-
兰科植物Orchidaceae是被子植物中仅次于菊科Asteraceae的第二大科,约800属28 000种,广泛分布于热带和温带地区[1−2]。兰科植物进化水平高,拥有丰富的物种多样性、高度特化的花结构,生存繁衍对生态系统依赖程度高,是研究生态学和分子生物学的重要材料[3−5]。且兰科植物观赏价值极高,具有药理作用,有较高的经济价值,市场需求量大[6]。萼脊蝴蝶兰Phalaenopsis japonica曾被命名为萼脊兰Sedirea japonica,为萼脊兰属Sedirea,近年来根据分子生物学数据萼脊兰属被并入蝴蝶兰属Phalaenopsis [7]。产于中国浙江文成和云南盈江,及日本琉球群岛和朝鲜半岛南部,生于海拔600~1 350 m的疏林树干和山谷崖壁上[8]。按照濒危等级,萼脊蝴蝶兰被世界自然保护联盟(IUCN)评定为易危(VN),列入濒危野生动植物种国际贸易公约(CITES)附录Ⅱ。其植株较小,叶片革质,总状花序,花期4月中旬至5月底,花形似蝴蝶,有香味,中萼片和花瓣呈淡黄绿色,侧萼片和唇瓣白紫相间,是蝴蝶兰Phalaenopsis aphrodite良好的育种材料。 KIM等[9]将萼脊蝴蝶兰与29个不同品种蝴蝶兰互为父母本进行杂交,得到3个萼脊蝴蝶兰做母本的杂交组合的后代,表型更接近蝴蝶兰。中国对萼脊蝴蝶兰组培快繁、分子生物学等进行了研究[10−12],然而萼脊蝴蝶兰开花进程观察和繁育系统的研究仍欠缺。
繁育系统是指所有影响后代遗传组成的有性特征总和,主要包含花器官特征和开花进程、花粉活力与柱头可授性、花粉组织化学、杂交指数、人工控制授粉等方面[13]。前人对喇叭唇石斛Dendrobium lituiflorum、长瓣兜兰Paphiopedilum dianthum等植物的繁育系统研究[14−17],为研究萼脊蝴蝶兰开花进程与繁育系统提供方法学基础。SUETSUGU等[18]通过观察自然条件下的萼脊蝴蝶兰,发现其授粉成功率仅为7.7%,认为萼脊蝴蝶兰可能无法自发进行自花授粉。本研究以温室栽培条件下的萼脊蝴蝶兰植株为研究对象,记录其开花进程,进行人工控制授粉,测定分析其繁育系统,为人工授粉和杂交育种提供依据,以期更好地保护和利用种质资源。
-
萼脊蝴蝶兰由野外采集的果实经无菌播种培育而成,随机选出长势良好的100株4年生萼脊蝴蝶兰。试验在中国农业科学院蔬菜花卉研究所无土栽培温室进行,常年保持温度26~30 ℃。春秋两季开天窗通风,夏季开风机配合水帘降温,冬季暖气供暖。全温室附60%遮阳网,夏季额外再附1层60%遮阳网。夏季每周浇水2次,春秋冬每周浇水1次。
-
随机选取10株,统计单株花序数与单花序花数。随机选取盛花期全天开放的花10朵,用直尺和游标卡尺测量植株性状(株高、最大叶长/宽/厚、冠幅、花序长/粗)和花部性状(中萼片长/宽、侧萼片长/宽、花瓣长/宽、唇瓣长/宽、合蕊柱长/宽、子房长/宽、药帽直径)。并在体视显微镜(Olympus SZX7)下观察花部特征形态结构,观察盛花期成熟花粉团,用扫描电子显微镜(Hitachi S-4800)进行拍照。具体步骤:取样后立即将花粉团投入FAA固定液中(100 mL FAA:体积分数为50%的乙醇 90 mL、冰乙酸 5 mL、甲醛 5 mL),上下颠倒保证花粉团完全浸入FAA固定液,4 ℃固定24 h,随后对其进行梯度乙醇脱水(体积分数分别为70%、80%、90%、95%、100%的乙醇各处理15 min),更换100%乙醇放入冰盒等待干燥。用二氧化碳临界点干燥仪(Leica CPD300)干燥5 h,将样品待观察部位朝上放置在有双面胶的金属台上,用洗耳球轻吹使其粘牢,用离子溅射仪(Hitachi E-1010)对金属台喷金镀膜后,进行扫描电镜观察。
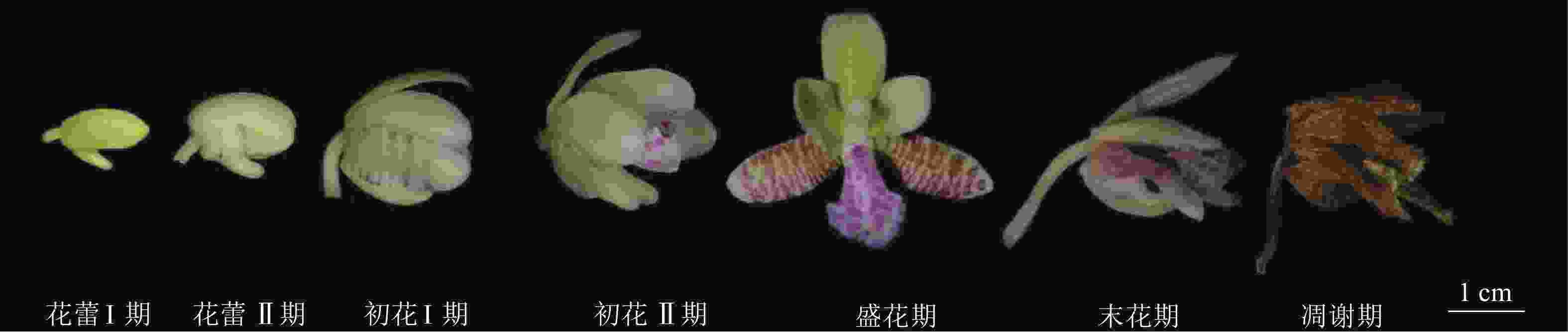
随机选取10 株萼脊蝴蝶兰,观察记录从花蕾显现至枯萎脱落的开花进程。单花花期为花瓣从两侧打开至凋谢的日期,单株花期为单个植株上第1朵花花瓣从两侧开放起至最后1朵花凋谢截止的日期,群体花期为群体内第1朵花花瓣从两侧打开起至群体内最后1朵花凋谢截止的日期。并依据DAFNI标准[19],用开花尺度和开花特征将开花进程细分为花蕾Ⅰ期、花蕾Ⅱ期、初花Ⅰ期、初花Ⅱ期、盛花期、末花期、凋谢期。
-
在晴天上午采集不同开花时期的花粉团,将花粉团置于载玻片上滴加2滴亚历山大染色液[20],用镊子和解剖针将花粉团分为若干花粉块,立即盖上盖玻片,并在其上方附1层滤纸,用带橡皮铅笔的橡皮端用力按压盖玻片,将花粉块压为单细胞层,染色5 min,用研究级正置显微镜(Olympus BX53)统计花粉活力。以FAA固定液浸泡24 h的花粉团作为对照,在显微镜下无活力的花粉细胞为绿色或淡粉色,有活力则为紫红色。每个开花时期取不同株3个花粉团,制片后取3个视野,统计有活力花粉细胞数和总花粉细胞数(每个视野内至少有50个花粉细胞)。花粉萌发率=萌发的花粉数/视野内总花粉数×100%。
采用联苯胺-过氧化氢法[21]测定萼脊蝴蝶兰柱头可授性。每个开花时期取3个柱头进行试验,将其浸入联苯胺-过氧化氢反应液(1%联苯胺∶3%过氧化氢∶水的体积比4∶11∶22)中,用体视显微镜(Olympus SZX7)观察并拍照。依据柱头周围是否有气泡出现判别其可授性,气泡越多表明可授性越好。
-
随机采取3个盛花期成熟花粉团,将每个花粉团分别置于载玻片上滴加2滴碘-碘化钾染色液[22],用镊子和解剖针将花粉团分为若干花粉块,立即盖上盖玻片,并在其上方附1层滤纸,用带橡皮铅笔的橡皮端用力按压盖玻片,将花粉块压为单细胞层,染色5 min,用研究级正置显微镜(Olympus BX53)观察花粉细胞染色情况。花粉细胞呈黄橘红色,表示其中含有脂质;呈蓝紫黑色,表示其中含有淀粉。
-
依据DAFNI标准[19],选取10株萼脊蝴蝶兰,测定指标并计算杂交指数。花直径<1 mm记为0,1~2 mm记为1,2~6 mm记为2,>6 mm则记为3;花药开裂时间和柱头可授粉期同时或雌蕊先熟记为0,雄蕊先熟记为1;柱头和花药处于同一空间位置记为0,空间分离记为1。三者之和为杂交指数(OCI)。评判标准:OCI为0表示闭花受精型;1表示专性自交型;2表示兼性自交型,有异交可能;3表示兼性异交型,有时需要传粉者;≥4表示异交型,部分自交亲和,多数需要传粉者。
-
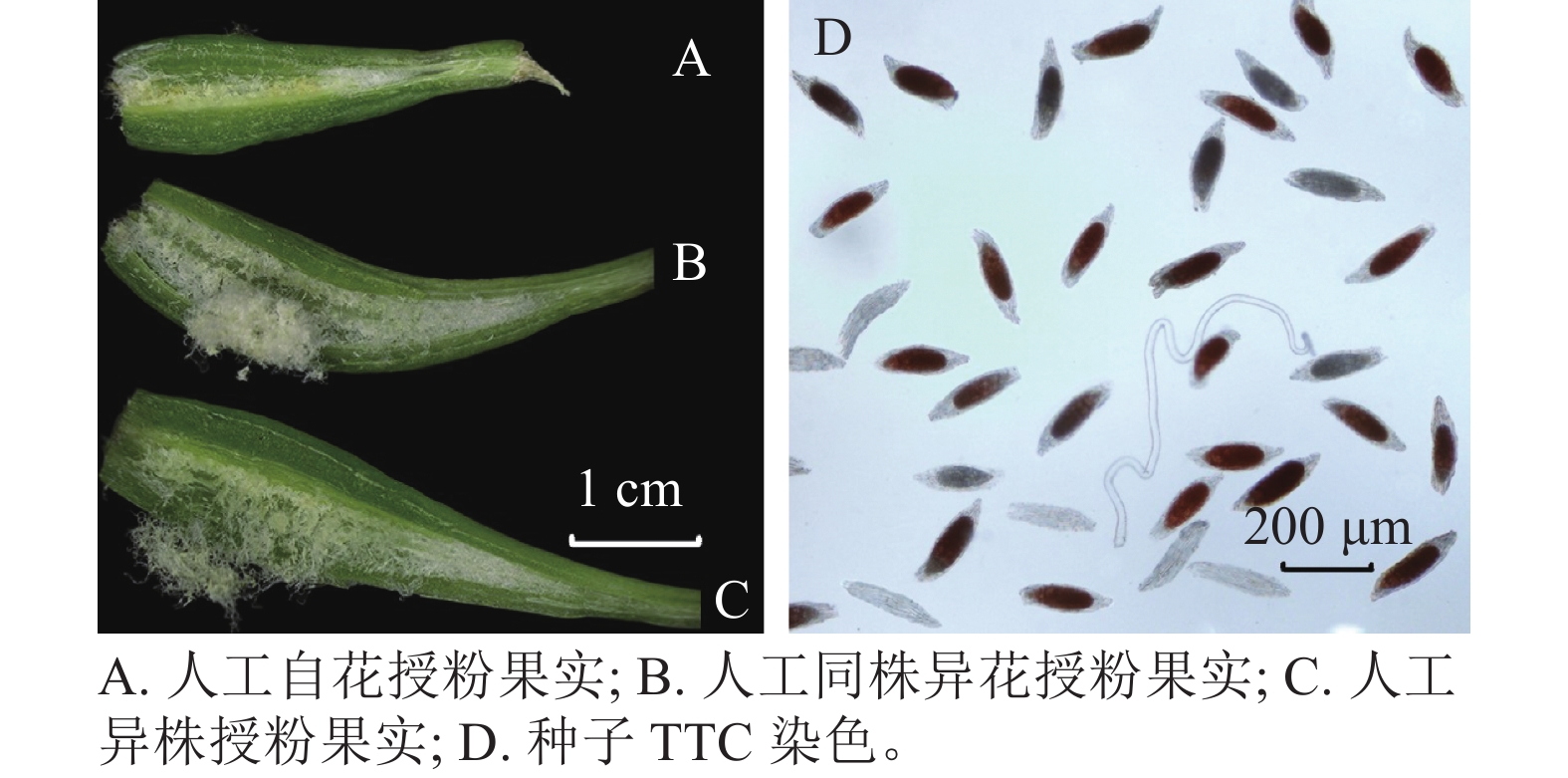
设置6种授粉方式:自然授粉(不去雄且不套袋)、自然自花授粉(不去雄并套袋)、去雄不授粉、人工自花授粉、人工同株异花授粉、人工异株授粉。每种授粉方式选择10朵初花Ⅱ期的花,授粉前后3 d不浇水,在晴天上午完成各处理,选不同植株重复3次。授粉成功后约7 d花朵凋谢,14 d子房膨大。授粉后100 d,果实成熟,统计结实率,并采摘。结实率=果实数/授粉朵数×100%。
每个授粉方式取3个果实测量各指标。用游标卡尺测量不同授粉方式得到的果实长和宽。将果实用手术刀剖开,把种子拨入2.0 mL离心管内,加蒸馏水1.5 mL,震荡后静置待种子沉底,挑出上层棉絮状物质,并将水吸出。向离心管中注入2.0 mL质量分数2.5%次氯酸钠溶液,常温浸泡75 min,用蒸馏水冲洗3次,再注入2.0 mL蒸馏水留在管中,室温浸泡24 h。用质量分数1% TTC溶液替换蒸馏水,避光浸泡24 h。吸出TTC溶液,蒸馏水冲洗3次,再注入1.0 mL蒸馏水,得到TTC染色后的种子悬浮液。用已剪枪头吸取悬浮液0.1 mL,制片用体视显微镜(Olympus SZX7)观察,种胚为红色即为有活力[23],在10倍镜下随机选取3个视野统计有活力种子数和种子总数,估算种子活力,种子活力=有活力种子数/种子总数。统计0.1 mL所有种子数,重复3次,并按比例尺测量种子长和宽,单果实种子总数=0.1 mL所有种子数×10。
-
利用Excel 2016绘制表格,SPSS 25.0处理数据,Photoshop 2021制图。
-
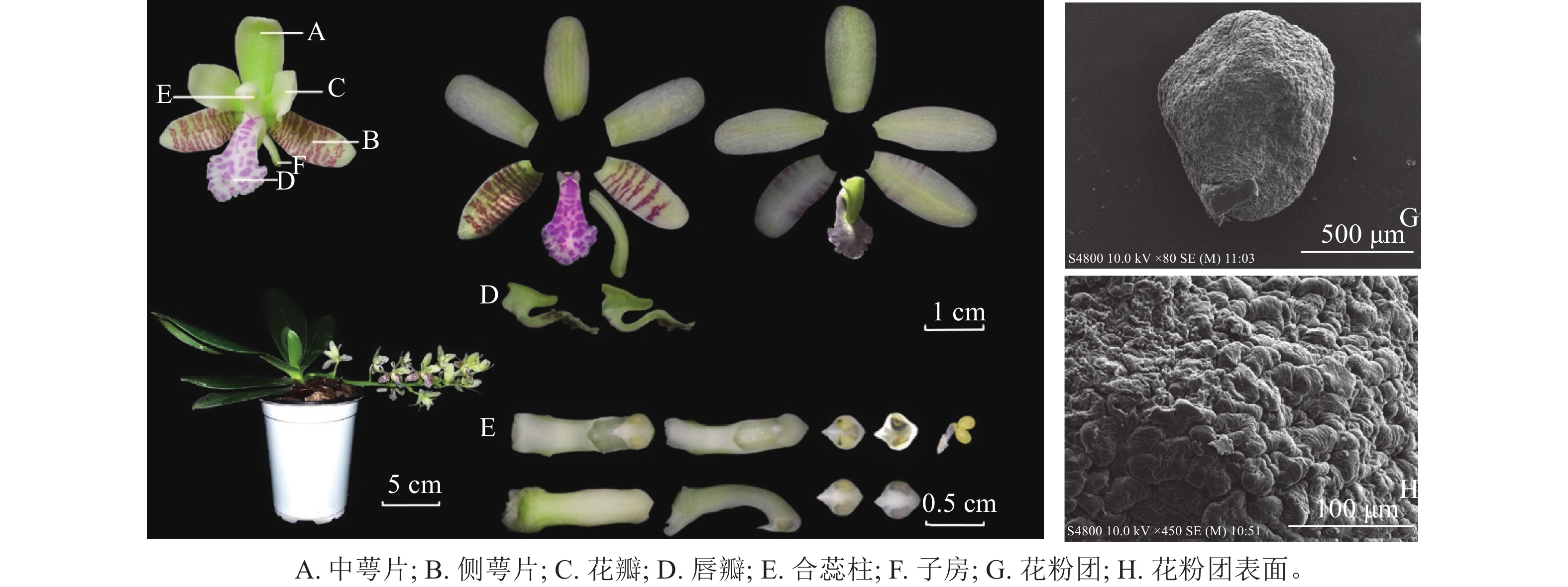
萼脊蝴蝶兰株高13.71 cm,冠幅24.01 cm;单株多为1~2个花序,单花序约10朵花。花由1枚中萼片、2枚侧萼片、2枚花瓣、1枚唇瓣、1个合蕊柱、1个子房组成(图1)。茎短,被宿存叶鞘包裹,叶4~6枚,叶片革质呈长圆倒卵状披针形,先端钝且有稍不等侧2裂,最大叶长13.76 cm、宽3.59 cm。总状花序微下垂,花序长12.61 cm;花苞片淡褐色,宽卵形,凹的,先端钝;萼脊蝴蝶兰呈淡黄绿色,侧萼片表面具褐紫色横向不规则条纹,唇瓣表面具粉紫色不规则斑纹,花具有香气。中萼片长圆形,先端钝,中萼片长14.27 mm、宽6.55 mm;侧萼片比中萼片稍窄,侧萼片长14.64 mm、宽6.46 mm;花瓣长圆状舌形,先端钝,花瓣长12.64 mm、宽6.81 mm;唇瓣3裂,唇瓣长14.91 mm、宽8.92 mm,侧裂片很小,近三角形,中裂片大,匙形,从基部向先端扩大为卵圆形的前部,边缘具不规则的圆齿,正面凹下而背面隆起,浅绿色距从唇瓣根部延伸,末端指向唇瓣中裂片,月牙形,内部光滑无毛,底端具有少量密状物质;合蕊柱淡黄绿色,细圆柱形,合蕊柱长13.01 mm、宽2.58 mm,药帽前端收窄成喙状,顶端增厚成短凸,直径3.31 mm;子房长8.52 mm,淡黄绿色,下位子房。通过电子扫描显微镜观察,萼脊蝴蝶兰花粉团表面不平整,有极多凸起,是由数万个花粉细胞紧密聚集而形成的(图1)。
-
萼脊蝴蝶兰从4月始零星开放,群体花期4中旬至5月底,单花花期约30 d,单株花期约35 d。依据DAFNI标准,将开花进程划分为7个阶段(图2)。①花蕾Ⅰ期(开花前4~7 d):花蕾未展开,整体呈不规则椭圆形,萼片和花瓣紧闭,呈淡黄绿色,可见距。②花蕾Ⅱ期(开花前1~4 d):花蕾变大,准备开放,萼片和花瓣微微分离,侧萼片根部至中部有紫色花纹显现。花蕾长大于1.0 cm,宽大于0.5 cm。③初花Ⅰ期(开花后1~3 d):花蕾初步开放,萼片和花瓣分离,花直径大于1.5 cm,白紫色唇瓣露出,合蕊柱被唇瓣包裹在内,紫色花纹继续在整个侧萼片延伸。④初花Ⅱ期(开花后4~5 d):花蕾进一步开放,中萼片向斜上方竖起,侧萼片紫色花纹更加明显,花瓣向外延展,唇瓣展开露出合蕊柱,花直径大于2.5 cm。⑤盛花期(开花后6~25 d):花蕾完全开放,中萼片直立,侧萼片完全展开,在内面具紫色横向不规则条纹,花瓣向前延伸,白紫相间唇瓣向内隆起,可见合蕊柱顶端明显药帽和中部具黏液柱头腔。⑥末花期(开花后26~30 d):花逐步失水,由淡黄绿色变为淡黄白色,但仍可见紫色花纹,花器官均卷缩。⑦凋谢期(开花后30~40 d):花完全失水,呈褐色,自然掉落。
-
萼脊蝴蝶兰花粉在花蕾期就具有一定活力,并随着开花进程,花粉活力在盛花期达到最大值84.98% (表1)。显微镜观察发现:萼脊蝴蝶兰花粉为近圆形四合花粉(图3A),花粉被碘-碘化钾染为黄橘红色(图3B),说明萼脊蝴蝶兰花粉主要由脂质组成。
时期 花粉活力/% 柱头可授性 花蕾Ⅰ期 12.80±0.53 f − 花蕾Ⅱ期 28.23±0.52 d +/− 初花Ⅰ期 46.19±0.56 c + 初花Ⅱ期 74.13±0.91 b ++ 盛花期 84.98±0.95 a ++ 末花期 22.14±0.59 e − 凋谢期 0±0 g − 说明:数值为平均值±标准误。不同小写字母表示不同时期花粉活力之间差异显著(P<0.05)。−表示柱头不具可授性,+/−指部分柱头具可授性,+表示柱头具可授性,++表示柱头可授性强。 Table 1. Test of pollen viability and stigma acceptability
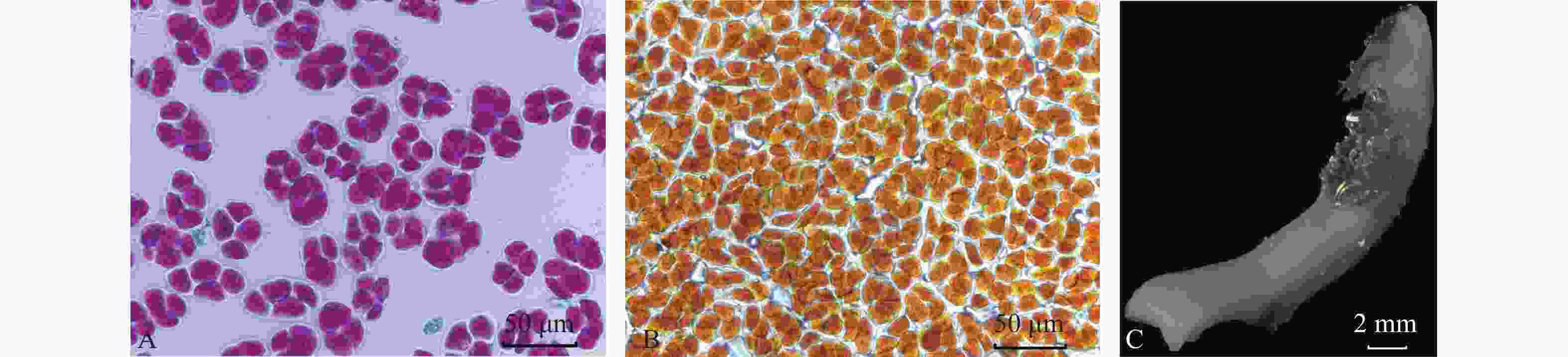
Figure 3. Alexander staining (A) and iodide-potassium iodide staining (B) of pollen viability, and benzidine- hydrogen peroxide method of stigma acceptability test (C)
萼脊蝴蝶兰初花Ⅱ期和盛花期时的柱头表面均变蓝且有大量气泡产生(图3C),表明此阶段可授性最强。从表1可以看出,萼脊蝴蝶兰雌雄同熟,在盛花期花粉活力最强,柱头可授性最强,因此在人工授粉时,应选择盛花期的花朵异株自交。
-
萼脊蝴蝶兰花直径约为3 cm (>6 mm),记为3;花粉和柱头同步成熟,可同期授粉,记为0;花粉位于合蕊柱顶端,与柱头空间分离,且花粉与柱头腔间的蕊喙发育,阻止自交授粉发生,记为1。由此得出萼脊蝴蝶兰杂交指数为4,其繁育系统是异交型,部分自交亲和,需要传粉者。
-
由表2可见:自然授粉、自然自花授粉、去雄不授粉的结实率均为0,人工自花授粉、人工同株异花收粉、人工异株授粉结实率分别为60.00%、80.00%、93.33%,均远高于前3种处理。说明萼脊蝴蝶兰繁育系统是需要传粉者传粉的且自交和异交混合的繁育系统,不存在无融合生殖现象。
处理方式 处理花数/朵 结实数/粒 结实率/% 自然授粉 30 0 0 c 自然自花授粉 30 0 0 c 去雄不授粉 30 0 0 c 人工自花授粉 30 18 60.00±1.15 b 人工同株异花授粉 30 24 80.00±0.58 ab 人工异株授粉 30 28 93.33±0.67 a 说明:数值为平均值±标准误;不同小写字母表示不同处理下结实率之间差异显著(P<0.05)。 Table 2. Percentage of fertile fruit in different pollination treatments
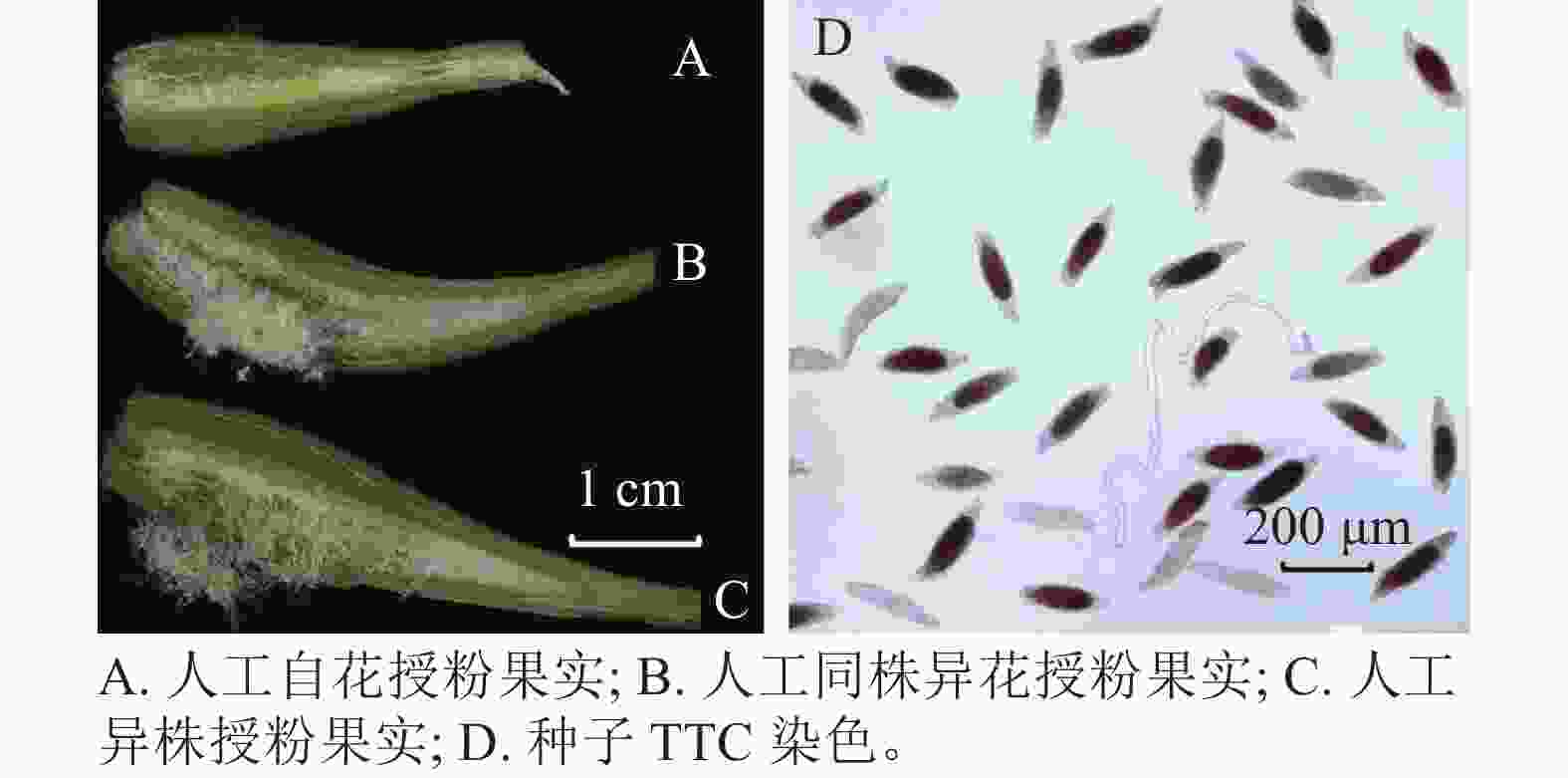
萼脊蝴蝶兰授粉成功后,除合蕊柱和子房外,各花器官会在5 d内迅速枯萎,寿命远短于正常花期。授粉后3 d内柱头腔逐渐向内闭合至包裹花粉,约7 d可发现子房开始膨大。授粉后100 d,果实成熟,果实绿色油亮。萼脊蝴蝶兰的种子为粉末状,且与棉絮状物质共同存在于果实中,种子没有胚乳,只有种皮和种胚。由图4和表3可见:人工自花授粉果实较小(图4A),且种子活力与种子数均较低;人工同株异花授粉果实大小其次(图4B);而人工异株授粉果实不仅果实体积大(图4C),其种子大小、种子活力(图4D)、种子数(单果实中种子数量约43 000粒)均最高。由此可得出人工异株授粉得到的果实质量最高。
处理方式 果实长/cm 果实宽/cm 种子长/μm 种子宽/μm 种子活力/% 单果实种子总数/粒 人工自花授粉果实 2.33±0.03 c 0.46±0.02 b 135.87±16.73 b 55.58±4.76 a 50.49±0.78 c 3 000±360 c 人工同株异花授粉果实 2.87±0.09 b 0.60±0.04 a 177.45±4.32 a 49.4±3.98 a 65.64±1.79 b 21 000±1 700 b 人工异株授粉果实 3.50±0.17 a 0.67±0.02 a 189.55±3.18 a 62.08±5.16 a 82.69±3.46 a 43 000±1 800 a 说明:数值为平均值±标准误;不同小写字母表示不同处理下结实率之间差异显著(P<0.05)。 Table 3. Phenotype of fruit obtained under the different pollination treatments
-
萼脊蝴蝶兰植株多为1~2个花序,单花序约有10朵花。花由1枚中萼片、2枚侧萼片、2枚花瓣、1枚唇瓣、1个合蕊柱、1个子房组成。单花花期约30 d,单株花期30~40 d,群体花期约45 d,其中单花盛花期为花朵展开后的6~25 d。侧萼片和唇瓣内面白紫相间的横向不规则条纹,与上萼片和花瓣的淡黄绿色形成鲜明对比。通过解剖位于萼脊蝴蝶兰唇瓣后的距,可发现其内部底端中具有少量蜜状物质。这与SUETSUGU等[18]研究相同,他们认定熊蜂属Bombus昆虫是萼脊蝴蝶兰的主要授粉者之一,并得出距内蜜状奖励物质含量与虫媒授粉成功率呈正相关关系,且花距长度与传粉者嘴部长度相关。萼脊蝴蝶兰花粉为四合花粉,即减数分裂形成的4个小孢子不分开而是结合在一起继续发育成成熟花粉。通过碘-碘化钾染色,萼脊蝴蝶兰花粉呈黄橘红色,说明萼脊蝴蝶兰花粉主要由脂质组成。通过扫描电镜对花粉团的观察,发现花粉是由多个花粉细胞紧密聚集而成,这种组成结构相对于由淀粉组成的风媒授粉植株的花粉而言,质量较大,富含营养物质较多。由此确定萼脊蝴蝶兰为虫媒授粉植株[24]
植物能否成功授粉取决于花粉活力与柱头可授性的高低。本研究通过对比不同花粉活力检测方法,得出亚历山大染色法是最适合检测萼脊蝴蝶兰花粉活力的方法。TTC染色法无法明显区别萼脊蝴蝶兰花粉活力,可能是因为萼脊蝴蝶兰对TTC染液不敏感所致,但TTC却能将长瓣兜兰[15]花粉染色,同样,醋酸洋红染色法有活力花粉和无活力花粉颜色差别不大,可能是TTC和醋酸洋红对兰科不同属间花粉的染色度不同所导致;花粉离体萌发法操作较复杂,本研究未采用此方法。本研究发现:萼脊蝴蝶兰花粉与柱头同期成熟,并且活力均先上升后下降,在盛花期达最高,这与细茎石斛Dendrobium moniliforme、葱叶兰Microtis unifolia、镰翅羊耳蒜Liparis bootanensis 等植物相似[25]。值得注意的是,萼脊蝴蝶兰的柱头在初花Ⅱ期和盛花期都具有较高的可授性,后期若进行萼脊蝴蝶兰人工授粉,可选用成熟的花粉对初花Ⅱ期的柱头进行授粉,使植株较早坐果。
在杂交指数估算时,不能以萼脊蝴蝶兰合蕊柱上花粉团与柱头的空间分布判断杂交指数。应着重观察萼脊蝴蝶兰花粉团与柱头腔间的蕊喙是否发育,以此判断植物是否能发生自交授粉。萼脊蝴蝶兰杂交指数为4,说明萼脊蝴蝶兰繁育系统是需要传粉者传粉的且自交和异交混合的繁育系统。
萼脊蝴蝶兰自然授粉、自然自花授粉、去雄不授粉3种处理结实率均为0,说明萼脊蝴蝶兰不能自主自花授粉,需要媒介授粉才能结实,因为其花粉均位于合蕊柱顶端,与柱头空间分离,但温室里没有能为其传粉的昆虫。人工自花授粉、人工同株异花授粉、人工异株授粉结实率分别为60.00%、80.00%、93.33%,且人工异株授粉果实的大小、含种子量、种子活力均高于人工自花授粉果实和人工同株异花授粉果实。这符合自然界杂种优势[26]。
-
萼脊蝴蝶兰为总状花序,单花花期约30 d,群体花期为4月中旬至5月底,开放时有香味。雌雄同株,且同步成熟,在盛花期(开花6~25 d )花粉活力与柱头可授性均达到最强,花粉活力高达84.98%。萼脊蝴蝶兰花粉为近圆形四合花粉,主要由脂质组成,适合虫媒授粉。杂交指数为4,其繁育系统是需要传粉者自交和异交混合的交配系统。人工异株授粉的萼脊蝴蝶兰结实率最高(93.33%),且果实质量最好,单果实中种子数量最大(约43 000粒),种子活力最高(82.69%)。
Flowering characteristics and breeding system of Phalaenopsis japonica
doi: 10.11833/j.issn.2095-0756.20230146
- Received Date: 2023-02-10
- Accepted Date: 2023-05-17
- Rev Recd Date: 2023-05-11
- Available Online: 2023-11-23
- Publish Date: 2023-11-23
-
Key words:
- Phalaenopsis japonica /
- breeding system /
- pollen viability /
- stigma acceptability /
- hybridization index (OCI)
Abstract:
| Citation: | CHEN Zhiguang, XU Yan, GE Hong, et al. Flowering characteristics and breeding system of Phalaenopsis japonica[J]. Journal of Zhejiang A&F University, 2023, 40(6): 1197-1204. DOI: 10.11833/j.issn.2095-0756.20230146 |









 DownLoad:
DownLoad: