-
瓯柑Citrus suavissima优良新品种‘无子瓯柑’C. suavissima ‘Seedless’是通过芽变选种获得的,其果实无核,遗传性状稳定[1]。与瓯柑相比,两者的花粉量相当,但‘无子瓯柑’花药不易开裂,自然散发的花粉少[2]。形态学和细胞学研究表明:小孢子母细胞减数分裂异常是‘无子瓯柑’雄性不育的重要原因之一[2−3]。瓯柑及‘无子瓯柑’花粉发育早期的花药转录组与蛋白质组关联分析结果表明:差异代谢通路主要富集在苯丙素生物合成、黄酮类化合物生物合成和苯丙氨酸代谢通路[4]。CsRNF217基因是瓯柑与‘无子瓯柑’在小孢子母细胞时期与苯丙素生物合成途径密切关联的重要基因,与瓯柑相比,该基因在同时期的‘无子瓯柑’中显著上调表达;CsRNF217基因的氨基酸序列中存在RING-HC_RBR和IBR 2个结构域,属于典型的单亚基RING-HC E3亚家族,定位于细胞核[5]。
泛素化是真核生物中一种高度通用的翻译后修饰,它介导蛋白酶体降解、细胞运输、蛋白质相互作用和细胞蛋白质的功能激活[6−8]。泛素化级联需要3种不同的酶催化:E1泛素激活酶、E2泛素结合酶和E3泛素连接酶[9]。其中,E3泛素连接酶具有显著的多样性,它决定了底物的特异性,并作为调节细胞反应的枢纽[10−11]。E3泛素连接酶分为单亚基和多亚基两类。单亚基组由3个主要的亚家族组成:RING、HECT以及U-box结构域家族[11−13]。CsRNF217基因即属于单亚基RING结构域家族。E3泛素连接酶在植物的生长发育过程中起着关键的作用,包括细胞程序性死亡和抗病防御反应[14]、成花调控[15]、生物[16]和非生物应激反应[14]以及花粉发育[17−19]等。其中,在水稻Oryza sativa[17]、拟南芥Arabidopsis thaliana[18]、白菜Brassica campestris ssp. chinensis[19]中发现的RING型E3泛素连接酶基因DSNP1、DAF、Bra015092等在花粉发育过程中起重要作用。
本研究以过表达‘无子瓯柑’CsRNF217的转基因烟草Nicotiana tabacum植株为材料,通过对基因表达分析、转基因自交1代植株(T1)花粉活力、转基因植株自交及与野生型烟草正反交的结实率等参数的测定,分析过表达CsRNF217对转基因烟草植株育性的影响,为进一步揭示CsRNF217基因在‘无子瓯柑’雌雄败育过程中的重要作用提供参考。
-
以野生型烟草(WT)作为对照,采用农杆菌Agrobacterium tumefaciens介导法获得过表达CaMV 35S :: CsRNF217的阳性转基因烟草[5](T0)。将T0自交种子穴盘播种获得转基因烟草自交1代植株(T1)。采集T1阳性烟草植株6#、35#、63#株系的叶片及含苞待放花蕾的花药,立即置于液氮中速冻并于−80 ℃保存,用于半定量RT-PCR分析。
-
采集野生型烟草及T1烟草植株的叶片,采用CTAB法提取DNA,以pCAMBIA 2300s : CsRNF217质粒作为阳性对照,ddH2O为阴性对照,用35S_F及基因特异引物CsRNF217_R838_S[5](表1)进行PCR检测筛选阳性植株,PCR程序为94 ℃,5 min;94 ℃,30 s,55 ℃,30 s,72 ℃,1 min,35次循环;72 ℃,10 min;12 ℃保存。
引物名称 引物序列(5′→3′) CsRNF217_R838_S GTCGACTTGCATAGAGCCAATAAA 35S_F ACGCACAATCCCACTATCCTTC CsRNF217_qL2 ACGTGCGAGGGTATGAAAGA CsRNF217_qR2 TACCCTCCATGCCACTTCAG EF1α-F TGGTTGTGACTTTTGGTCCCA EF1α-R ACAAACCCACGCTTGAGATCC Table 1. Specific primers used in this study
-
于晴天10:00采集烟草含苞待放的花蕾,采用亚历山大染色法[20]对野生型烟草及经PCR鉴定的T1阳性植株进行花粉染色活力检测,采用花粉离体培养法[21]对花粉染色活力显著下降的烟草单株进行花粉萌发试验,每个植株随机挑选3朵花作为生物学重复。花粉粒被染为紫红色的视为有活力的花粉粒,花粉管长度大于或等于花粉粒直径视为萌发。花粉染色活力=着色花粉粒数/视野中的花粉粒总数×100%,花粉萌发率=萌发花粉粒数/视野中的花粉粒总数×100%。
使用MiniBEST Plant RNA Extraction Kit (TaKaRa,日本)试剂盒,提取野生型烟草及花粉活力显著下降的T1烟草阳性植株花药RNA,采用EASYScript one-step gDNA removal and cDNA synthesis supermix (TransGen Biotech code#AE311- 03)试剂盒进行cDNA合成,使用CsRNF217基因对引物和烟草内参基因EF1α特异引物(表1)进行半定量RT-PCR分析,程序为94 ℃,5 min;94 ℃,30 s,55 ℃,30 s,72 ℃,1 min,29次循环;72 ℃,10 min;12 ℃保存。
-
于晴天10:00收集野生型和T1已开花,但未散粉植株的花药于离心管,4 ℃干燥保存备用。于傍晚用干净的小镊子摘去即将开放花蕾的花瓣和雄蕊。次日10:00蘸取花粉轻点已分泌黏液的柱头,套袋,每个植株进行3朵花的正反交重复,3 d后摘去袋子。正反交组合配置中,正交授粉以转基因株系为父本,野生型为母本;反交授粉以野生型为父本,转基因株系为母本。种子成熟时统计每个单株采收的蒴果数,测量蒴果横径、纵径及种子总质量。
-
采用SPSS软件对种子萌发率、花粉染色活力、花粉离体萌发率、蒴果横纵径及种子数量等进行了单因素方差分析(one-way ANOVA,LSD)。
-
T0烟草种子穴盘播种共获得137个单株,其中6#株系16株,35#株系80株,63#株系41株。以野生型及T0种子播种获得的烟草叶片DNA为模板,经PCR检测,共获得68株阳性植株(表2)。其中,6#株系10株,35#株系33株,63#株系25株,阳性率分别为62.5%、41.2%、61.5%。
株系 株数/
株阳性
株数/株植株阳
性率/%花粉染色活力
均值/%6# 16 10 62.5 19.5±2.7 b 35# 80 33 41.2 31.9±2.8 b 63# 41 25 61.5 21.8±2.3 b WT 3 0 0 94.5±2.4 a 说明:同列不同字母表示不同株系间花粉染色活力差异显著(P<0.05)。WT为野生型株系。 Table 2. Positive rate and pollen viability of wild type and T1 tobacco plants
-
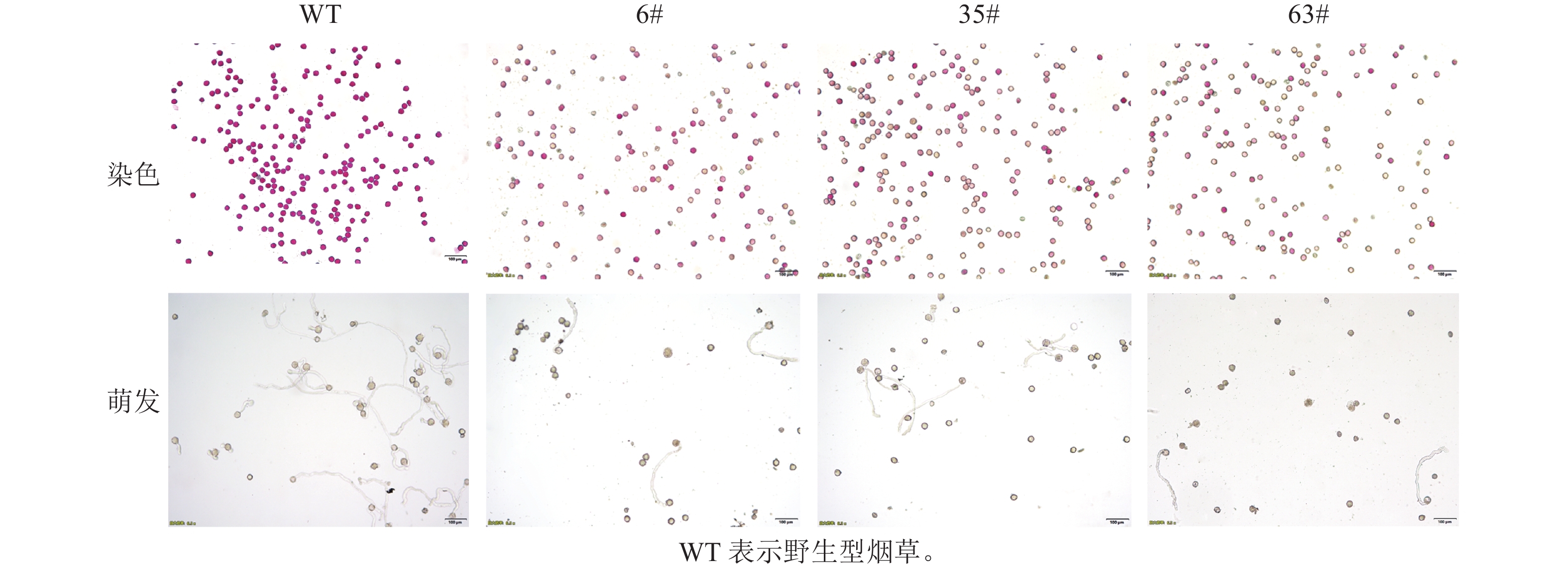
T1阳性烟草植株花粉的散粉量与野生型存在差异(图1)。野生型植株花药开裂后,散粉量多,可见大量花粉散布于花瓣及柱头;T1阳性烟草植株花药裂开后,散粉量少,几乎无可见花粉散出。经染色法和花粉离体萌发培养(图2):T1阳性烟草植株花粉染色活力显著低于野生型植株(图3,P<0.05)。有活力的花粉粒经亚历山大染色后呈紫红色,无活力的花粉粒呈黄褐色。野生型植株经亚历山大染色后花粉粒大多呈紫红色(94.5%),阳性植株呈紫红色的花粉粒数量显著少于野生型(图3,P<0.05)。其中6#株系的14号单株(6#14)、35#株系的4号单株(35#4)、63#株系的4号单株(63#4)的花粉经亚历山大染色后着色率最低,分别为9.6%、12.0%、9.7%。有活力的花粉粒能在适宜的离体条件下萌发,T1阳性植株花粉粒的萌发率显著低于野生型植株(60.3%)。其中6#株系的9号单株(6#9)、6#株系的14号单株(6#14)、63#株系的4号单株(63#4)的花粉粒萌发率在各株系中最低,分别为30.6%、29.0%、33.4%。半定量RT-PCR分析显示(图4):CsRNF217基因在过表达的各株系中均能表达,其中,外源基因在63#株系各单株花药中的表达量最高。
-
对花粉染色活力、萌发率都显著低于野生型的T1转基因阳性单株进行授粉(表3,图5)表明:自交和与野生型进行正反交的各授粉组合均能结实,但蒴果大小及种子数量存在较大差异。蒴果横径的比较结果显示:转基因63#株系自交、与野生型进行正反交的蒴果横径均显著小于野生型自交的蒴果(P<0.05);35#株系自交蒴果的横径显著小于野生型自交(P<0.05)。蒴果纵径的比较结果显示:转基因63#株系自交和与野生型反交的蒴果纵径均显著小于野生型自交(P<0.05),其余组合与野生型自交无显著差异。根据烟草原始种的种子千粒重(0.087 g)[22],计算每个株系的单果种子数量。阳性株系自交、与野生型正反交获得的种子数量都显著低于野生型自交种子数(P<0.05),但转基因株系之间无显著差异。
处理 株系 蒴果横径/mm 蒴果纵径/mm 种子数量/(粒·果−1) 自交 WT 9.2±0.2 a 15.8±0.2 a 1 525.7±19.9 a 6# 8.9±0.1 ab 14.9±0.1 ab 1 030.4±105.2 b 35# 8.4±0.1 bcd 14.9±0.1 ab 948.5±37.3 b 63# 8.4±0.0 bcd 14.6±0.1 b 1 006.7±36.0 b 正交 WT (♀) × 6# (♂) 8.5±0.1 abcd 15.2±0.4 ab 845.5±69.4 b WT (♀) × 35# (♂) 8.6±0.2 abc 14.3±0.2 b 924.6±26.7 b WT (♀) × 63# (♂) 8.1±0.2 cd 15.0±0.4 ab 1 049.8±8.0 b 反交 6# (♀) × WT (♂) 8.6±0.2 abc 15.1±0.5 ab 1 103.4±119.8 b 35# (♀) × WT (♂) 9.1±0.3 ab 14.8±0.5 ab 992.3±185.9 b 63# (♀) × WT (♂) 7.8±0.3 d 14.4±0.4 b 914.4±90.7 b 说明:WT表示野生型烟草。同列不同字母表示株系间差异显著(P<0.05)。 Table 3. Capsule and seed number in selfing and hybridization test
-
本研究表明:过表达CsRNF217的烟草植株在花粉染色活力、花粉萌发率、蒴果大小及种子数量上均显著低于野生型,说明过表达CsRNF217降低了转基因烟草的小孢子育性,甚至对胚囊育性也存在一定的影响。
近年来的研究表明:RING型E3泛素连接酶基因参与了植物花粉发育[19]、花药开裂[18]、胚囊发育[17]等生命过程。水稻中的RING型E3泛素连接酶DSNP1与水稻减数分裂过程中的联会复合体组装和同源重组关系密切,其突变体dsnp1中形成的稳定同源配对和重组交叉严重减少,并最终形成活性较低的花粉[17]。然而,白菜中过表达E3泛素连接酶基因Bra015092,也降低了转基因白菜的花粉染色活力和萌发率,并导致了花粉外部形态的畸形[19]。前期研究表明:‘无子瓯柑’成熟花粉的染色活力和离体萌发率均显著低于有籽瓯柑,其小孢子母细胞减数分裂异常[2−3],CsRNF217的表达在小孢子发育早期显著上调[5]。本研究中过表达CsRNF217的烟草花粉离体萌发率为野生型的一半,花粉染色活力则更低。推测CsRNF217基因可能通过负调控小孢子母细胞的减数分裂过程参与‘无子瓯柑’的花粉发育。拟南芥E3泛素连接酶基因DAF在雄蕊中特异表达,并通过正向调控茉莉酸生物合成途径来促进花药开裂,通过干涉实验抑制其表达的植株则表现为雄性不育[18]。‘无子瓯柑’的花药自然开裂难、散粉量低[2−3],实时定量PCR结果显示:CsRNF217基因在雄蕊的表达丰度显著高于其他花器官[5]。本研究中,过表达CsRNF217的转基因烟草也表现出自然散粉较弱的特性,推测CsRNF217可能参与了花药开裂的调控。
以野生型花粉对水稻不育突变体dsnp1进行授粉,突变体不能结实,表明该突变体既是雄性不育的,也表现出雌性不育[17],说明该RING型E3泛素连接酶DSNP1对水稻的雌雄育性都有影响。本研究表明:转基因烟草不仅花粉活力显著下降,其种子数量也显著减少,说明CsRNF217的超量表达在负调控花粉育性的同时,对胚囊的育性也存在一定的调控作用。在生产实践中,‘无子瓯柑’在同其他有籽柑橘品种混栽时也表现无籽,说明胚囊败育是‘无子瓯柑’果实无核的重要原因,因此CsRNF217对‘无子瓯柑’胚囊育性的影响值得进一步研究。
-
E3泛素连接酶基因CsRNF217对转基因烟草的育性存在显著影响,过表达‘无子瓯柑’CsRNF217的T1烟草阳性植株雌雄育性均显著下降,推测CsRNF217对‘无子瓯柑’的育性存在负调控作用。
Fertility effect of E3 ubiquitin ligase gene CsRNF217 in transgenic tobacco plants
doi: 10.11833/j.issn.2095-0756.20220710
- Received Date: 2022-11-21
- Accepted Date: 2023-07-11
- Rev Recd Date: 2023-05-04
- Available Online: 2023-11-23
- Publish Date: 2023-11-23
-
Key words:
- Citrus /
- E3 ubiquitin ligase /
- male sterility /
- embryo sac abortion
Abstract:
| Citation: | YE Xiaoling, ZHAO Yuhong, JIANG Nan, et al. Fertility effect of E3 ubiquitin ligase gene CsRNF217 in transgenic tobacco plants[J]. Journal of Zhejiang A&F University, 2023, 40(6): 1181-1187. DOI: 10.11833/j.issn.2095-0756.20220710 |















 DownLoad:
DownLoad: