-
中国是世界三大氮沉降区之一,自20世纪80年代以来陆地生态系统氮沉降增加了60%[1]。中国中东部森林生态系统大气氮沉降已超过20 kg·hm−2·a−1,并呈现逐年加重的趋势[2]。长期的氮输入使许多陆地生态系统氮含量增加,引发土壤矿化速率增加、土壤酸化和生物多样性降低等问题[3−4]。丛枝菌根(arbuscular mycorrhizal, AM)真菌能与绝大多数陆地植物的根系形成互惠共生体,既从宿主植物获得自身生长所需的碳源,也帮助宿主植物吸收营养物质、提高宿主植物对胁迫条件的耐性和减轻病虫害等,其农学和生态学意义都十分重大[5−6]。然而,氮沉降增加会导致土壤氮含量及酸碱度发生改变,也会影响AM真菌群落及其与宿主植物的相互关系,进而影响植物群落组成、土壤微生物类群及相关土壤生态过程[7]。近年来开展了许多模拟氮沉降对AM真菌群落影响的试验,发现氮添加可能增加[8]、降低[9−10]或不影响[11]AM真菌的群落组成和多样性,这可能与土壤养分含量、宿主植物类型、氮添加量和持续时间以及测定方法差异等有关[12]。同时,不同季节土壤温湿度的变化,以及寄主植物能为AM真菌提供碳源的差异也会对AM真菌群落产生影响[13]。
杨树Populus spp.是一类能与AM真菌共生的树种,这在一定程度上促进了其在各种胁迫环境中的生长[14]。杨树人工林在中国氮沉降最严重的地区,即苏北沿海地区,广泛分布,在木材生产和生态环境保护方面发挥着巨大的作用[15]。氮添加会影响杨树人工林土壤有机碳、土壤动物和微生物群落结构等[16−18],但氮添加对杨树根际土壤AM真菌群落的影响及其季节性差异目前尚不明确。鉴于此,本研究以江苏省盐城市东台林场杨树人工林为研究对象,在连续施氮6 a后,于春、夏、秋、冬4个季节分别采集根际土壤,通过高通量测序解析AM真菌群落结构和多样性的动态变化特征;同时监测土壤理化性质,分析AM真菌群落与环境因子之间的关系,以期为认识氮沉降背景下杨树人工林地下生态过程和生态系统功能提供数据支撑。
-
研究区位于江苏省盐城市东台林场(32°33′~32°57′N,120°07′~120°53′E),该区地处长江中下游冲积平原,属北亚热带季风气候。年均气温为14.6 ℃、相对湿度为88.3%,无霜期为220.0 d,年日照时数为2 169.6 h,年太阳辐射总量为493.5 J·cm−2,年均降水量为1 059.8 mm。林场内杨树人工林占地面积达2 000 hm2,土壤为砂质壤土,弱碱性。
-
杨树人工林氮沉降样地设立于2012年5月,采用人工施氮的方式模拟大气氮沉降。选择2003年营造的黑杨派无性系I35 Populus deltoides CL35人工林,设置3块25 m×190 m的样地。采用随机区组设计,每块样地设置5个25 m×30 m样方,样方间设置10 m的缓冲带。参照该地区大气氮沉降水平(6.3~12.6 kg·hm−2·a−1[19])设置5种氮添加量,分别为0、5、10、15和30 g·m−2·a−1,依次标记为N0、N1、N2、N3和N4。每年5—10月进行人工施氮,将年施氮量平均分配到上述6个月,每次将所需的硝酸铵(NH4NO3)溶解在20 L水中(相当于0.027 mm降水),用喷雾器均匀洒入样方中,对照喷洒等量水。
分别于2018年7月(夏季)、2018年10月(秋季)、2019年1月(冬季)和2019年4月(春季),在每个样方中随机选择生长一致的杨树4~5株,去除凋落物层后,采集位于0~20 cm土层的杨树细根(直径小于2 mm)根际土壤样品(根系抖落后的土壤),在野外迅速过2 mm筛。将同一样方中的根际土壤混合均匀,放入自封袋中,共取得5(施氮水平)×3(重复)×4(采样季节)=60个土壤样品。将取得的土壤样品保温箱冷藏带回实验室,每个土壤样品分为2份,一份放入−80 ℃冰箱,用于AM真菌群落结构和多样性的测定;另一份风干,用于测定土壤理化性质。
-
土壤pH值采用玻璃电极法测定(土水质量比为1∶5);土壤总碳(TC)、总氮(TN)使用元素分析仪(Perkin Elmer 2400 Ⅱ)测定;土壤总磷(TP)和速效磷(AP)采用钼锑抗比色法测定;硝态氮(NO3 −-N)和铵态氮(NH4 +-N)采用紫外分光光度法和靛酚蓝比色法测定[20]。4个采样时间的月平均土壤温度和湿度采用土壤温湿度仪测定。
-
AM真菌群落结构组成采用Illumina Miseq高通量测序技术测定。具体步骤为:称取0.5 g冷冻土样,用Fast DNA SPIN Kit for soil(MP)试剂盒进行总DNA抽提,以Nano Drop 2000进行DNA浓度和纯度的检测;以AML1/AML2[21]和AMV4-5NF/AMDGR[22]进行2轮PCR扩增(ABI Gene Amp® 9700型)。第1轮PCR反应参数为:95 ℃预变性3 min,32个循环(95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸45 s),最后72 ℃延伸10 min。第2轮PCR利用第1次PCR产物作为模板,反应参数为:95 ℃预变性3 min,30个循环(95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸30 s),最后72 ℃延伸10 min。PCR扩增体系为20.0 μL,5×FastPfu 缓冲液4.0 μL,2.5 mmol·L−1 dNTPs 2.0 μL,引物(5 μmol·L−1) 0.8 μL,FastPfu聚合酶0.4 μL,BSA 0.2 μL,DNA模板10 ng。扩增的PCR产物以Axy Prep DNA Gel Extraction Kit (Axygen Biosciences)纯化后,利用Quanti Fluor™-ST(Promega)进行定量检测;送至上海美吉生物医药科技有限公司的Miseq PE250平台(Illumina)进行测序。60个样本的测序原始数据已上传至美国国家生物技术信息中心(NCBI)数据库(序列登录号:PRJNA904933)。将测序后的数据去除非目的序列,再以软件Usearch归类操作,依据序列相似性(相似性水平为97%)归为不同的分类操作单元(OTU),并根据MaarjAM库中的参考序列对OTU进行种属鉴定(相似性水平为97%)。
-
将所有样本序列抽平至10 000条后,用Mothur软件计算AM真菌群落Chao指数和Simpson指数[23],在属水平统计各样本的群落组成。采用R语言vegan软件包对AM真菌群落结构(基于OTUs组成)进行非度量多维度分析(non-metric multidimensional scaling,NMDS),并采用ANOSIM进行不同氮添加和季节间以及同一季节不同氮处理间差异显著性分析;AM真菌群落结构与各环境因子间的关系用冗余分析(redundancy analysis,RDA)评价,RDA分析只保留方差膨胀因子(variance inflation factor,VIF)小于10的环境因子,并用蒙特卡洛分析(Monte Carlo)进行环境因子与AM真菌群落结构的显著性检验。采用SPSS 18.0对季节和氮添加处理下AM真菌群落多样性指数、主要类群相对丰度和环境因子的影响进行多元方差分析(MANOVA);对同一季节不同氮添加处理下AM真菌群落多样性指数和环境因子进行单因素方差分析;并用Spearman相关系数分析AM真菌群落多样性指数与环境因子间的相关关系。用Origin 2017作图。
-
双因素(氮添加和季节)方差分析(表1)表明:季节对所测的10个土壤理化性质均有显著影响,而氮处理对土壤pH、碳氮比、铵态氮和硝态氮质量分数有显著影响(P<0.01)。随着氮添加量的增加,土壤pH和碳氮比均有降低的趋势,且N0、N1和N2处理下两者显著高于N3和N4处理(P<0.05);N4处理下的土壤硝态氮质量分数显著高于其他处理,而铵态氮质量分数在N1处理下最大。不同氮组分随着季节变化呈不同的变化方式:秋季和夏季总氮质量分数显著高于冬季和春季(P<0.05);铵态氮和硝态氮质量分数的最大值分别出现在冬季和秋季,且显著高于其余季节(P<0.05)。此外,随季节的变化土壤温度从大到小依次为夏季、秋季、春季、冬季,而土壤湿度从大到小依次为冬季、春季、夏季、秋季,且各季节间均有显著差异(P<0.05)。
季节 氮添加 pH 总氮/
(g·kg−1)总磷/
(mg·kg−1)总碳/
(g·kg−1)碳氮比 速效磷/
(mg·kg−1)铵态氮/
(mg·kg−1)硝态氮/
(mg·kg−1)湿度/% 温度/℃ 夏季 N0 8.29±0.07 ab 1.73±0.06 b 923±6 a 18.1±1.4 a 10.5±1.1 a 36.8±11.6 a 5.98±0.56 b 2.48±0.48 d 20.1±0.2 a 20.2±0.1 a N1 8.19±0.02 b 1.83±0.12 b 893±17 a 16.4±1.6 ab 9.0±1.5 ab 37.2±22.7 a 7.45±0.24 a 7.85±0.54 b 20.5±0.5 a 20.2±0.2 a N2 8.31±0.03 a 2.27±0.15 a 878±10 a 17.4±1.3 ab 7.7±0.6 b 28.4±3.9 a 6.20±0.38 b 4.06±0.33 c 21.0±0.6 a 20.2±0.3 a N3 8.20±0.07 ab 1.97±0.40 ab 893±13 a 15.0±1.7 b 7.7±0.8 b 43.3±3.3 a 7.41±0.49 a 3.61±0.21 c 21.0±0.5 a 20.2±0.1 a N4 7.99±0.08 c 1.97±0.06 ab 884±50 a 15.4±0.4 b 7.8±0.4 b 44.5±1.0 a 7.42±0.50 a 25.25±0.41 a 20.6±0.5 a 20.2±0.1 a 秋季 N0 8.65±0.02 a 2.13±0.45 a 778±23 a 17.4±1.2 b 8.5±2.4 a 13.8±5.7 a 5.43±0.42 a 12.38±0.97 b 17.7±0.2 a 17.3±0.2 a N1 8.55±0.03 b 2.40±0.50 a 808±25 a 17.2±0.7 b 7.4±1.4 a 18.4±4.8 a 5.56±0.21 a 2.50±0.49 c 17.7±0.6 a 17.3±0.3 a N2 8.43±0.02 c 2.40±0.46 a 772±64 a 15.2±1.0 c 6.5±1.4 a 13.4±1.5 a 5.28±0.40 a 10.52±1.52 c 17.9±0.7 a 17.3±0.1 a N3 8.55±0.04 b 3.33±1.64 a 806±23 a 17.9±0.4 b 6.2±2.7 a 13.6±6.7 a 3.95±0.30 b 10.80±1.98 c 17.8±0.8 a 17.3±0.2 a N4 8.37±0.03 d 3.63±1.40 a 792±22 a 19.7±1.2 a 5.9±2.0 a 13.9±1.8 a 5.45±0.20 a 58.91±2.09 a 17.6±0.5 a 17.3±0.4 a 冬季 N0 8.46±0.03 bc 1.33±0.21 a 757±8 a 16.5±1.8 a 12.4±0.7 a 19.0±3.6 a 8.86±0.61 a 11.83±0.09 b 33.9±0.2 a 5.5±0.1 a N1 8.51±0.03 ab 1.37±0.25 a 705±6 bc 17.1±1.4 a 12.7±1.5 a 19.2±1.7 a 8.14±0.19 b 11.86±0.41 b 33.9±0.4 a 5.5±0.1 a N2 8.55±0.04 a 1.40±0.10 a 693±22 c 16.4±0.5 a 11.8±0.8 a 19.4±3.6 a 7.46±0.19 c 11.83±0.19 b 33.8±0.2 a 5.5±0.1 a N3 8.42±0.05 c 1.43±0.15 a 727±9 b 15.2±1.0 a 10.6±0.4 a 20.6±4.8 a 7.75±0.05 bc 11.66±0.73 b 34.0±0.2 a 5.5±0.0 a N4 8.46±0.04 bc 1.47±0.21 a 708±9 bc 14.9±0.8 a 10.4±1.9 a 22.1±5.6 a 6.73±0.23 d 14.72±0.19 a 34.0±0.4 a 5.6±0.1 a 春季 N0 8.54±0.06 bc 1.40±0.00 bc 647±10 a 17.1±0.2 b 12.2±0.1 ab 21.8±4.3 a 5.65±0.08 b 1.10±0.24 a 27.9±0.4 a 14.6±0.1 a N1 8.62±0.01 a 1.50±0.20 b 558±147 a 19.6±0.2 a 13.2±1.9 a 25.5±7.9 a 7.47±0.79 a 1.19±0.08 a 27.8±0.6 a 14.6±0.2 a N2 8.57±0.03 ab 1.17±0.06 c 639±12 a 16.9±0.6 b 14.5±0.5 a 22.4±3.2 a 5.17±0.14 b 1.06±0.14 a 27.7±0.6 a 14.6±0.2 a N3 8.50±0.01 c 1.50±0.17 b 668±11 a 18.6±1.2 ab 12.6±2.4 a 21.2±3.5 a 5.51±0.22 b 1.11±0.41 a 27.3±0.5 a 14.6±0.1 a N4 8.60±0.02 ab 1.83±0.15 a 673±1 a 18.1±1.7 ab 9.9±0.2 b 19.8±2.5 a 6.96±0.25 a 1.53±0.07 a 27.9±0.9 a 14.6±0.1 a 氮添加 ** ns ns ns ** ns ** ** ns ns 季节 ** ** ** ** ** ** ** ** ** ** 氮添加×季节 ** ns ns ** ns ns ** ** ns ns 说明:表中数值为3个重复的平均值±标准差。同列不同小写字母表示同一季节下氮添加处理间差异显著(P<0.05)。ns表示差异不显著,*表示差异达5%显著水平,**表示差异达1%显著水平。 Table 1. Soil physicochemical properties in the poplar plantations
-
60个土壤样本共获得1 337 714条原始序列,去除低质量序列后,得AM真菌有效序列1 307 513条(每个样本12 998~24 496条),分属于196个分类单元(OUT)。所有样本的稀释曲线在测序量为4 000条时已趋于平稳,表明本研究中的土壤样品测序深度足够反映样本中的AM真菌群落结构。双因素方差分析结果(表2)表明:氮处理对Chao指数(P=0.193)和Shannon指数(P=0.725)均无显著影响,而不同季节间差异显著(P<0.01)。Chao和Shannon指数最大值均出现在秋季,平均值分别为98.31和3.07。秋季、冬季和春季间的Shannon指数差异不显著,且显著高于夏季(P<0.01);对Chao指数而言,除夏季和冬季差异不显著外,其他季节间均有显著差异,从大到小依次为秋季、春季、夏季、冬季。
处理 Chao指数 Shannon指数 夏季 秋季 冬季 春季 夏季 秋季 冬季 春季 N0 90.1±2.6 a 105.0±10.7 a 68.5±2.2 a 94.9±16.2 a 2.58±0.23 a 3.20±0.04 a 2.78±0.36 a 3.12±0.10 a N1 76.0±11.7 a 98.4±14.8 a 78.4±3.9 a 93.8±7.5 a 2.63±0.51 a 3.22±0.08 a 3.00±0.06 a 2.86±0.05 c N2 73.5±11.3 a 89.3±11.4 a 77.2±15.9 a 77.3±5.5 a 2.65±0.58 a 2.84±0.80 a 2.88±0.25 a 2.90±0.15 bc N3 71.5±20.0 a 97.9±3.5 a 68.6±13.4 a 82.2±10.2 a 2.45±0.24 a 3.16±0.05 a 2.70±0.12 a 3.04±0.16 abc N4 74.3±19.6 a 101.4±18.6 a 67.2±2.7 a 91.7±4.9 a 2.24±0.40 a 2.92±0.48 a 2.83±0.21 a 3.10±0.05 ab 氮添加 ns ns 季节 ** ** 氮添加×季节 ns ns 说明:表中数值为3个重复的平均值±标准差。同列不同小写字母表示同一季节下氮添加处理间差异显著(P<0.05)。ns表示差异不显著,*表示差异达5%显著水平,**表示差异达1%显著水平。 Table 2. Soil AM fungal community richness and diversity index
-
主要群落(平均相对丰度≥0.05%)组成如表3所示:主要有球囊霉属Glomus、多胞囊霉属Diversispora、盾巨孢囊霉属Scutellospora和原囊霉属Archaeospora。双因素方差分析表明:季节和氮添加处理均对球囊霉属和多胞囊霉属相对丰度有显著影响(P<0.05)。N4处理下的球囊霉属相对丰度显著高于N1和N2处理(P<0.05);从N1到N4处理,多胞囊霉属相对丰度逐渐降低,除N2和N3处理差异不显著外,其他处理间均有显著差异(P<0.05)。球囊霉属相对丰度随着季节变化从大到小依次为冬季、秋季、夏季、春季,除夏季和秋季差异不显著外,其余季节间差异均达显著水平(P<0.05);而多胞囊霉属相对丰度在冬季出现最小值(4.8%),且显著低于其他3个季节。
季节 氮添加 优势AM真菌相对丰度/% 球囊霉属
Glomus多胞囊霉属
Diversispora盾巨孢囊霉属
Scutellospora球囊菌纲
Glomeromycetes
(未分类)多样孢囊霉科
Diversisporaceae
(未分类)原囊霉属
Archaeospora其他 夏季 N0 89.6±4.9 a 10.0±4.9 b 0.090±0.078 a 0.000±0.000 c 0.027±0.029 a 0.067±0.090 a 0.157±0.271 a N1 76.3±5.7 b 23.3±5.8 a 0.223±0.387 a 0.007±0.012 c 0.133±0.154 a 0.000±0.000 a 0.000±0.000 a N2 85.8±6.3 a 13.5±5.9 b 0.550±0.470 a 0.003±0.006 c 0.043±0.006 a 0.000±0.000 a 0.030±0.052 a N3 85.9±2.5 a 13.3±2.9 b 0.643±0.772 a 0.080±0.070 b 0.003±0.006 a 0.040±0.069 a 0.000±0.000 a N4 90.9±2.3 a 8.8±2.3 b 0.000±0.000 a 0.213±0.025 a 0.070±0.082 a 0.053±0.051 a 0.000±0.000 a 秋季 N0 88.9±0.4 a 10.1±1.1 b 0.757±1.250 a 0.060±0.010 a 0.030±0.026 a 0.000±0.000 a 0.123±0.205 a N1 74.7±8.6 b 24.6±8.7 a 0.100±0.173 a 0.340±0.150 a 0.160±0.156 a 0.090±0.123 a 0.090±0.147 a N2 91.1±5.9 a 6.3±2.9 b 1.763±2.470 a 0.790±1.340 a 0.057±0.029 a 0.000±0.000 a 0.000±0.000 a N3 89.1±3.2 a 10.6±3.1 b 0.063±0.110 a 0.027±0.038 a 0.123±0.021 a 0.013±0.023 a 0.007±0.006 a N4 89.9±0.9 a 9.1±1.2 b 0.070±0.113 a 0.730±0.729 a 0.127±0.110 a 0.000±0.000 a 0.033±0.049 a 冬季 N0 91.6±1.7 bc 7.82±1.80 a 0.233±0.404 a 0.060±0.026 b 0.000±0.000 a 0.010±0.017 b 0.317±0.107 a N1 95.7±1.2 ab 4.25±1.21 bc 0.000±0.000 a 0.020±0.020 b 0.007±0.006 a 0.000±0.000 b 0.010±0.017 b N2 93.1±2.7 b 6.79±2.59 ab 0.000±0.000 a 0.077±0.098 b 0.007±0.012 a 0.057±0.049 a 0.013±0.023 b N3 97.3±0.7 a 1.88±0.26 c 0.000±0.000 a 0.793±0.435 a 0.000±0.000 a 0.000±0.000 b 0.000±0.000 b N4 96.7±1.2 a 3.29±1.18 bc 0.000±0.000 a 0.043±0.059 b 0.007±0.012 a 0.003±0.006 b 0.000±0.000 b 春季 N0 83.0±1.5 ab 16.4±1.4 ab 0.127±0.219 a 0.037±0.032 a 0.190±0.329 a 0.020±0.026 a 0.173±0.300 a N1 83.6±5.6 ab 15.7±5.3 ab 0.203±0.352 a 0.263±0.040 a 0.057±0.051 a 0.023±0.032 a 0.137±0.237 a N2 74.0±2.0 b 24.3±2.8 a 0.893±1.030 a 0.357±0.592 a 0.067±0.076 a 0.453±0.777 a 0.000±0.000 a N3 82.6±9.5 ab 16.2±8.5 ab 0.000±0.000 a 0.593±0.370 a 0.537±0.657 a 0.013±0.006 a 0.010±0.017 a N4 88.1±4.6 a 11.2±3.9 b 0.430±0.745 a 0.107±0.032 a 0.060±0.072 a 0.153±0.129 a 0.000±0.000 a 氮添加 ** ** ns ns ns ns ** 季节 ** ** ns ns ns ns ns 氮添加×季节 ** ** ns ns ns ns ns 说明:表中数值为3个重复的平均值±标准差。同列不同小写字母表示同一季节下氮添加处理间差异显著(P<0.05)。ns表示差异不显著,*表示差异达5%显著水平,**表示差异达1%显著水平。 Table 3. Relative abundances of the AM fungal groups (relative abundance ≥0.05%)
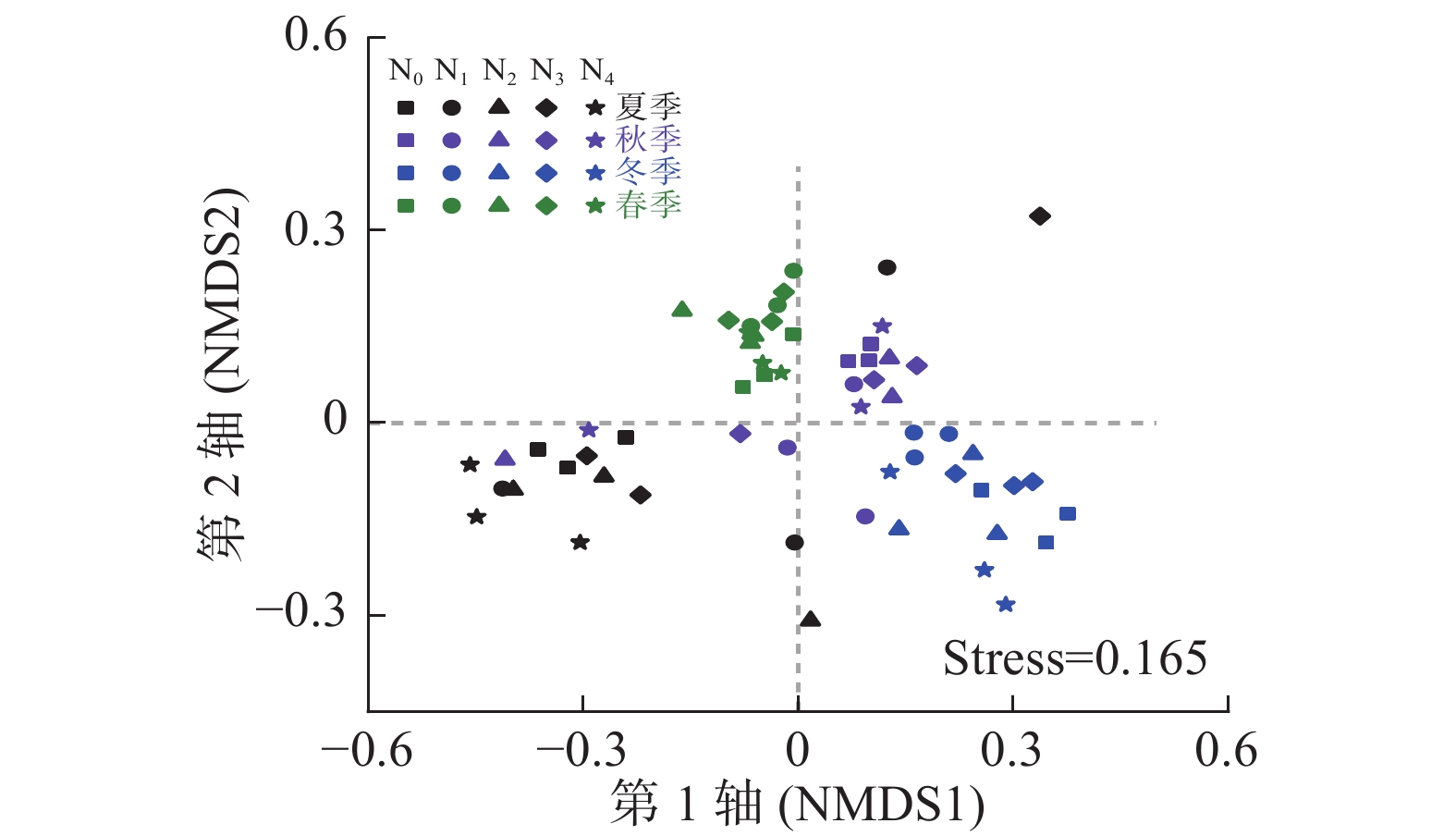
基于OTUs的NMDS排序分析结果(图1)表明:季节显著影响AM真菌群落结构(r=0.695, P=0.001),而氮处理对其影响不显著(r=0.027, P=0.163)。对同一季节下不同氮处理间进行相似性检验发现:春季(r=0.529, P=0.001)、冬季(r=0.479, P=0.002)和秋季(r=0.310, P=0.016)不同氮添加处理间AM真菌群落结构差异显著,而夏季氮添加处理间差异不显著(r=0.124, P=0.125)。
-
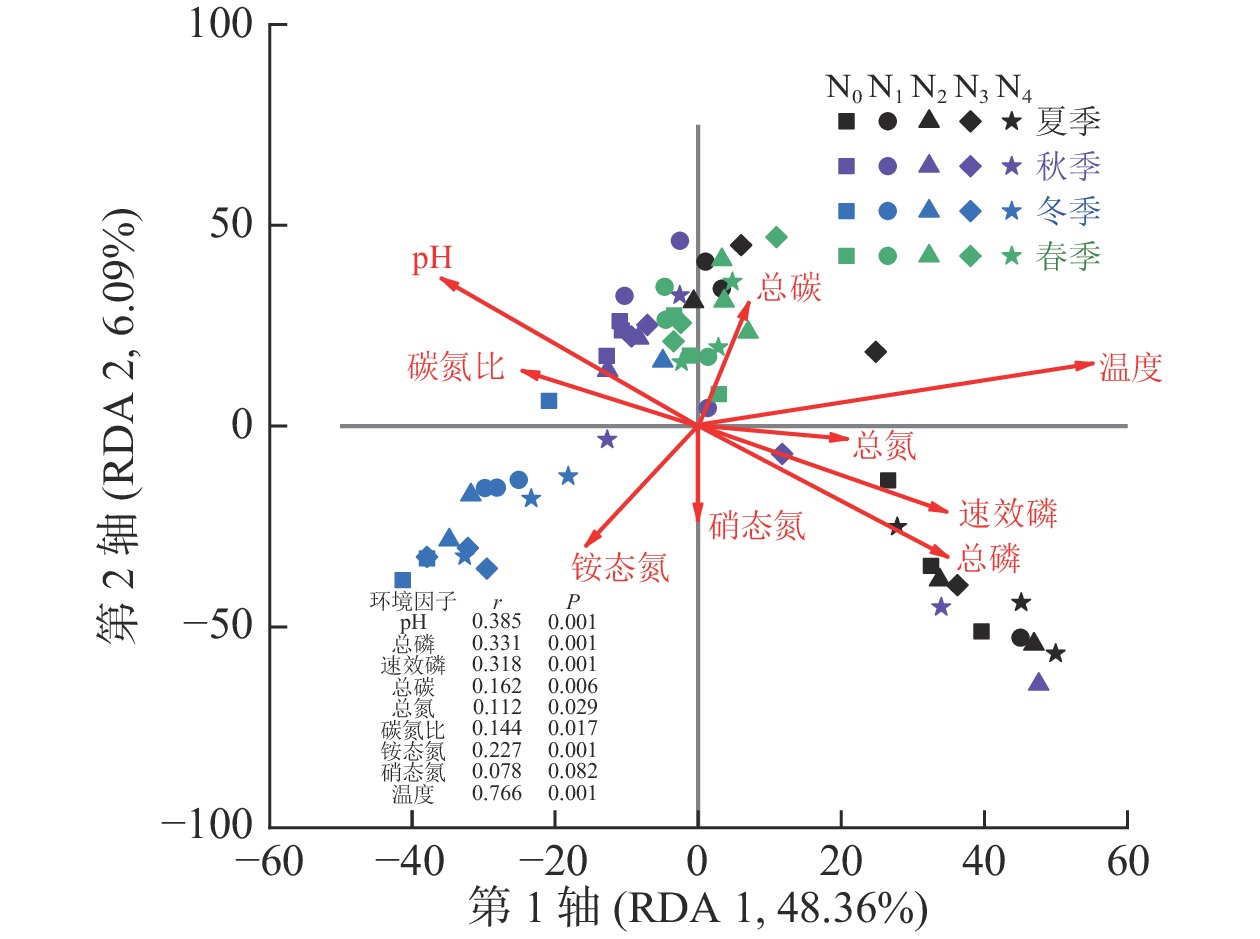
测定的所有环境因子中有9个因子VIF小于10,分别是pH、总磷、速效磷、总碳、总氮、碳氮比、铵态氮、硝态氮和温度,它们对AM真菌群落组成变化的解释量为57.6% (P=0.001)。Monte Carlo检验结果表明:除硝态氮外,所有环境因子均与AM真菌群落组成有显著相关关系(P<0.05,图2)。其中,AM真菌群落组成与温度相关关系最大(r=0.766),其次为pH (r=0.385)和总磷(r=0.331,P<0.01)。
土壤AM真菌群落多样性指数与土壤理化性质间的相关分析(表4)发现:Chao指数与pH、总碳、碳氮比和温度显著正相关(P<0.05),与总磷、铵态氮和湿度显著负相关(P<0.05);Shannon指数则与pH和总碳显著正相关(P<0.05),与总氮、总磷和铵态氮显著负相关(P<0.05)。
指数 pH 总氮 总磷 总碳 碳氮比 速效磷 铵态氮 硝态氮 湿度 温度 Chao指数 0.344** −0.090 −0.291* 0.505** 0.316* −0.190 −0.511** 0.110 −0.449** 0.325* Shannon指数 0.596** −0.396** −0.548** 0.271* 0.020 0.110 −0.390** −0.096 0.016 −0.149 说明:n=60,*P<0.05; **P<0.01。 Table 4. Correlation coefficients between AM fungal community Chao and Shannon indexes and soil physicochemical properties
-
AM真菌属球囊菌门Glomeromycota球囊菌纲Glomeromycetes,由4目11科27属约300种组成[24]。本研究中,杨树人工林根际土壤中AM真菌群落分属10属,优势属为球囊霉属(平均相对丰度87.4%)和多胞囊霉属(11.9%)。MAITRA等[25]在亚热带常绿阔叶混交林的研究发现:土壤AM真菌优势群落为球囊霉科Glomeraceae (78.8%)和原囊霉科Archaesporaceae (11.1%);而朱亮等[26]研究表明:亚高山岷江冷杉Abies faxoniana次生林土壤AM真菌优势群落为球囊霉属、无梗囊霉属Acaulospora和多孢囊霉属。由此可见,不同区域不同类型森林生态系统中的优势AM真菌群落组成具有一定差异。
不同AM真菌类群在生活习性、形态结构和生态功能等方面具有较大差异[6]。氮沉降增加导致的土壤氮等性质变化,将会直接影响寄主植物与不同AM真菌类群间的博弈关系[27−28]。随着氮添加量增加,植物氮限制降低,相对于那些具有大量菌丝或大孢子的AM真菌类群,根外菌丝少或孢子较小的AM真菌类群需要从植物获取的碳源少,可能会被选择性的保留下来;而较高的氮含量促进植物生长后,会加速植物的磷限制,这时植物可能加大对那些具有大量菌丝或大孢子的AM真菌类群(更强的磷吸收能力)的碳投入,加速其生长[29]。本研究中,氮添加处理对杨树人工林根际AM真菌群落整体结构和多样性影响均不显著,但是随着氮添加水平的变化,不同AM真菌类群呈现不同响应:随着氮添加水平的增加,多胞囊霉属相对丰度逐渐降低;高氮处理下的球囊霉属相对丰度显著高于低氮处理。这与其他研究者的结果一致,氮沉降增加后,巨孢囊霉属Gigaspora和多孢囊霉属AM真菌生长受到限制,而球囊霉属和无梗囊霉属丰度增加[8, 30−31]。相比之下,多孢囊霉属和巨孢囊霉属AM真菌会将更多的能量分配给根外菌丝,而不是根内结构;球囊霉属AM真菌的能量分配方式则与之相反[12]。更多的根外菌丝代表AM真菌具有更强的养分吸收能力,同时为了维持这一结构,其从植物吸收的碳源也更多[32]。随着氮添加水平增加,植物依靠AM真菌为其吸收养分的程度逐渐降低,将会减少对碳需求多的AM真菌类群的投资,从而使得多胞囊霉属相对丰度逐渐降低。
不同季节AM真菌群落结构和多样性具有显著差异。由于AM真菌是活营养体真菌,只能通过与具有生命力的植物建立共生关系才能完成生命历程,有研究发现:在草地生态系统中的AM真菌的生长与植物物候期一致[33]。本研究中,秋季和春季AM真菌群落Chao和Shannon指数均显著高于夏季,不同季节群落聚集在NMDS排序图中的不同位置。AM群落组成常常是种间竞争和环境变化作用的结果[13]。在夏季,由于土壤温度较高,湿度相对较为适宜,微生物活性强,土壤总磷、有效磷和铵态氮质量分数均显著高于秋季和春季。鉴于AM真菌与植物间的功能关系,在贫瘠和胁迫条件下植物更易支持AM真菌生长[5, 34]。相关分析也表明:AM真菌多样性指数与总磷和铵态氮质量分数显著负相关(P<0.05)。AM真菌群落组成与土壤温度相关性最大(r=0.766),原因是土壤温度可以直接或通过改变植物碳分配的方式间接影响AM真菌的生长[7]。除此以外,土壤pH和总碳与AM真菌群落结构和多样性均显著相关,且AM真菌群落多样性随着土壤pH和总碳的增加而增加。本研究中杨树人工林是由沿海滩涂围垦形成,土壤pH 7.99~8.65,pH增加对植物形成的胁迫越大,可能会使植物加大对AM真菌群落的投入使其多样性增加。需要说明的是,本研究中氮添加时间设置在5—10月,尽管避免了氮添加后立即采样,但季节效应也可能会受到氮添加时间的影响,后续可针对这一问题展开研究。本研究用于RDA分析的9个环境因子对AM真菌群落组成变化的解释量为57.6% (P=0.001),表明还有一些影响AM真菌群落组成的因素没有被考虑到,如植物群落组成和结构等,在今后的研究中需进一步探讨。
-
杨树人工林根际优势AM真菌属为球囊霉属Glomus和多胞囊霉属Diversispora。氮添加处理对AM真菌群落整体结构和多样性影响均不显著,但是随着氮添加水平的增加,多胞囊霉属相对丰度逐渐降低;高氮处理下的球囊霉属相对丰度显著高于低氮处理。
不同季节间AM真菌群落Chao、Simpson指数和群落结构差异显著,秋季和春季AM真菌群落多样性指数均显著高于夏季。土壤温度、pH、总磷、总碳和铵态氮是影响杨树人工林AM真菌群落结构和多样性动态变化的主要驱动因素。
Seasonal dynamic responses of soil arbuscular mycorrhizal fungal community to nitrogen additions in a poplar plantation
doi: 10.11833/j.issn.2095-0756.20220640
- Received Date: 2022-10-17
- Accepted Date: 2023-04-10
- Rev Recd Date: 2023-04-10
- Available Online: 2023-07-13
- Publish Date: 2023-08-20
-
Key words:
- nitrogen deposition /
- seasonal dynamics /
- arbuscular mycorrhizal fungi /
- community composition /
- diversity /
- poplar plantation
Abstract:
| Citation: | PENG Sili, ZHANG Xin, WU Renjie, et al. Seasonal dynamic responses of soil arbuscular mycorrhizal fungal community to nitrogen additions in a poplar plantation[J]. Journal of Zhejiang A&F University, 2023, 40(4): 792-800. DOI: 10.11833/j.issn.2095-0756.20220640 |









 DownLoad:
DownLoad: