄母树的插穗设置3次重复,各重复105个插穗,共315个。所有年龄处理共900个插穗,采用完全随机区组设计,在相对一致的环境下,进行统一浸泡处理。
-
扦插前用小棒在基质内引孔,深度为2~3 cm。每个容器扦插1根插穗。扦插后用小棒将插穗周边的基质稍加压实,浇透水,将容器摆放在塑料托盘中。扦插完成后在插床搭建高约45 cm白色塑料薄膜拱棚,扦插基质湿度保持为55%~65%,拱棚内相对空气湿度保持为85%以上,天气晴朗时插穗上方30 cm处覆盖50%遮阳网。
-
扦插60 d后,统计各处理的生根株数和每个插穗的生根数量,测量各插穗不定根的直径与长度。计算扦插生根率(%)。从各处理已生根插穗中随机抽取5个插穗,计算平均不定根数量、不定根长及不定根直径。
-
栓皮栎生根过程中,扦插前进行第1次取样,每个处理随机取3根插穗,重复取样3次,取其基部2~3 cm茎段部分。10 d后再次取样,之后隔7 d取样1次,直到9月1日结束取样工作。所有样品均保存在FAA溶液(V甲醛∶V冰醋酸∶V70%酒精=1∶1∶18) 里,抽真空。常规石蜡切片,切片厚度为10~12 μm,甲苯胺蓝染色,在光学显微镜(Olympus BX51)下观察插穗基部组织结构并拍照。
-
栓皮栎生根过程中,扦插前第1次取样,取其基部3~4 cm范围内的韧皮部,每次取样10根,设置3次重复,用锡箔纸包好后放入液氮中,再转移至−80℃冰箱进行保存;10 d后再次取样,之后7 d取样1次,直至9月1日结束。参考李墨野[14]和刘俊芳[15]方法测定样品PPO和POD活性,参考王小虎[16]测量IAAO活性。
-
采用单因素方差分析和Duncan法进行多重比较,采用SPSS 19.0软件进行数据统计处理。
-
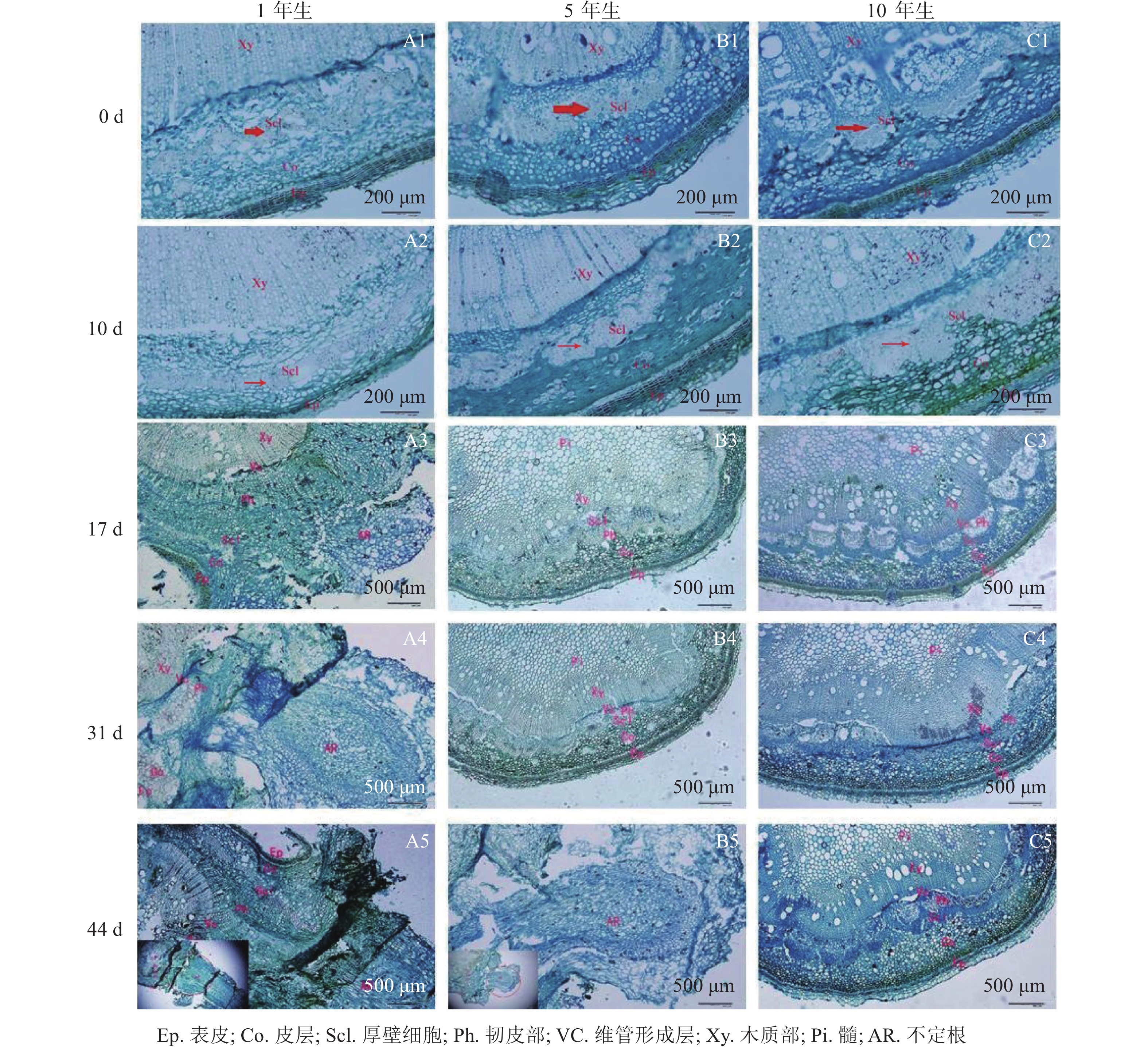
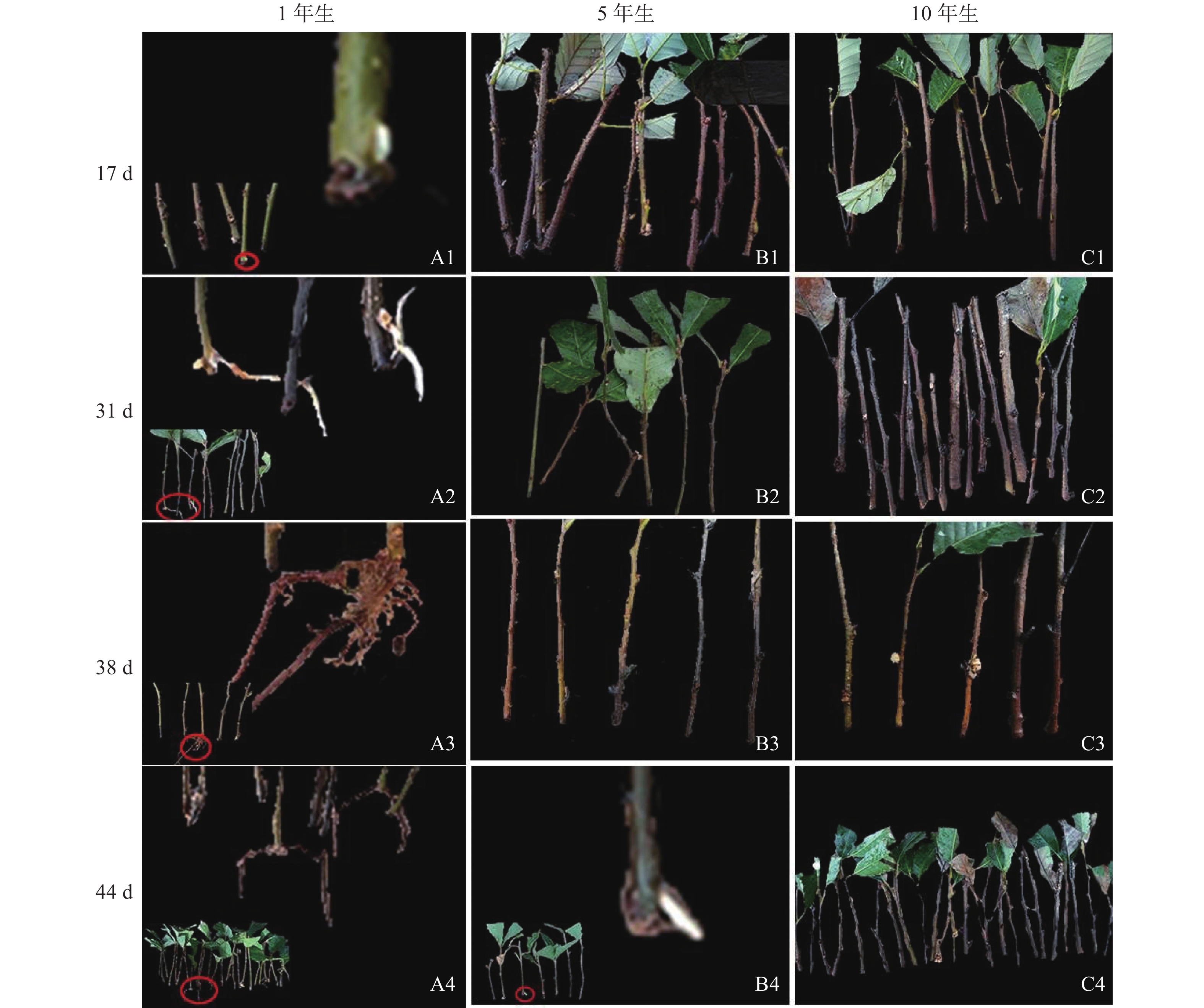
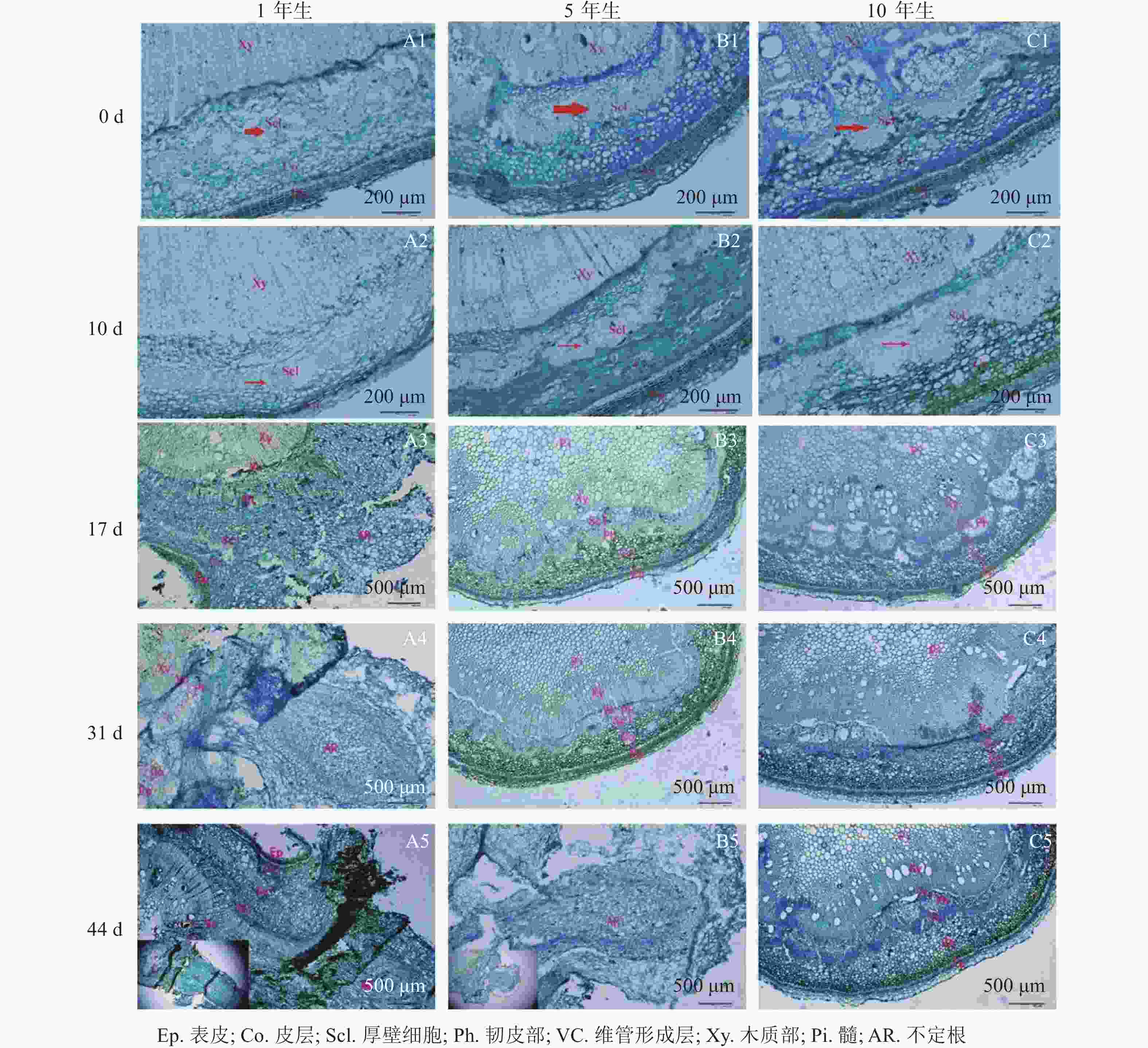
由图1可知:栓皮栎生根部位为插穗基部,不定根从皮层长出。1年生栓皮栎嫩枝插穗在扦插17 d后发现白色不定根突出(图1A1),31 d不定根形成(图1A2),38 d不定根由白色发育为褐色,并出现根伸长的现象(图1A3),44 d不定根数目增多(图1A4)。5年生栓皮栎嫩枝插穗在扦插44 d才发现白色的不定根形成(图1B4),此前的插穗叶子长势良好,插穗顶部有愈伤组织形成。10年生栓皮栎插穗在整个实验期间未见不定根形成,且叶子逐渐干枯,部分插穗变黑,直至死亡(图1C4)。
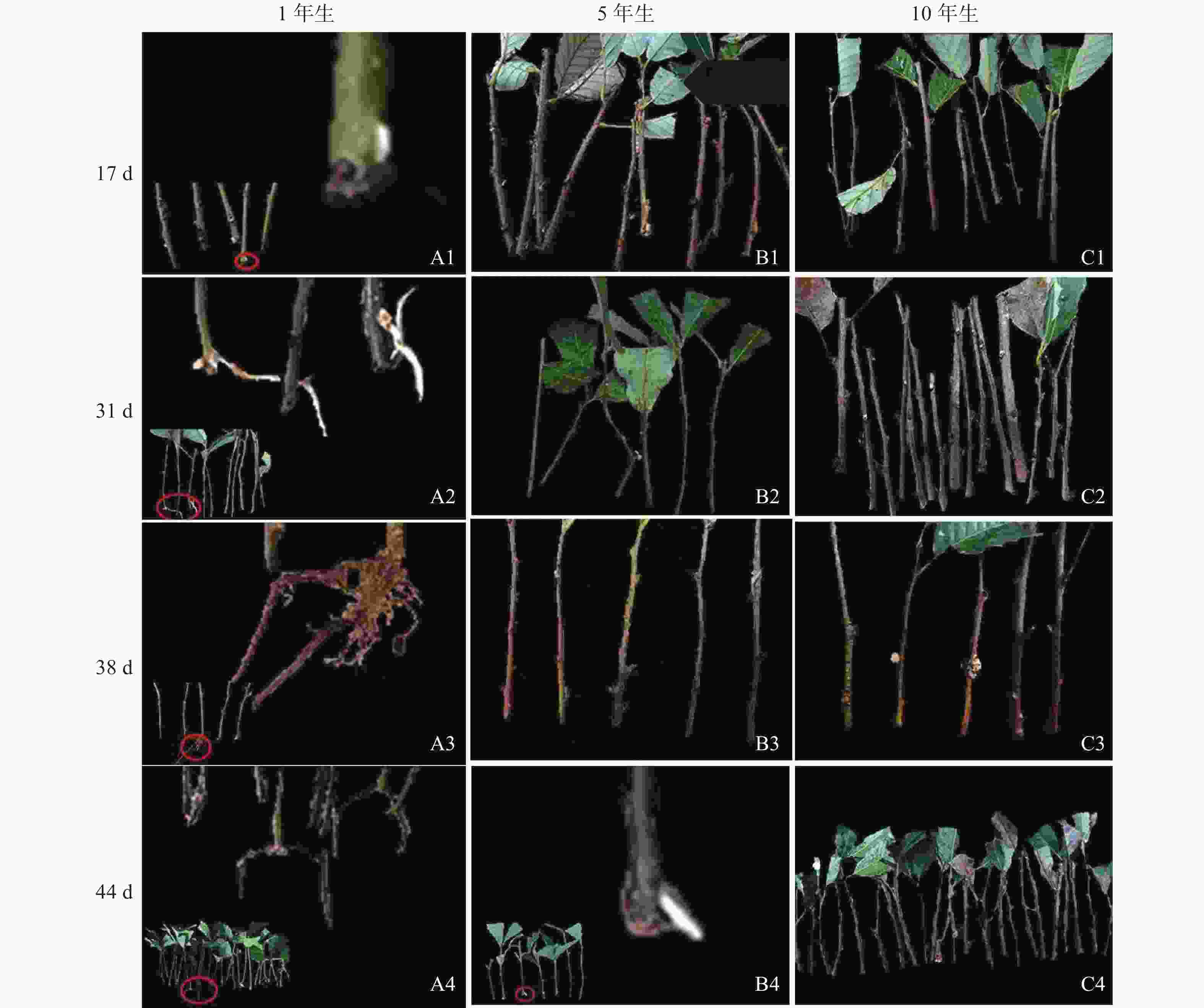
Figure 1. External morphological of mother cuttings of Q. variabilis at different ages during rooting
从表1可以看出:栓皮栎嫩枝扦插生根率及其根系特征存在明显的年龄效应。栓皮栎嫩枝扦插生根率和平均不定根数量从多到少依次均为1年生、5年生、10年生,各年龄插穗差异显著(P<0.05),平均不定根长和根直径从大到小依次均为5年生、1年生、10年生,各年龄插穗差异显著(P<0.05)。
年龄 生根率/% 平均不定根数量/条 平均不定根长/mm 平均不定根直径/mm 1年生 92.69±0.02 b 3.6±2.15 b 40.22±10.09 b 1.19±0.25 b 5年生 10.79±0.10 a 2.6±0.49 b 52.82±8.35 c 1.42±0.21 b 10年生 0 a 0 a 0 a 0 a 说明:数值为平均值±标准差,同列不同字母表示差异显著(P<0.05) Table 1. Rooting of cuttings of Q. variabilis mother tree at different ages
-
解剖学观察(图2)可知:插穗基部由外向内依次为表皮、皮层、厚壁组织、韧皮部、形成层及木质部等。扦插0 d时3个年龄的栓皮栎厚壁组织宽度不一,表现为5年生和10年生较大,1年生较小(图2A1、图2B1和图2 C1)。1年生栓皮栎嫩枝扦插生根约需44 d,经历诱导期—表达期—形成期—伸长期等阶段;5年生栓皮栎嫩枝扦插44 d后插穗生根才进入形成期,而10年生栓皮栎嫩枝则一直处于诱导期,未发生生根迹象。1年生和5年生栓皮栎嫩枝最终都形成了不定根,其根原基是在不定根形成后期产生,由此判断栓皮栎根原基为诱生型。1年生栓皮栎嫩枝扦插10 d时,插穗没有发现根原基的存在,处于不定根诱导期(图2A2);扦插17 d后靠近表皮位置出现不定根,此时为不定根表达期(图2A3);扦插31 d后不定根明显形成,此时为不定根形成期。不定根主要由靠近形成层附近的薄壁细胞分化而来,依次向外突破韧皮部、厚壁组织、皮层及表皮(图2A4)。扦插44 d后,不定根向外延伸,处于伸长阶段(图2A5)。5年生栓皮栎嫩枝扦插44 d后不定根出现,其由维管形成层附近的薄壁细胞分化而来,向外伸展,处于不定根的形成期(图2B5)。10年生栓皮栎嫩枝在整个生根过程中插穗基部的组织没有任何明显变化。
-
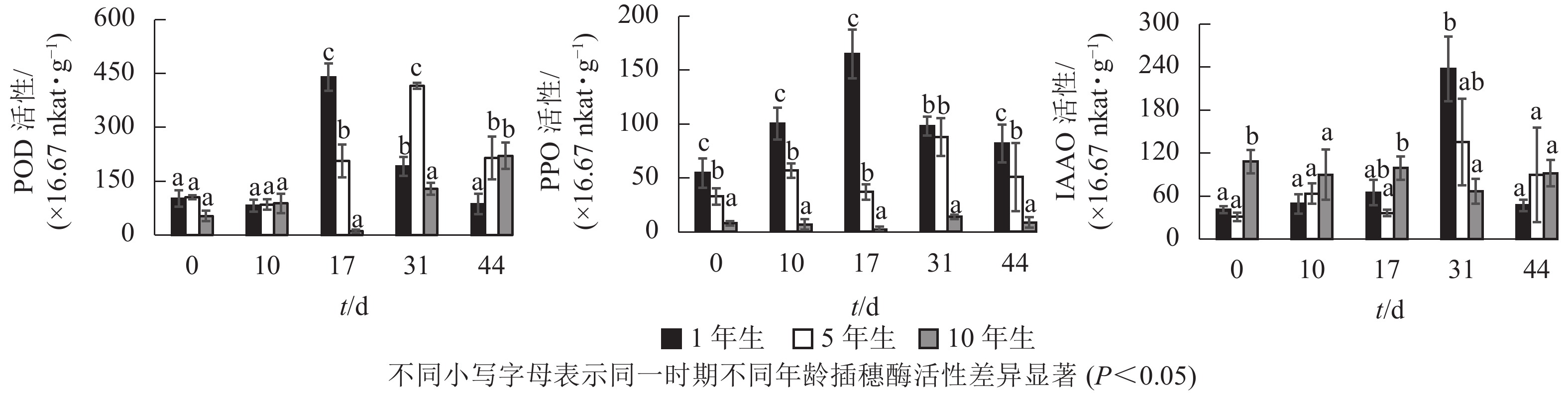
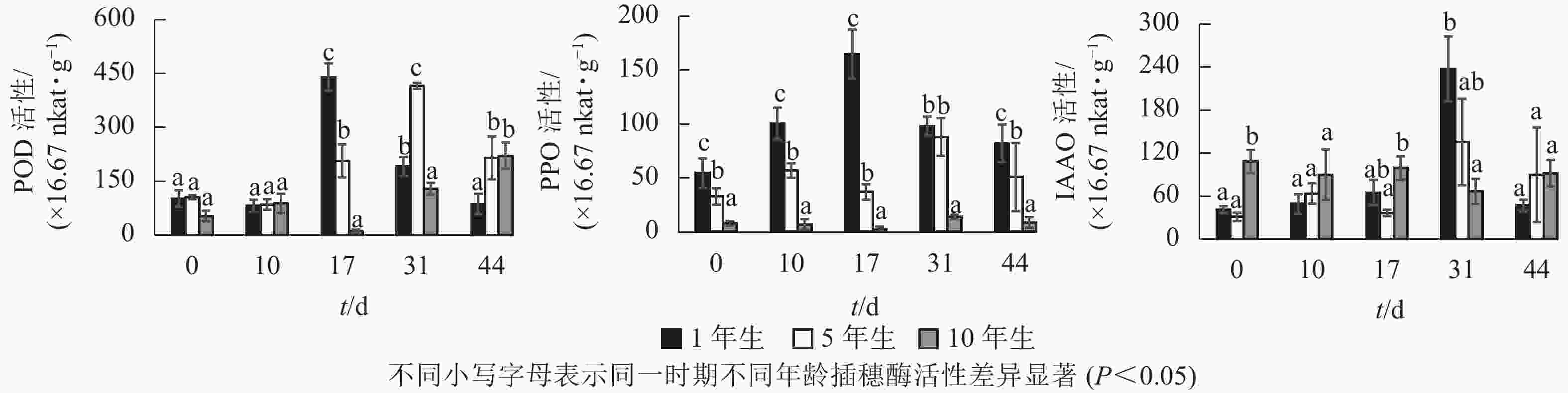
不同年龄栓皮栎嫩枝生根过程中的POD、PPO及IAAO活性变化规律存在差异。由图3可知:不同年龄插穗POD活性水平1年生和5年生要高于10年生;PPO活性水平从大到小依次为1年生、5年生、10年生;扦插17 d之前IAAO活性水平10年生大于5年生和1年生,17 d后从大到小依次为1年生、5年生、10年生。1年生和5年生插穗POD活性整体呈先上升后下降的单峰趋势,1年生在扦插17 d时达到峰值;5年生在扦插31 d时达到峰值,此时插穗都处于不定根的表达期(图2 A3、图2B4)。10年生插穗POD活性呈先下降后上升的趋势,在扦插17 d时达最低值。
1年生插穗的PPO活性呈先上升后下降的单峰趋势,在扦插17 d时出现峰值,此时PPO活性显著高于5年生和10年生插穗;5年生插穗的PPO活性则出现了双峰趋势,最大值出现在扦插31 d时,此时PPO活性与1年生插穗差异不显著,却显著高于10年生插穗;10年生插穗的PPO活性整体变化趋势不明显。
1年生和5年生插穗的IAAO活性整体上呈先上升后下降的单峰趋势,在扦插31 d时出现峰值,1年生IAAO活性显著高于5年生和10年生。此时1年生栓皮栎进入不定根形成期(图2 A4),5年生进入不定根表达期(图2 B4)。10年生插穗IAAO活性整体变化呈现先下降后上升趋势。
-
本研究发现:1年生和5年生栓皮栎扦插均产生了不定根,10年生无不定根;插穗生根率从高到低依次为1年生、5年生、10年生,且差异显著。可见植物插穗的母树年龄越小,不定根形成时间越短,生根率也越高。原因可能是母本年龄越小,代谢更加活跃,酶活性含量会增加,POD活性增加有利于增加细胞壁的强度[17],能更好地抵御病原体入侵,有助于插穗生根,这与十月樱Prunus subhirtella ‘Autumnalis’和夏栎Quercus robur的研究类似[4, 18]。母树年龄越大,插穗的组织分化程度会更高,进行再分化的能力下降[19];而随着年龄增加,韧皮纤维厚度会加大,韧皮纤维细胞壁厚,会增加生根的机械阻力[20],这些都会影响不定根的生成。
-
对插穗组织解剖结构的观察发现:栓皮栎的不定根根原基是在生根过程中形成的,由此判定栓皮栎根原基类型为诱生型。通常来说,诱生型根原基的出现需要一定时间,导致植物插穗难以生根[20],这可能是栓皮栎插穗生根难的原因之一。栓皮栎插穗的皮层和韧皮部间存在连续的环状厚壁组织,观察发现5年生和10年生厚壁组织的宽度大于1年生,这可能也是造成栓皮栎5年生和10年生生根率低的原因。在沙棘Hippophae rhamnoide[21]及冬青llex×altaclerensis ‘Belhica Aurea’[22]扦插繁殖过程中也发现厚壁组织的存在和生根率不高存在一定相关性。
-
POD、PPO和IAAO的活性与不定根形成关系密切[7]。扦插前POD活性升高,可以保护插穗免受活性氧自由基的侵害[12]。高活性POD有利于细胞分化产生不定根[23],1年生和5年生插穗在不定根表达期POD活性出现峰值,使得插穗内的IAA含量降低,从而有助于不定根的形成[24]。随后POD活性开始下降,至不定根伸长期后又开始升高。10年生插穗则出现先下降后上升的趋势,其POD活性在扦插下降,预示其对插穗保护效果下降,自由基及活性氧对插穗细胞侵害加大,不利于不定根的形成。
PPO可以催化酚类物质,在根原基诱导和发育过程中起至关重要作用[25]。本研究中1年生栓皮栎生根过程中PPO活性呈单峰趋势,在扦插17 d(不定根表达期)出现峰值,此时高活性的PPO有助于催化酚类物质与IAA形成生根辅助因子“IAA-酚酸复合物”,以此促进生根[26-27]。5年生栓皮栎生根过程PPO活性水平呈双峰趋势,扦插31 d(形成期),峰值明显大于扦插第10 d(诱导期)。诱导期(10 d)时插穗的切口暴露在外,高水平PPO活性可降低其被细菌侵害的可能[28];至不定根形成期(31 d),插穗需要更高的PPO活性来形成生根辅助因子促进生根。10年生的PPO活性整体趋势变化不明显,这可能是其难生根的原因。
IAAO通过调节内源IAA的水平来影响不定根的形成。1年生和5年生IAAO活性均呈现单峰趋势,且1年生的IAAO活性显著高于5年生。IAAO活性升高有助于消除插穗体内多余的IAA[29],利于生根诱导[30]。10年生插穗IAAO的酶活性整体上趋势变化不大,并且在31 d出现下降的趋势,使得IAAO活性降低,内源IAA水平过高,导致生根率低。
总体上,1年生和5年生插穗在不定根表达期不定根开始形成,这时的POD和PPO活性最高;1年生IAAO活性低,5年生IAAO活性偏高,导致插穗体内IAA被大量降解,最终影响生根率。同时,ABT属于外源激素,对内源激素也会产生影响,所以IAAO活性也会受到一定影响。
-
母树年龄影响插穗不定根的生成,栓皮栎嫩枝扦插生根率和平均不定根数量从高到低依次为1年生、5年生、10年生,平均生根率1年生为92.69%,5年生为10.79%,10年生为0。栓皮栎的生根类型为皮部生根型,其根原基属于诱生型根原基,不定根主要由靠近形成层附近的薄壁细胞分化而来,最终突破韧皮部、厚壁组织、皮层及表皮向外伸长,但皮层与韧皮部中间存在的连续环状厚壁组织对不定根的形成有一定的阻碍作用。POD活性增加有利于增加细胞壁强度,使植物能更好地抵御病菌,有助于插穗生根;PPO活性增加能促进生根辅助因子“IAA-酚酸复合物”的形成,IAAO活性增加有助于减少体内IAA含量,较低质量浓度的IAA有助于促进生根;栓皮栎插穗1年生和5年生POD、PPO及IAAO活性整体呈现“先上升后下降”趋势,POD和PPO活性升高有利于不定根的表达,IAAO活性升高有助于不定根的生成。
Rooting, anatomical analysis and changes of enzyme activity of softwood cuttings of Quercus variabilis at different ages
doi: 10.11833/j.issn.2095-0756.20220143
- Received Date: 2022-01-25
- Accepted Date: 2022-07-11
- Rev Recd Date: 2022-05-30
- Available Online: 2023-01-18
- Publish Date: 2023-01-17
-
Key words:
- Quercus variabilis /
- cutting /
- age effect /
- rooting rate /
- anatomical structure /
- enzyme activity
Abstract:
| Citation: | QIAN Jialian, LI Yingchao, XU Huihui, et al. Rooting, anatomical analysis and changes of enzyme activity of softwood cuttings of Quercus variabilis at different ages[J]. Journal of Zhejiang A&F University, 2023, 40(1): 107-114. DOI: 10.11833/j.issn.2095-0756.20220143 |









 DownLoad:
DownLoad: