-
三叶青Tetrastigma hemsleyanum为葡萄科Vitaceae 崖爬藤属Tetrastigma三叶崖爬藤,全株均具有药用价值[1],多以块根入药,味微苦,性平,具有清热解毒、消肿止痛、化痰散结等功效[2−4]。三叶青中的主要活性成分为酚类和黄酮类物质,在植物中不仅表现出各种生理和药理活性,包括抗氧化[5]、抗炎[6]和公认的抗癌活性[7−8],并且具有防御功能,可以保护植物免受紫外线的伤害[9]。
高剂量的紫外线辐射会诱导产生活性氧(ROS),进而损害多种细胞器和大分子物质(如DNA和光合蛋白),破坏光合功能,导致生长减缓[10−11]。但近年来的研究显示:紫外辐射可以诱导植物加速次生代谢物的合成进程[12−14],例如,紫外线促进铁线莲Clematis terniflora[15]、黄花蒿Artemisia annua[16]、黄连Coptis chinensis[17]等植物合成酚类和黄酮类化合物,从而降低紫外辐射的伤害。类黄酮质量分数与中波紫外线(UV-B)的持续辐射时间有关[18],例如,BAI等[19]研究发现:适当的UV-B和短波紫外线(UV-C)辐射30~180 min 可提高三叶青中黄酮类成分质量分数,黄酮类物质的种类和质量分数差异还与辐射时间有关。同时,UV-B辐射也有利于植物抗逆能力的增加,如GAO等[20]研究发现:低剂量UV-B辐射能提高植物抗逆酶活性,LÜ等[21]发现:经UV-B处理后类黄酮生物合成酶基因(PAL、C4H、4CL、CHS1和DTX41)和抗逆性基因(MYB、WRKY、APX3和EX2)表达水平均上调。此外,适当的黑暗处理并施加UV-B辐射对植物有积极影响,如处理后的茶树Camellia sinensis[22]可显著增加叶片中游离氨基酸质量分数,降低可溶性蛋白质量分数,处理后的苹果Malus pumila[23]可增加体内有效成分质量分数。但是,UV-B辐射及黑暗处理对三叶青次生代谢的调控和有效成分影响还不清晰。
本研究探究UV-B辐射及黑暗补充处理对三叶青总酚和总黄酮类物质质量分数、抗氧化能力、抗逆酶活性及相关基因表达的影响,以期为有效利用环境因素提高三叶青药用价值提供新的方法。
-
选取3年生三叶青成苗(购自浙江省丽水市遂昌县种植基地)作为研究材料,经浙江农林大学周爱存博士鉴定为三叶青。使用盆栽(直径为20 cm)方式种植于浙江省中药资源保护与创新重点实验室试验基地(30°15′30.39″N, 119°43′26.92″E)。单株种植,种植所用土壤购自杭州锦海农业有限公司,隔1 d浇水1次,浇水量为400 mL,缓苗2个月后于2022年5—10月进行取样。
选取生长健壮、无病虫害、苗高大小近似的三叶青苗,随机分为6组,分别移入置物架中,由不透光黑布完全覆盖,置物架中配置40 W的UV-B (280~320 nm)灯管(南京华强电子有限公司),UV-B辐射强度为10 W·m−2。持续辐射12 h并分别选取0、0.5、1.0、3.0、6.0和12.0 h 共6个时间节点观察表型。另取三叶青苗5组,以未经过UV-B辐射的植株作为对照(ck),分为2种处理(UV-B处理和UV-B+暗处理)进行,即经过UV-B辐射1.0、3.0 h后,一部分立即取样(处理代号分别为T1和T3),另一部分转移进行暗处理至完整处理时间为24.0 h (代号分别为T1+23和T3+21)。处理结束后取样,放入密封袋,立即液氮中冷冻并超低温冰箱保存(−80 ℃),备用。
-
精密称取新鲜三叶青块根和叶片各1 g,参考韩敏琪[24]的方法制备提取液;参考AL-KHAYRI等[25]的方法测定总酚质量分数;参考BAI等[19]的方法测定总黄酮质量分数。
-
取1.2中提取液,参考刘希达等[26]的方法测定三叶青叶片及块根中1,1-二苯基-2-三硝基苯肼(DPPH)清除率,计算抗氧化能力。
-
参考李世玉等[27]的方法测定过氧化氢酶(CAT)、过氧化物酶(POD)、超氧化物歧化酶(SOD)活性及丙二醛(MDA)质量摩尔浓度。
-
收集ck、T1、T1+23、T3和T3+21处理叶片样本在液氮中速冻。样品在−80 ℃的冰箱中保存,24 h后送至南京派森诺基因技术有限公司进行RNA提取和转录组测序。通过Oligo (dT)富集mRNA,通过Agilent 2100 Bioanalyzer对文库进行质检。样品经过RNA抽提、纯化、建库之后,采用第2代测序技术(next-generation sequencing,NGS),基于Illumina测序平台,进行双末端(paired-end,PE)测序。对原始下机数据中带接头、低质量的读长(reads)进行过滤,以获得去除接头和低质量reads后的数据(clean reads),并组装成非冗余序列(unigene),计算每个样品的unigene表达水平。对于多个样品,根据要求检测样品间的差异表达基因,并对差异表达基因进行深入的聚类和功能富集分析(|log2CF|≥2,P<0.05。CF为差异倍数)。
-
利用Origin 2021和SPSS 27计算平均值±标准差,运用单因素方差分析法(one-way ANOVA)和最小显著性差异法(LSD)进行方差分析和多重比较,用Origin 2021进行皮尔逊相关性分析,利用TB tools进行热图分析。
-
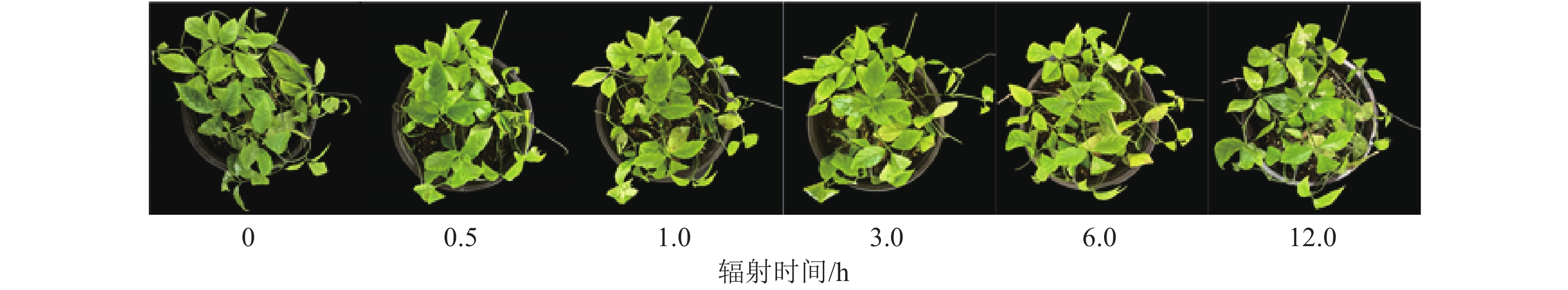
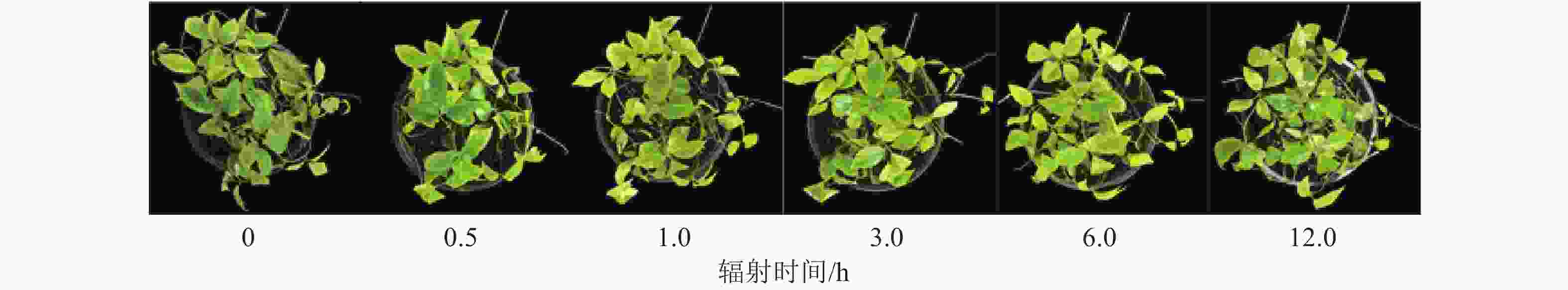
UV-B持续辐射下,三叶青苗的生长情况是反映其对胁迫响应最直观的指标。与对照组相比,辐射时长为0.5和1.0 h的三叶青苗并没有明显的外观差异,辐射3.0 h的叶片有局部发黄,辐射6.0和12.0 h的三叶青叶片有明显发黄和略微卷曲(图1)。因此本研究选用紫外光UV-B辐射1.0和3.0 h及补充黑暗处理至24.0 h的三叶青进行酚类化合物质量分数及抗氧化能力变化测定。
-
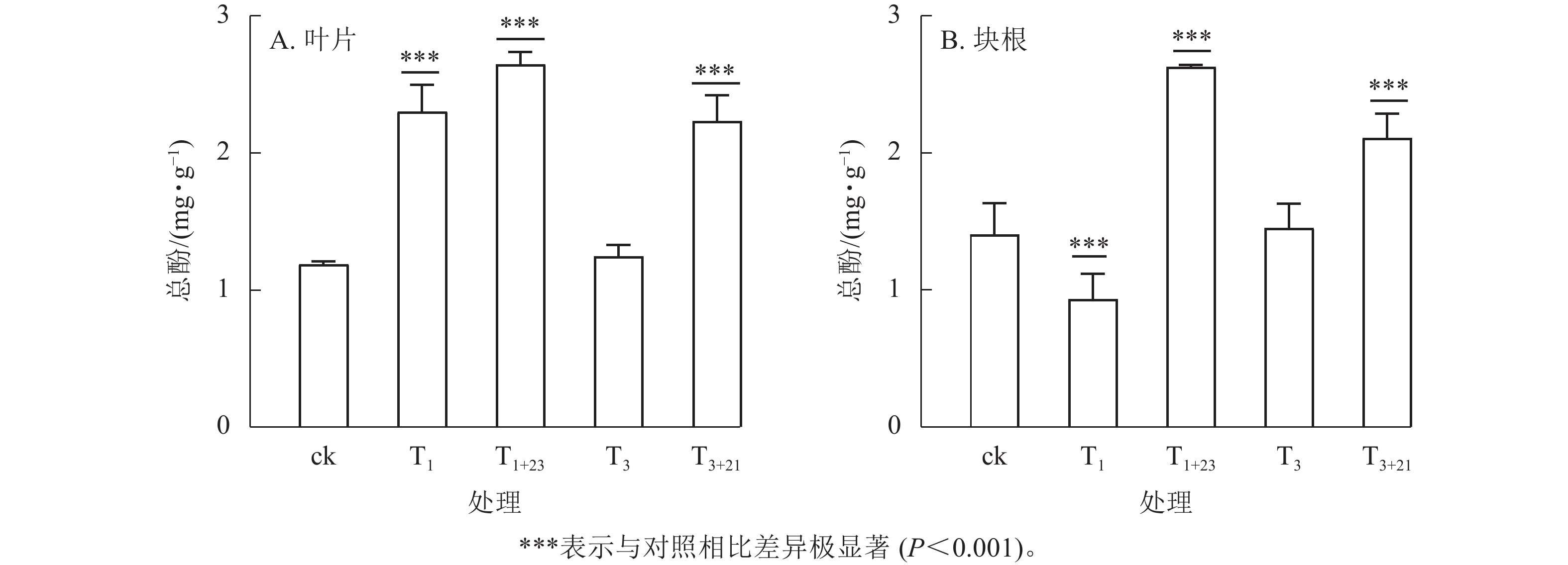
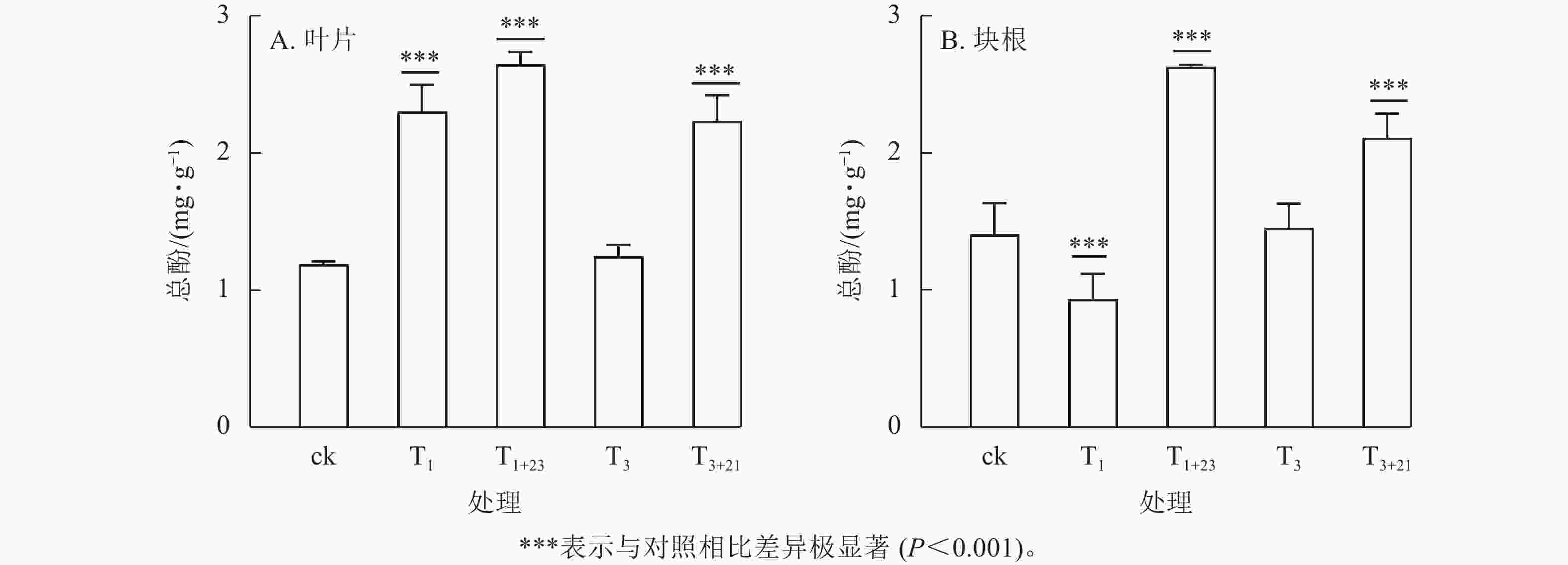
除T1处理外,三叶青总酚质量分数在叶片(图2A)和块根(图2B)中的变化趋势基本一致。在叶片中,T1处理的总酚质量分数是T3处理的1.84倍,分别为2.30、1.25 mg·g−1;在块根中,T3处理的总酚质量分数是T1处理的1.56倍,分别为1.45、0.93 mg·g−1。说明块根和叶片对于UV-B辐射的响应是不同步的。UV-B辐射后经过黑暗处理,总酚质量分数都有明显提升。T1+23处理的叶片和块根中的总酚质量分数都达到了最大值,分别为ck的2.23和1.87倍,差异极显著(P<0.001)。
-
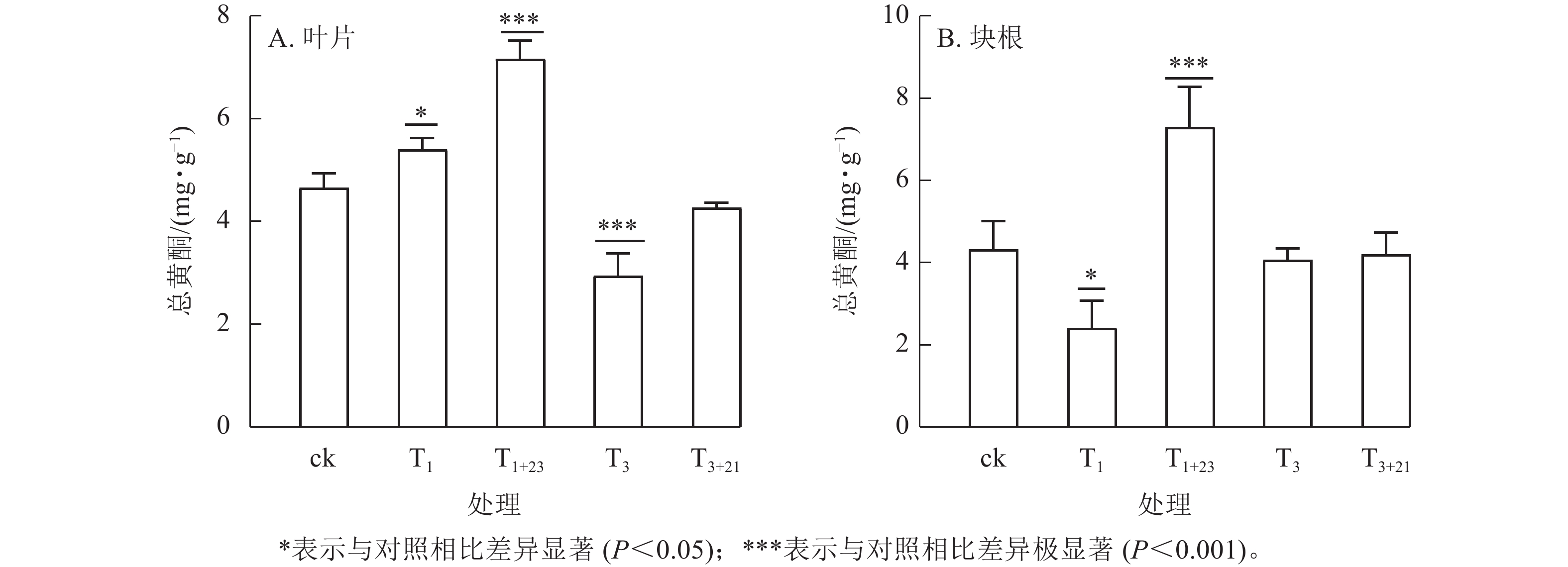
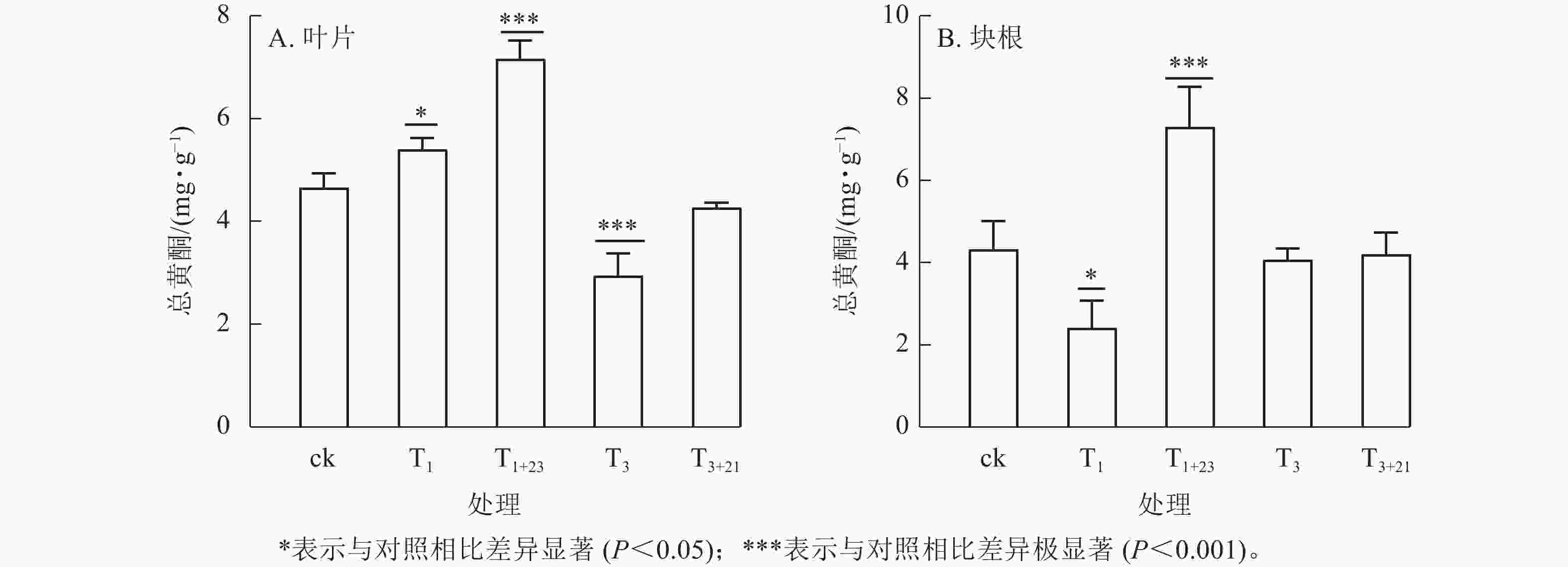
经T1处理后,叶片中总黄酮质量分数比ck增加,为5.40 mg·g−1 (图3A),块根中黄酮质量分数比ck减少,为2.42 mg·g−1 (图3B),分别是ck的1.16和0.56倍,且均差异显著(P<0.05)。T1+23处理的叶片和块根中的总黄酮质量分数都达到了最大值,叶片中的总黄酮质量分数为7.16 mg·g−1,是T1处理的1.33倍;块根中的总黄酮质量分数为7.30 mg·g−1,是T1处理的3.02倍。在T3处理及后续的T3+21处理中,叶片和块根中的总黄酮质量分数均低于ck。
-
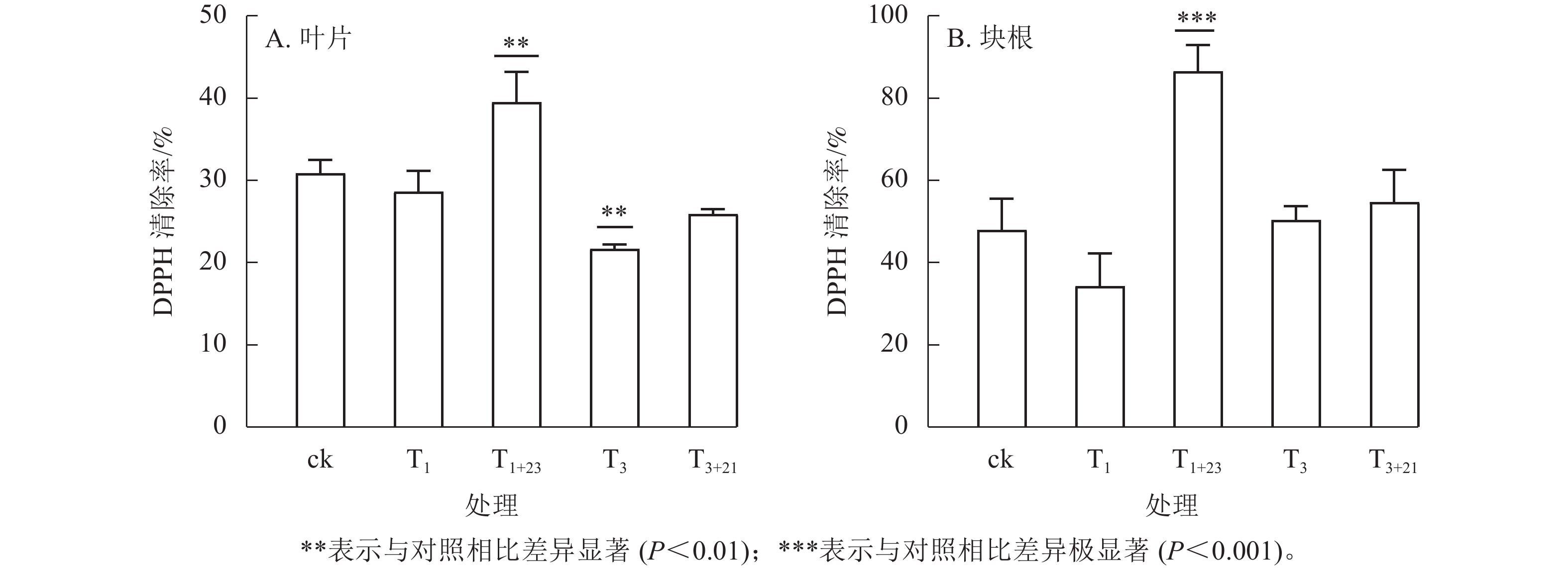
如图4所示:在ck及各处理组中,三叶青块根的抗氧化能力较高,约为叶片相应处理组的1.20~2.33倍。仅使用UV-B辐射对叶片和块根的抗氧化能力提升作用并不明显,而增加黑暗处理后抗氧化能力得到明显改善,尤其是块根的T1+23处理呈极显著差异(P<0.001)。T1+23处理的叶片和块根中DPPH清除率均达到了最大值,分别比T1处理增加39.48%和86.56%,是ck的1.28和1.80倍。
-
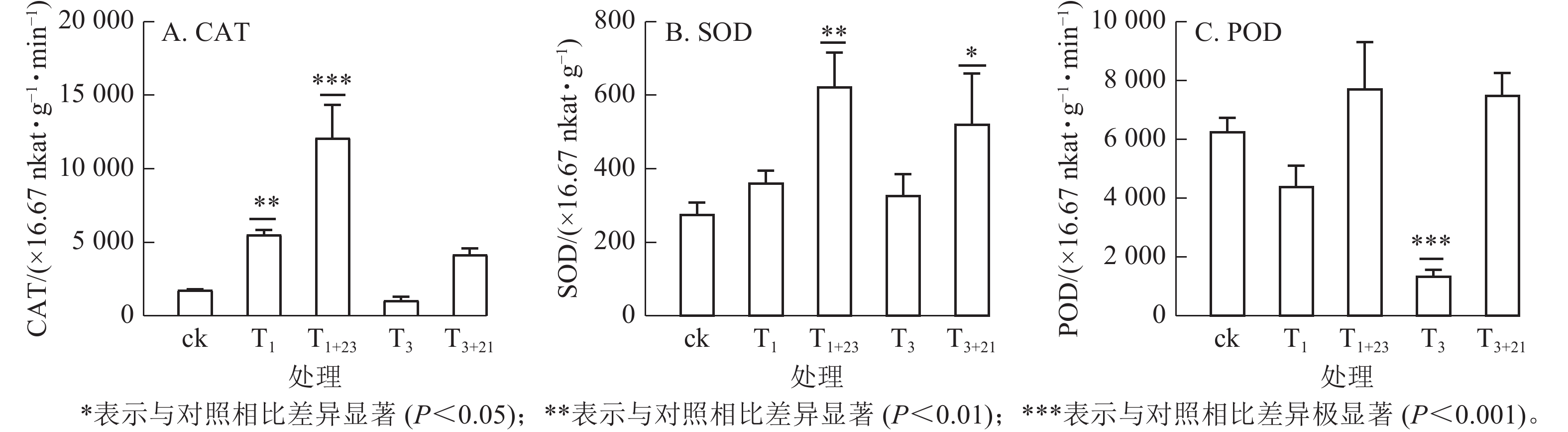
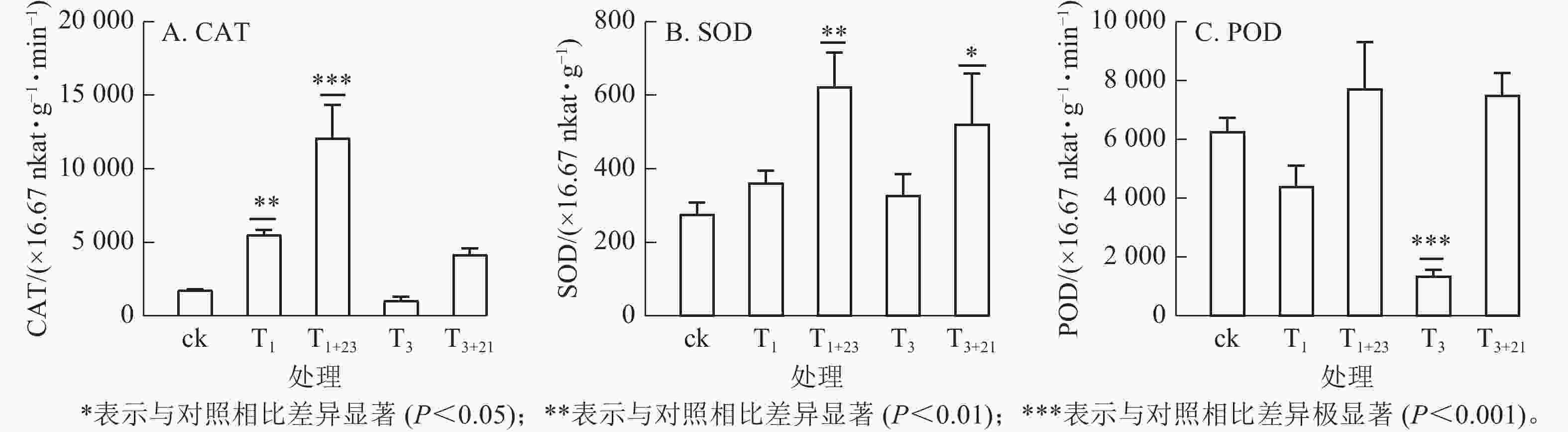
如图5所示:T1处理的CAT活性和SOD活性提高,分别是ck的3.15和1.31倍,其中CAT差异显著(P<0.01);而T3处理的CAT活性和POD酶活性降低,分别是ck的0.60和0.22倍,其中POD酶活性差异极显著(P<0.001)。在紫外辐射后加上黑暗处理,3种抗逆酶活性都有了大幅度的提升,其中T1+23处理后,CAT、SOD和POD活性均达到最大值,分别为725.66×16.67 nkat·g−1·min−1、37.40×16.67 nkat·g−1和463.94×16.67 nkat· g−1·min−1,分别是ck的6.89、2.26和1.23倍。
-
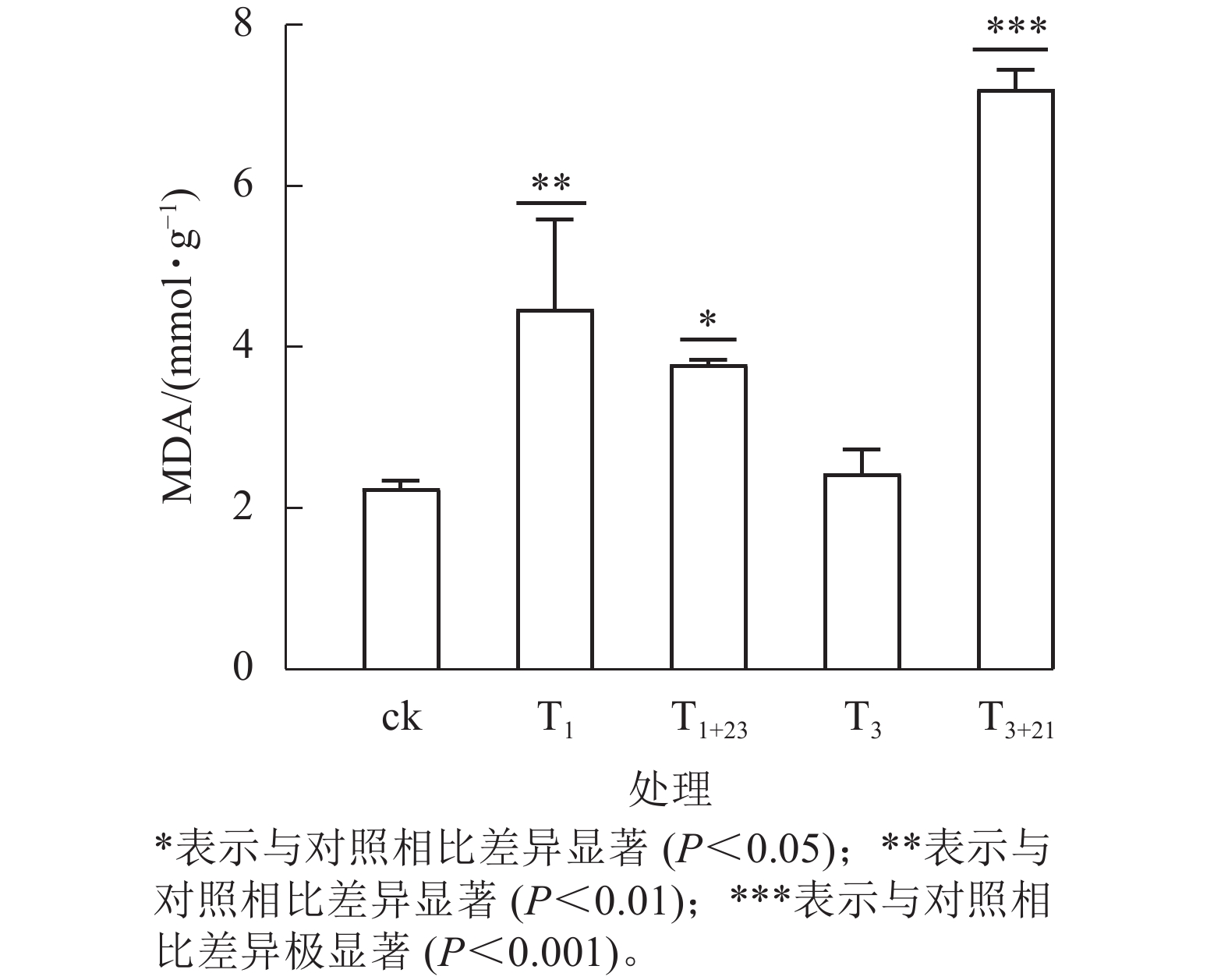
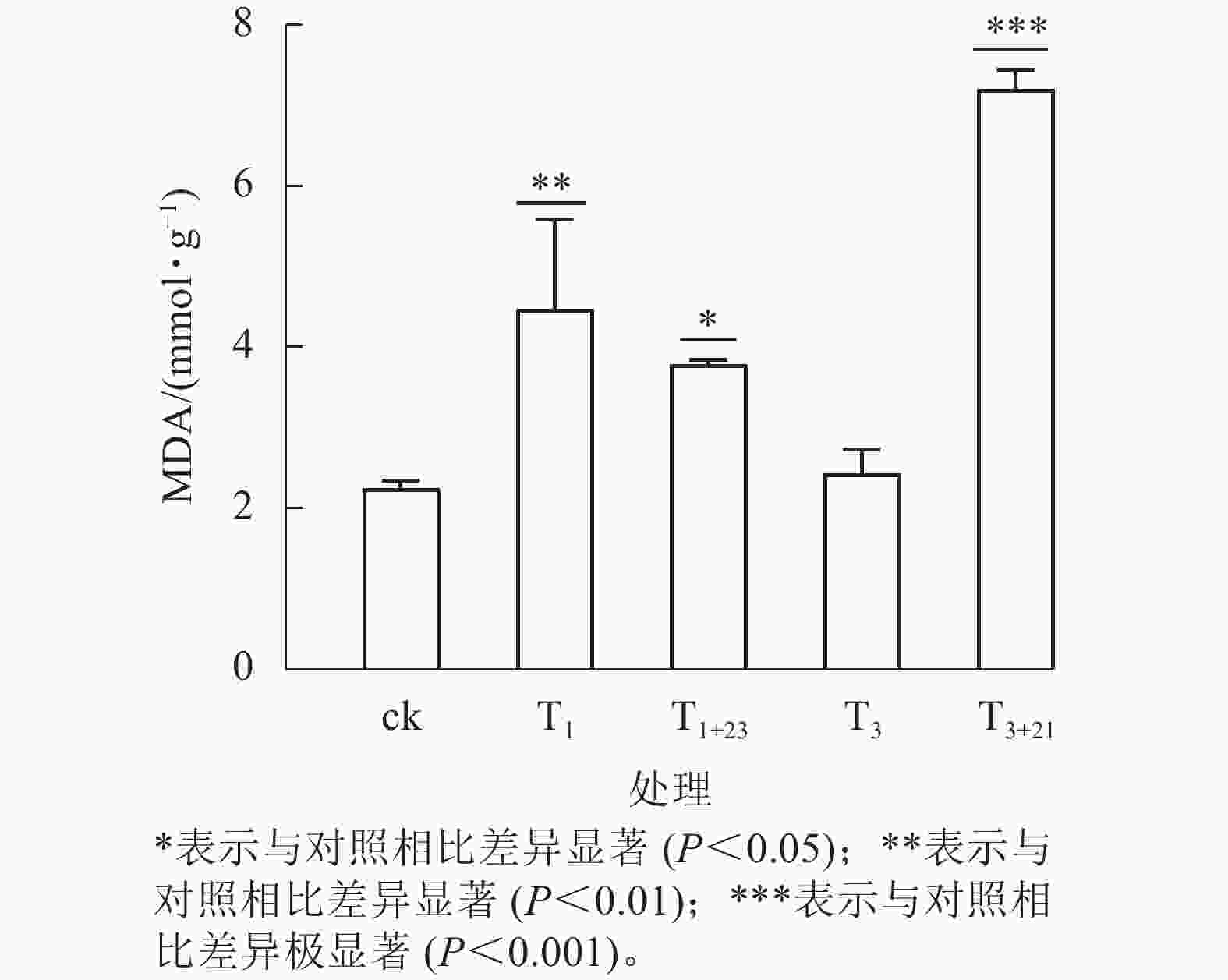
UV-B 辐射增加了各组三叶青叶片中的MDA质量摩尔浓度(图6)。T3+21处理后,三叶青叶片中的MDA质量摩尔浓度与ck相比差异极显著(P<0.001),且高于其他处理,达7.21 mmol·g−1,分别是ck的3.21倍、 T3处理的2.97倍。
-
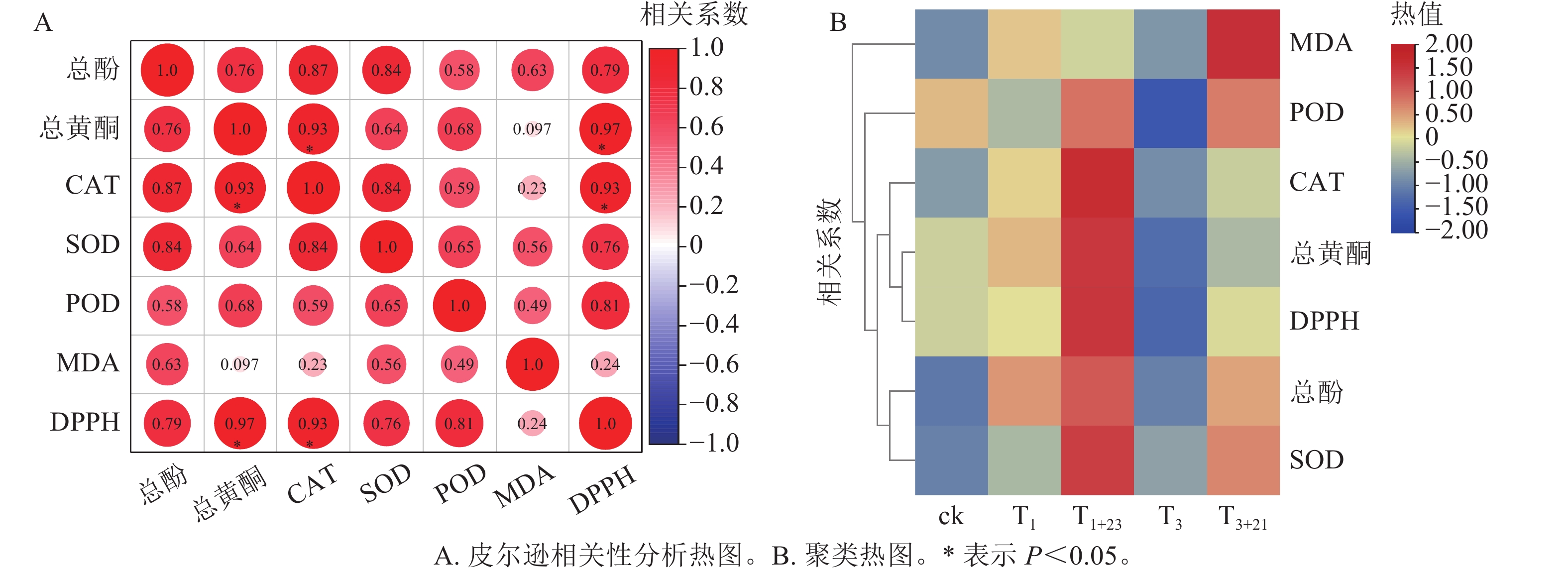
如图7所示:总酚、总黄酮、CAT、SOD、POD和MDA均与DPPH呈正相关,其中总黄酮质量分数、CAT酶活性与三叶青抗氧化能力(DPPH清除率)呈现显著正相关(P<0.05);CAT活性与总黄酮质量分数呈显著正相关(P<0.05)。对三叶青上述成分进行热图聚类分析发现:总酚、总黄酮质量分数,POD、CAT、SOD活性和DPPH清除率均在T1+23处理达到最高,说明黑暗处理对这些指标具有促进作用,MDA质量摩尔浓度在T3+21处理达到最高。
-
对UV-B胁迫处理后的三叶青叶片做转录组差异表达unigene的京都基因与基因组百科全书(KEGG)通路富集分析,黄酮代谢通路中的差异基因如表1所示。12个结构基因中,ck-T1组的ThHCT (DN17271)和ThCYP98A3(DN156339)、ck-T1+23组的ThF3H (DN10838)和ThF3H (DN7985)、ck-T3+21组的ThANS (DN12973)、ThF3H (DN7985)和ThF3H (DN10838)以及T3-T3+21组的ThF3H (DN7985)、ThCHS (DN734)、ThANR(DN145313)、ThFLS (DN10864)和ThCYP75B1 (DN1106)基因的表达量均下降了2倍以上(log2CF为−2.08~−3.71),ThCHS (DN734)和ThANR (DN145313)基因的表达量下降了4倍以上,差异极显著(|log2CF|>4)。
组别 基因编码 基因注释 log2CF 调节 ck-T1 TRINITY_DN17271_c0_g1 羟基肉桂酰基转移酶(HCT) −2.59 下调 ck-T1 TRINITY_DN156339_c0_g1 5-O-(4-香豆酰基)-D-奎喹酸酯 3′-单加氧酶(CYP98A3) −2.08 下调 ck-T3 TRINITY_DN10864_c0_g1 黄酮醇合酶(FLS) 3.28 上调 ck-T1+23 TRINITY_DN10838_c0_g1 黄烷酮3-羟化酶(F3H) −2.60 下调 ck-T1+23 TRINITY_DN7985_c0_g1 黄烷酮3-羟化酶(F3H) −2.56 下调 ck-T3+21 TRINITY_DN12973_c0_g1 花青素合酶(ANS) −3.71 下调 ck-T3+21 TRINITY_DN7985_c0_g1 黄烷酮3-羟化酶(F3H) −2.14 下调 ck-T3+21 TRINITY_DN10838_c0_g1 黄烷酮3-羟化酶(F3H) −2.73 下调 T3-T3+21 TRINITY_DN7985_c0_g1 黄烷酮3-羟化酶(F3H) −2.38 下调 T3-T3+21 TRINITY_DN734_c0_g1 查耳酮合酶(CHS) −4.74 下调 T3-T3+21 TRINITY_DN145313_c0_g1 花青素还原酶(ANR) −6.33 下调 T3-T3+21 TRINITY_DN10864_c0_g1 黄酮醇合酶(FLS) −3.21 下调 Table 1. Differential genes in the flavonoid metabolic pathway
此外,KEGG 富集分析中T1-T1+23组和T3-T3+21组的过氧化物酶体(peroxisome)通路差异基因如表2所示。其中T1-T1+23组结果显示:抗氧化系统的ThCAT (DN105637)基因上调了2.97倍;T3-T3+21组结果显示:ThXMP2 (DN16875)、ThSOD (DN4487)、ThICDH (DN19733)和FMN依赖型α-羟基酸脱氢酶基因均上调。
组别 基因编码 基因注释 log2CF 调节 T1-T1+23 TRINITY_DN105637_c0_g1 过氧化氢酶(CAT) 2.97 上调 T3-T3+21 TRINITY_DN16875_c0_g1 过氧化物酶体膜蛋2(XMP2) 1.65 上调 T3-T3+21 TRINITY_DN4487_c0_g1 超氧化物歧化酶(SOD) 1.07 上调 T3-T3+21 TRINITY_DN19733_c0_g2 异柠檬酸脱氢酶(ICDH) 1.46 上调 T3-T3+21 TRINITY_DN48302_c0_g1 FMN依赖型α-羟基酸脱氢酶 2.10 上调 Table 2. Differential genes in the peroxisome pathway
-
长时间的紫外光照射对于生长在林下的三叶青属于致胁迫因素。本研究对三叶青持续UV-B辐射发现:3.0 h后三叶青表型发生变化,叶片开始略微发黄,初步判断此时三叶青已受到轻度胁迫。这与小麦Triticum aestivum[28]、苦荞Fagopyrum tataricum[29]、黄瓜Cucumis sativus[30]等植物受到胁迫后的表型变化趋势相似。此外,多种植物在表型产生胁迫变化的同时,会在体内做出一系列适应紫外胁迫的生理生化反应。例如,鸡毛菜Brassica rapa显著提高了多种类黄酮代谢通路上游合成酶的活性[13],榛子Corylus avellana增加了花粉中多酚和黄酮类次生代谢产物的质量分数[31],三叶青改变了黄酮类化合物质量分数及相关合成酶、抗逆酶活性[19]等。在本研究中,经UV-B辐射1.0 h后,叶片中总酚和总黄酮质量分数均升高,这是因为黄酮类物质对紫外光极其敏感[32],可减少紫外线对植物组织的穿透作用,保护叶片免受伤害[33]。经UV-B辐射3.0 h后,三叶青总酚和总黄酮质量分数均较UV-B辐射1.0 h下降,分析认为可能是植物光合作用受到影响,合成速率降低,光合产物减少,导致黄酮类化合物合成减少。与GAO等[20]的研究结果一致,即较低水平的UV-B辐射比高水平的UV-B辐射更能促进植物黄酮类化合物的合成和积累。而在块根中,UV-B辐射1.0 h的总酚和总黄酮质量分数均小于ck,参考SURJADINATA等[34]和ÁLVAREZ-GÓMEZ等[35]的研究发现:刚开始遭受UV-B胁迫,块根中还原糖质量分数降低以供运输到叶片进行酚类物质合成,导致块根中合成酚类的前体物质减少,最终直接影响酚类化合物的积累。此外,在多种药用植物研究中发现:UV-B辐射后施以黑暗处理,铁线莲Clematis terniflora会通过增加黄酮类物质[36]、桑树Morus alba积累桑黄素N和黑木耳素[37]、长春花Catharanthus roseus积累生物碱[38]来提高耐胁迫能力。本研究结果与以上研究相似。单纯的紫外处理并未显著增加三叶青中的次生代谢产物,UV-B辐射后给予黑暗处理可大幅度增加总酚和总黄酮质量分数,并显著提升其抗氧化能力。相关性分析结果也表明:总酚和总黄酮质量分数与三叶青抗氧化能力有显著正相关性。
对多种植物的研究结果均显示:光照和生物钟会影响植物对UV-B胁迫的敏感性,并且一些基因(如ELIP1、CHS/PRR9和ELF4等)在不同环境中对UV-B的敏感度是不同的[39],例如,适当的紫外光辐射会提高彩色马铃薯Solanum tuberosum的抗氧化能力,从而增加花青素结构基因(F3'5'H、F3'H、DFR和ANS等)的表达[40];UV-B加黑暗处理对拟南芥Arabidopsis thaliana叶片光合相关基因(CAB)的表达、叶绿素质量分数和光合效率的影响较大[41];黑暗处理对茶树不同基因的影响存在差异,经黑暗处理后的CsPAL、CsCHS、Cs4CL、CsLAR、CsANR和CsANS基因表达下调,而CsFLS基因表达上调,表达结果较为复杂[42]。本研究的转录组结果进一步表明:与ck组相比,经UV-B和黑暗处理后多数黄酮合成酶基因,如ThF3H、ThANS和ThCHS等均下调。分析认为:这与植物在黑暗处理时会快速且大量合成次生代谢产物从而形成反馈抑制有关,并进一步下调了相关合成酶基因表达[42]。孟凡来等[43]对紫甘薯Ipomoea batatas叶片的转录组分析发现:UV-B辐射增强类黄酮合成通路中的各关键酶(如查尔酮合成酶、花青素合成酶、4-香豆酸-CoA连接酶6、类花青素3-O-葡萄糖基转移酶7)基因主要以下调表达为主,与本研究结果一致。而ThFLS基因经UV-B辐射3.0 h后上调,表明其在UV-B辐射增强中起关键的正向调控作用[44]。
当植物(如黄岑Scutellaria baicalensis[45]、葡萄Vitis vinifera[46]等)遭受紫外光照射时,抗逆酶系统启动,逐步清除体内的游离氧离子、自由基及一些有害物质,对叶片起到较好的保护作用。SOD处于抵御活性氧伤害的第1道防线,主要清除对植物毒性较大的超氧阴离子($\mathop {\rm{O}}\nolimits_2^{{\rm{\cdot}}-}$),将其转化成为毒性较小的H2O2、CAT和POD,H2O2进一步转化为无毒的H2O和O2[47]。本研究中,三叶青在遭受UV-B辐射1.0 h后CAT和SOD活性增加,并且CAT活性较SOD和POD活性大幅提升,说明三叶青中各种酶的作用机理不同,进而在细胞内的活性变化趋势略有不同。而在UV-B辐射1.0 h加黑暗处理后,酶活性达到最大,表明了一定时间的黑暗处理可提高H2O2清除能力,对三叶青有修复功能[48]。当辐射时间达3.0 h时,胁迫进一步加重,3种酶活性下降,分析认为可能是保护酶系统受损。进一步转录组分析显示:UV-B辐射并增加黑暗处理能提高三叶青中一些氧化应激反应相关基因(如CAT和SOD)表达上调,与褚润等[49]对香蒲Typha orientalis的研究结果一致。MDA作为膜质过氧化分解的重要产物,可以对受胁迫细胞再次造成伤害,在一定程度上其质量摩尔浓度的高低可以表示细胞的膜质过氧化水平和细胞受损程度[50]。本研究中,经UV-B辐射1.0 h后MDA质量摩尔浓度升高,这与先前的研究结果相同[51];增加黑暗处理后,MDA质量摩尔浓度小幅降低,提示黑暗有利于膜的修复。UV-B辐射3.0 h时MDA质量摩尔浓度较UV-B辐射1.0 h呈下降趋势,表明UV-B辐射3.0 h内三叶青中抗氧化系统产生应激反应,$\mathop {\rm{O}}\nolimits_2^{{\rm{\cdot}}-} $被迅速清除,缓解了膜受损程度。而UV-B辐射3.0 h加黑暗处理后,MDA质量摩尔浓度达到最高,可能的原因是MDA的影响相对于抗逆酶系统有滞后效应,这也与GONCHARUK等[52]的研究结果相同,即此时抗逆酶系统虽在缓慢修复,但$\mathop {\rm{O}}\nolimits_2^{{\rm{\cdot}}-} $仍超出了植物自身防御系统的清除能力,此时,即使给予黑暗处理,MDA质量摩尔浓度也会显著升高。
-
UV-B辐射1.0 h加23.0 h黑暗处理能更好地增加三叶青中次生代谢物质及其抗氧化能力,同时下调黄酮合成酶和上调氧化相关调节因子的基因表达,并且使各物质达到最大值,既能增加次生代谢产物,又能将紫外胁迫的伤害控制在一定范围内,保证植物正常生长。同时,暗处理对紫外照射后的三叶青是极其重要的,其影响机理仍未探明,今后的研究将予以持续关注。
Effect of UV-B radiation on mass fraction of phenolic substances, antioxidant capacity and genes expression in Tetrastigma hemsleyanum
doi: 10.11833/j.issn.2095-0756.20230385
- Received Date: 2023-06-30
- Accepted Date: 2023-11-14
- Rev Recd Date: 2023-11-06
- Available Online: 2024-03-21
- Publish Date: 2024-04-01
-
Key words:
- Tetrastigma hemsleyanum /
- UV-B stress /
- phenolic substances /
- antioxidant capacity /
- differential expression
Abstract:
| Citation: | XIA Jingqing, LIU Hairong, GU Yiwen, et al. Effect of UV-B radiation on mass fraction of phenolic substances, antioxidant capacity and genes expression in Tetrastigma hemsleyanum[J]. Journal of Zhejiang A&F University, 2024, 41(2): 223-233. DOI: 10.11833/j.issn.2095-0756.20230385 |











 DownLoad:
DownLoad: