-
薄壳山核桃Carya illinoensis属于胡桃科Juglandaceae山核桃属Carya,原产于北美密西西比河流域[1],集坚果、油料、用材于一体,树形高大挺秀,株型紧凑[2],极具经济价值。中国引种薄壳山核桃的历史始于19世纪末[3],随着种植规模的不断扩大,薄壳山核桃病害的发生越来越多,已报道的约21种,包括叶部病害11种,其中,薄壳山核桃黑斑病、褐斑病及白粉病[4]等叶部病害较为严重,影响薄壳山核桃产量和品质[5]。薄壳山核桃叶部黑斑病及褐斑病病害统称为叶斑病[6]。INGRAM[7]认为:炭疽菌Colletorichum spp.是引起薄壳山核桃黑斑病的主要菌种,邓蕾等[8]认为山核桃黑斑病病原为小孢拟盘多毛孢Pestalotiopsis microspora,2种菌都被证实对叶片和果实具有侵染性。杨莉等[9]对四川的核桃褐斑病进行柯赫氏法则的验证,明确其病原为链格孢属的Alternaria alternata。
前期从薄壳山核桃叶斑病组织中分离鉴定出4种病原菌分别是共享镰孢菌Fusarium commune、暗球腔菌属Phaeosphaeria的P. fuckelii、茶藨子葡萄座腔菌Botryosphaeria dothidea和灰葡萄孢Botrytis cinerea[6]。这4种菌已被确认为植物病原菌且寄主多样化,共享镰孢菌有报道可引起龙牙百合Lilium brownii var. viridulum枯萎病[10]和烟草Nicotiana tabacum根腐病[11];暗球腔菌属Phaeosphaeria spp.对淡竹Phyllostachys glauca、桂竹Phyllostachys reticulata和香蕉Musa spp.[12−13]具有较大危害;茶藨子葡萄座腔菌寄生性较广,可以侵染山核桃Carya cathayensis、杨树Populus spp.、桉树Eucalyptus spp.等经济树种并对其造成严重危害[14−15];灰葡萄孢可引起草莓灰霉病等[16]。
目前这4种病原菌的相关生物学特性和农药毒力测定的研究较浅。由于同种病原不同菌株因环境差异而导致敏感性不同,因此有必要对该4种致病菌株进行生物学测定。化学防治仍然是植物病害防治的主要手段,合理使用农药可以降低病原菌的抗药性,增加防治效果[17]。本研究对4种病原菌的生物学特性进行研究,并用5种化学试剂进行室内毒力测定,旨在为薄壳山核桃叶斑病的防控提供可靠的理论依据。
-
薄壳山核桃叶斑病病原菌共享镰孢、P. fuckelii、 茶藨子葡萄座腔菌和灰葡萄孢在南京六合区、镇江句容市、安徽省滁州市采集感病薄壳山核桃叶片分离纯化获得。
-
5种供试药剂包括250 g·L−1吡唑醚菌酯悬浮剂(河北中保绿农作物科技有限公司)、450 g·L−1咪鲜胺水乳剂(湖南新长山农业发展股份有限公司)、400 g·L−1百菌清悬浮液(天津艾格福农药科技有限公司)、250 g·L−1氰烯菌酯悬浮剂(江苏省农药研究所股份有限公司)、300 g·L−1丙硫菌唑悬浮剂(安徽久易股份有限公司)。
-
将4种致病菌株用无菌打孔器打成直径5 mm的菌块,接种至马铃薯葡萄糖琼脂培养基(PDA)培养基上,设置5、10、15、20、25、30、35 ℃等7个温度处理,每个处理设置3次重复,采用十字交叉法分别测量培养7 和14 d的菌落直径,计算生长速率。
-
以马铃薯葡萄糖琼脂培养基(PDA)为基本培养基,用l mol·L−1的HCl溶液和l mol·L−1的NaOH溶液调整PDA培养基的pH值,设4.0、5.0、6.0、7.0、8.0、9.0、10.0等7个处理,将5 mm的菌块接入不同pH的培养基,27 ℃恒温培养,每个处理设置3次重复。除P. fuckelii分别于培养7 和14 d测量直径外,其余3种分别于培养3 和7 d时测量供试菌株直径,计算生长速率。
-
以基础培养基(0.25 g·L−1硫酸镁,0.30 g·L−1磷酸二氢钾,2.00 g·L−1硝酸钾,30.00 g·L−1葡萄糖,20.00 g·L−1琼脂, 1 000 mL水)为供试培养基。保持其他试剂不变,分别以30.00 g·L−1葡萄糖、蔗糖、麦芽糖、乳糖、可溶性淀粉作为不同碳源,进行碳源试验;保持其他试剂不变,分别以2.00 g·L−1硝酸钾、硫酸铵、尿素、牛肉浸膏、胰蛋白胨作为不同氮源,进行氮源试验。将5 mm菌块接入培养基,27 ℃恒温培养,每个处理设置3次重复,测量方法同1.2.2,计算生长速率。
-
采用生长速率法测定不同杀菌剂对薄壳山核桃叶斑病病原菌的抑菌作用。将5种药剂加入无菌水中,按照一定比例稀释设置5个质量浓度梯度(表1),再将稀释后的制剂与融化的PDA培养基以体积比1∶99混合均匀,配置出不同质量浓度的含药培养基并计算含药培养基中原药的质量浓度,含药培养基原药质量浓度=药剂体积×药剂中有效成分质量浓度/含药培养基体积。每种药剂每个质量浓度梯度设置3个重复,设置不含药剂的PDA培养基为空白对照。将5 mm的菌块移入培养基中,27 ℃恒温培养,每个处理3次重复,培养3~7 d后,采用十字交叉法测量供试菌株的菌落直径。抑制率=(对照菌落直径—处理菌落直径)/对照菌落直径×100%。将5种药剂5个质量浓度梯度下菌落生长的抑菌率的值换算成抑制几率值,作为因变量(y),药剂质量浓度的自然对数值作为自变量(x),利用最小二乘法建立“浓度对数—几率值”直线方程[18]。用Excel和DPS软件求出相关系数(r)、抑制中浓度(EC50)及毒力回归方程(y=ax+b),比较不同药剂对病原菌的毒力效果。
病菌 稀释质量浓度/(mg·L−1) 吡唑醚菌酯 咪鲜胺 百菌清 氰烯菌酯 丙硫菌唑 共享镰刀菌 1 000.0、100.0、10.0、1.0、0.2 10.0、2.0、1.0、0.2、0.1 10.0、2.0、1.0、0.2、0.1 100.0、10.0、1.0、0.2、0.1 1 000、100、10、2、1 P. fuckelii 10 000、100、10、2、1 10.0、2.0、1.0、0.2、0.1 100、10、4、2、1 10 000、100、40、20、10 100、10、4、2、1 茶藨子葡萄座腔菌 10 000、100、10、2、1 100.0、10.0、1.0、0.2、0.1 10 000、100、10、2、1 10 000、100、40、20、10 1 000、100、10、2、1 灰葡萄孢 10 000、1 000、400、200、100 1.00、0.20、0.10、0.02、0.01 10 000、100、10、2、1 10 000、100、40、20、10 1.00、0.20、0.10、0.02、0.01 Table 1. Dilution mass concentration of reagents
-
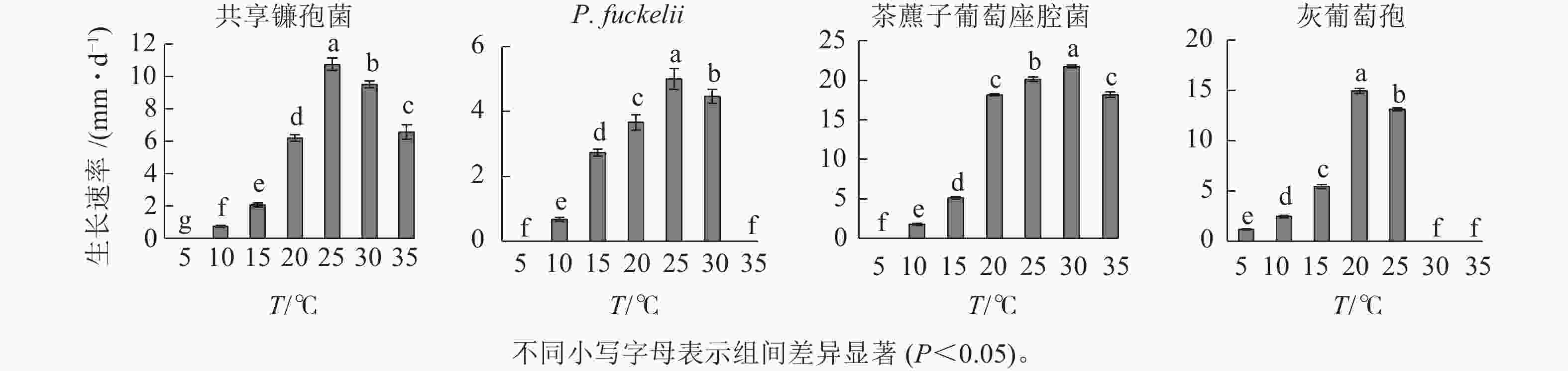
如图1所示:共享镰孢菌在10~35 ℃下均能生长,最适生长温度为25 ℃,菌落的生长速率为10.76 mm·d−1,温度在10 ℃以下时,菌落受到抑制;P. fuckelii菌丝在10~30 ℃下均能生长,最适生长温度为25 ℃,菌落的生长速率为5.00 mm·d−1,温度在5和35 ℃时生长速率为0;茶藨子葡萄座腔菌在10~35 ℃下均能生长,最适生长温度为30 ℃,菌落的生长速率为21.76 mm·d−1,温度在5 ℃及以下时生长受到抑制;灰葡萄孢在5~25 ℃下均能生长,最适生长温度为20 ℃,菌落的生长速率为14.95 mm·d−1,温度在30 ℃及以上生长受到抑制。
-
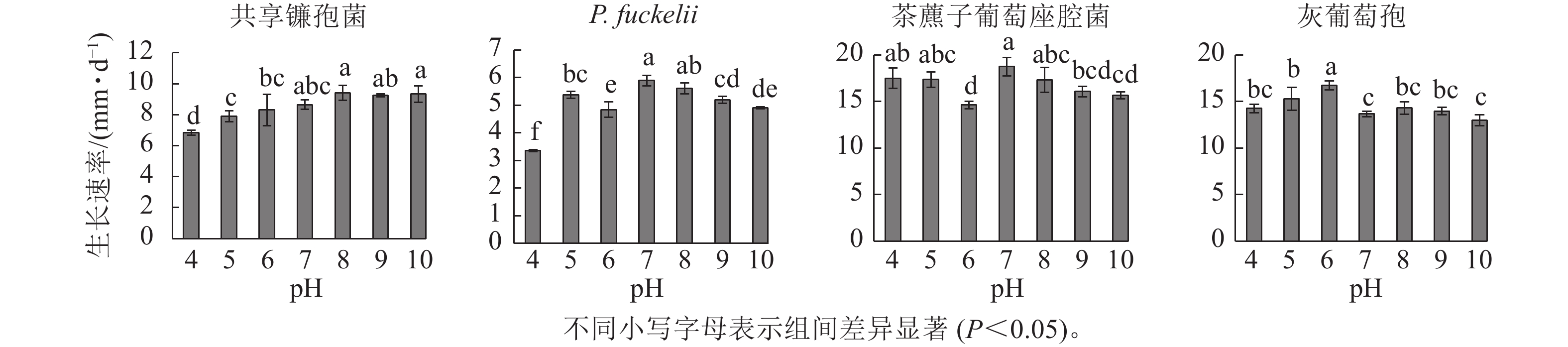
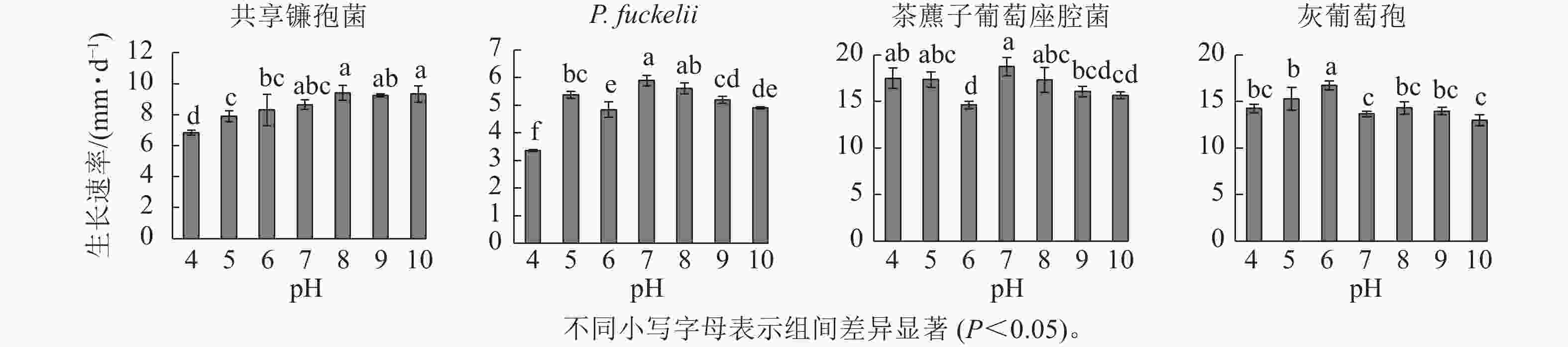
如图2所示:共享镰孢菌在pH为4.0~10.0的条件下均可生长,最适宜菌丝生长的pH为8.0,菌丝生长速率为9.40 mm·d−1,在pH 4.0的条件下,菌丝生长受限;P. fuckelii在pH 4.0~10.0的条件下均可生长,最适宜菌丝生长的pH为7.0,菌丝生长速率为5.90 mm·d−1,在pH 4.0的条件下,菌丝生长受限;茶藨子葡萄座腔菌在pH 4.0~10.0的条件下均可生长,最适宜菌丝生长的pH为7.0,该病原菌适于中性环境下生长,菌丝生长速率为18.74 mm·d−1,茶藨子葡萄座腔菌在pH 4.0~10.0的条件下无明显被抑制的现象,pH的波动对其生长影响不大;灰葡萄孢在pH 4.0~10.0的条件下均可生长,最适宜菌丝生长的pH为6.0,菌丝生长速率为10.69 mm·d−1。
-
如图3所示:共享镰孢菌在含葡萄糖的培养基上菌丝生长最快,生长速率为11.89 mm·d−1,但共享镰孢菌对这5种碳源的利用效果差异不显著;P. fuckelii对可溶性淀粉的碳源利用效果最好,菌丝生长速率为4.32 mm·d−1,P. fuckelii利用效果最差的碳源为乳糖,菌丝生长速率为2.87 mm·d−1;茶藨子葡萄座腔菌利用效果最差的碳源为麦芽糖,菌丝生长速率为8.11 mm·d−1;灰葡萄孢对可溶性淀粉的碳源利用效果最好,菌丝生长速率为10.79 mm·d−1,灰葡萄孢利用效果最差的碳源为麦芽糖,菌丝生长速率为8.7 mm·d−1。
-
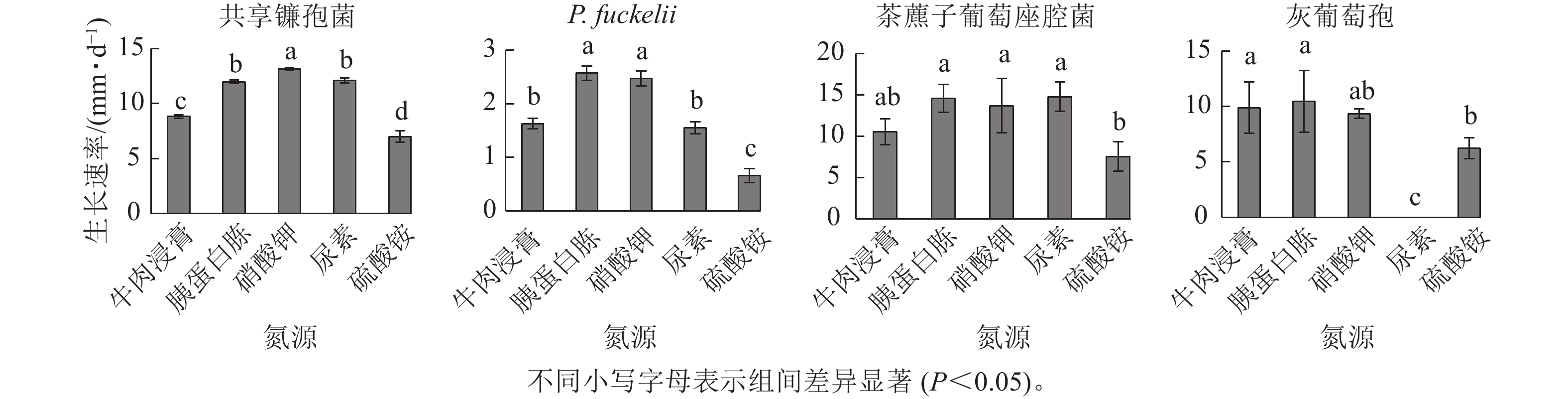
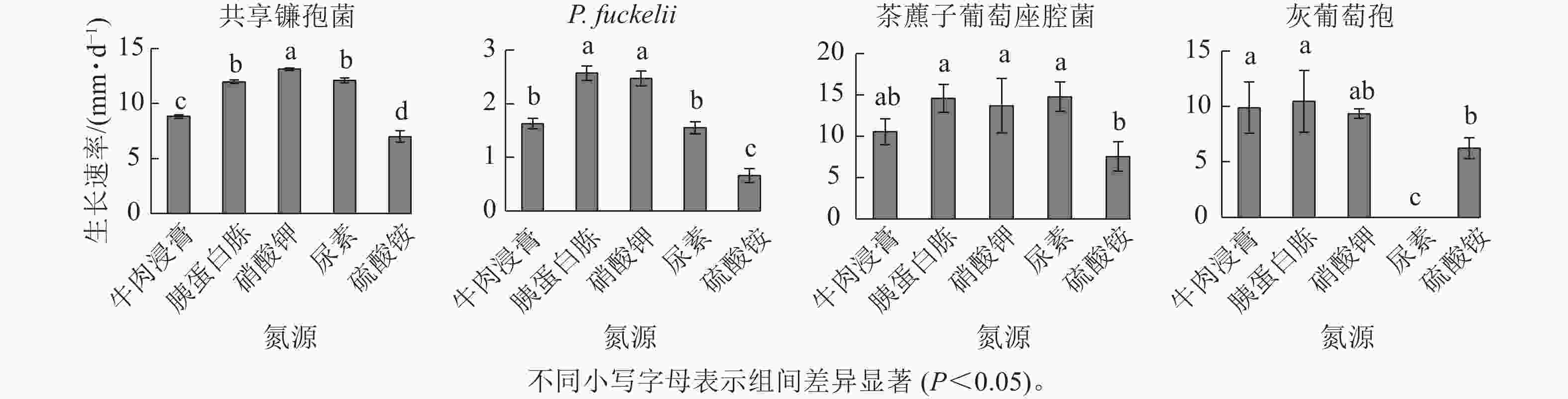
如图4所示:共享镰孢菌在5种不同氮源上均可以生长,其中利用效果最好的是硝酸钾,菌丝生长速率为13.11 mm·d−1,与其他氮源差异显著,在硫酸铵中生长最慢,菌丝生长速率为7.00 mm·d−1;P. fuckelli氮源利用效果最好的是胰蛋白胨,其菌丝生长速率为2.57 mm·d−1,在硫酸铵中生长最慢,生长速率为0.66 mm·d−1;茶藨子葡萄座腔菌氮源利用效果最好的是尿素,其菌丝生长速率为14.77 mm·d−1,利用效果最差的氮源为硫酸铵,菌丝生长速率为7.54 mm·d−1;灰葡萄孢在除尿素外的4种不同氮源上均可以生长,其中该病原菌利用效果最好的是胰蛋白胨,其菌丝生长速率为10.43 mm·d−1,与其他氮源差异显著,在尿素中该菌不生长。
-
由表2可知:5种杀菌剂对共享镰孢菌的EC50由小到大依次为咪鲜胺、氰烯菌酯、百菌清、丙硫菌唑、吡唑醚菌酯。因此,咪鲜胺对共享镰孢菌室内毒力最强,氰烯菌酯次之,吡唑醚菌酯最弱。5种杀菌剂对P. fuckelii的EC50由小到大依次为吡唑醚菌酯、咪鲜胺、丙硫菌唑、百菌清、氰烯菌酯。因此,吡唑醚菌酯对P. fuckelii室内毒力最强,咪鲜胺次之,氰烯菌酯最弱。5种杀菌剂对茶藨子葡萄座腔菌的EC50由小到大依次为咪鲜胺、吡唑醚菌酯、百菌清、丙硫菌唑、氰烯菌酯。因此,咪鲜胺对 茶藨子葡萄座腔室内毒力最强,吡唑醚菌酯次之,氰烯菌酯最弱。5种杀菌剂对灰葡萄孢的EC50由小到大依次为咪鲜胺、丙硫菌唑、百菌清、氰烯菌酯、吡唑醚菌酯。因此,咪鲜胺对灰葡萄孢室内毒力最强,丙硫菌唑次之,吡唑醚菌酯最弱。
药剂 共享镰孢菌 P. fuckelii 茶藨子葡萄座腔菌 灰葡萄孢 毒力回归
方程r EC50/
(mg·L−1)毒力回归
方程r EC50/
(mg·L−1)毒力回归
方程r EC50/
(mg·L−1)毒力回归
方程r EC50/
(mg·L−1)吡唑醚菌酯 y=0.15x+4.76 0.97 39.222 9 y=0.14x+5.34 0.99 0.0041 y=0.47x+5.20 0.90 0.367 5 y=0.45x+3.91 0.93 275.052 7 咪鲜胺 y=0.68x+6.34 0.97 0.010 8 y=0.47x+6.12 0.97 0.0043 y=0.69x+5.72 0.87 0.091 5 y=0.64x+6.07 0.97 0.021 0 百菌清 y=0.55x+4.96 0.99 1.178 9 y=0.57x+4.96 0.96 1.1676 y=0.57x+5.00 0.96 0.990 4 y=0.28x+4.74 0.98 8.238 0 氰烯菌酯 y=0.72x+5.38 0.75 0.294 7 y=0.88x+3.40 0.92 65.7059 y=0.79x+3.66 0.94 50.702 6 y=0.80x+3.34 0.95 118.136 7 丙硫菌唑 y=0.73x+4.00 0.97 23.363 3 y=0.31x+5.14 0.95 0.3582 y=0.60x+4.43 0.97 8.845 4 y=0.73x+5.64 0.98 0.134 1 说明:y为抑制几率值,x为药剂质量浓度的对数值。 Table 2. Toxicity test of 5 chemicals against 4 pathogens
-
本研究对共享镰孢菌、P. fuckelii、茶藨子葡萄座腔菌和灰葡萄孢进行生物学特性测定和室内药剂的毒力测定,探究其最适生长环境,筛选最佳防治药剂。共享镰孢菌对温度、pH等适应范围广,在10~35 ℃,pH 4.0~10.0时菌丝均能生长,其中最适温度为25 ℃,在pH 8时菌丝生长最快,此结论与曾莉莎等[19]研究结果相似。其最适碳源为葡萄糖,最适氮源为硝酸钾,与李润根等[10]的研究结果差异较大,这可能与共享镰孢菌所侵染的寄主不同有关[20]。目前对P. fuckelii生物学特性的研究尚且未见报道,本研究中P. fuckelii最适生长温度为25 ℃和最适生长pH为7.0,P. fuckelii适宜在较温暖且酸碱度呈中性的环境中生长。最适碳源为可溶性淀粉,最适氮源为胰蛋白胨。茶藨子葡萄座腔菌菌丝生长温度范围为10~35 ℃,最适生长温度为30 ℃,最适pH为7,在pH 4.0~10.0生长速率差别不大,与刘琪等[21]的研究结果一致。最适碳源为蔗糖,与李诚等[22]的研究结果相似,最适氮源为尿素,与何靖柳等[23]的研究结果差别较大,其原因可能在于菌株取自不同植物寄主,其对营养物质的利用方式略有差异。灰葡萄孢菌丝生长温度范围为5~25 ℃,最适温度为20 ℃,最适pH为6.0,与殷辉等[24]的研究结果相同。在本研究中,灰葡萄孢菌丝生长最适碳源为可溶性淀粉,最适氮源为胰蛋白胨,与来自其他寄主的灰葡萄孢生物学特性基本相同[25]。4种致病菌在以硫酸铵为氮源的培养基上生长缓慢且菌丝稀疏,生产种植上可选择用硫酸铵作为氮肥,以达到减缓病害发生的目的。
本研究5种药剂对4种致病菌抑菌效果和室内毒力作用存在差异,其中,咪鲜胺对共享镰刀菌抑菌效果最好,与周鑫钰等[26]研究结果相似。咪鲜胺作为一种咪唑类杀菌剂,其作用机制在于抑制生物体麦角甾醇的合成,破坏细胞膜功能[27],对包括镰孢属Fusarium等多种子囊菌和半知菌有显著的作用[28];对P. fuckelii来说,吡唑醚菌酯抑菌效果最好,据周英等[29]报道,25%咪鲜胺对葡萄座腔菌抑菌效果较好,其EC50为0.08 mg·L−1,与本研究结论相似;姜莉莉等[16]在对草莓灰霉病病原菌灰葡萄孢的室内药剂毒力测定中发现咪鲜胺的EC50为0.99 mg·L−1,毒力作用较强,本研究得出的结论与前者相似,咪鲜胺对灰葡萄孢抑菌效果最好,吡唑醚菌酯最弱。田间的药剂防治效果受多种因素影响,需后期进一步田间试验证实。目前有研究报道复配药剂比单剂防治效果更佳[30−31]。本研究的5种杀菌剂复配是否具有增效作用有待后续研究。
-
综上,对这4种病原菌来说,在室内温度为20~30 ℃,pH 6.0~8.0的环境中都可以生长得较好。这4种病原菌在以葡萄糖和可溶性淀粉为碳源,以尿素、硝酸钾和胰蛋白胨为氮源的环境下生长旺盛。咪鲜胺的杀菌效果在5种杀菌剂中最好,EC50均在0.10 mg·L−1以下,对病原菌具有很强的毒力作用,因此咪鲜胺是防治薄壳山核桃叶斑病的首选药剂,另外,吡唑醚菌酯也具有较好的抑菌作用。在今后的薄壳山核桃叶斑病防治中,为了防止长期使用单一药剂产生抗药性,可交替或混合使用咪鲜胺与吡唑醚菌酯,以达到良好的防治效果。
Biological characteristics and toxicity test of the pathogen of Carya illinoinensis leaf spot
doi: 10.11833/j.issn.2095-0756.20230029
- Received Date: 2023-02-01
- Accepted Date: 2023-06-25
- Rev Recd Date: 2023-06-19
- Available Online: 2023-09-26
- Publish Date: 2023-09-26
-
Key words:
- Carya illinoinensis /
- pathogen of leaf spot /
- biological characteristics /
- virulence test
Abstract:
| Citation: | ZHOU Jielu, WU Tianhao, JU Yunwei, et al. Biological characteristics and toxicity test of the pathogen of Carya illinoinensis leaf spot[J]. Journal of Zhejiang A&F University, 2023, 40(5): 1018-1025. DOI: 10.11833/j.issn.2095-0756.20230029 |











 DownLoad:
DownLoad: