-
金线莲为兰科Orchidaceae多年生药用植物金线兰Anoectochilus roxburghii新鲜或干燥全草,味甘、性平,具有保肝护肝[1]、降血糖[2]、抗骨质疏松[3]、抗氧化[4]等药理学功效,用于治疗肺结核咳血、糖尿病、重症肌无力等症,在民间具有“神草”“药王”之称。 金线莲苷(kinsenoside, KD)是金线兰特征性活性成分,对于酒精性肝损伤[5]、胆汁淤积性肝损伤[6]、放射性肝损伤[7]、非酒精性脂肪肝炎[8]、自身免疫性肝炎[9]、肝纤维化[10]等肝损伤具有良好的保护作用,于2023年7月28日获得国家药品监督管理局批示进入药物临床试验,但是,市场上金线莲苷奇缺,价格昂贵(标品:30~75元·g−1),难以满足临床需求。开展金线莲苷工业化生产势在必行,化学合成金线莲苷因成本高、产率低难以进行工业化生产[11]。从金线兰中分离提取是工业化制备金线莲苷的唯一方法。金线莲苷差向异构体——斑叶兰苷(goodyeroside A,GD)和金线莲苷分子量相同,理化性质相似,药理学活性不同[12−17],是金线莲苷制备过程极难去除的杂质。但是,目前尚无金线兰中金线莲苷及其差向异构体如何分布的报道。
金线兰野生资源濒危,是《濒危野生动植物种国际贸易公约》(CITES)附录Ⅱ的保护物种和《国家重点保护野生植物名录》(第二批)二级保护植物,目前市场上野生金线兰资源稀少,多以人工种植为主。人工种植金线兰受品种基因型和产区生态因子的影响[18−20],产量和质量极不稳定,难以作为药源工业化生产金线莲苷。明确不同品种、产地金线兰中金线莲苷及其差向异构体的分布是金线兰研究或金线莲苷工业化生产研究中的热点问题。
大多数次生代谢产物对植物细胞有毒害作用,在植物中含量很低,而金线莲苷在金线兰中含量相对较高[21],对植物细胞几乎无毒,在植物生长发育的不同时期呈现“S”型变化趋势[22]。金线莲苷积累势必与植物中内源激素积累相关,但目前尚无相关报道。
本研究分析金线兰中金线莲苷、斑叶兰苷、内源生长素(IAA)、水杨酸(SA)和脱落酸(ABA)的含量及分布模式;并分析了生长素抑制剂(2, 3, 5-三碘苯甲酸,TIBA)处理下茎段中内源IAA及金线莲苷分布模式的变化,研究结果将为金线兰质量评价提供基础数据,为工业化生产金线莲苷优质药源的筛选提供思路和技术支撑。
-
金线兰‘大叶’‘Daye’、‘小叶’‘Xiaoye’、‘尖叶’‘Jianye’和‘红霞’‘Hongxia’4个品系经鉴定为金线兰。‘尖叶’品系购自浙江省金华市农业科学研究院,‘红霞’‘大叶’‘小叶’品系购自于福建省南靖县金线兰种植企业,4个品系金线兰都在大棚中种植5个月。采集金线兰全草、根、茎、叶,经液氮研磨成粉末,用于分析金线莲苷及其差异构体含量。采集不同部位茎段及叶,叶分别为顶端叶、中部叶(第1、2位结节处叶片)和底部叶(第3个及以下结节处叶);茎段分为顶端茎段(第3个节以上茎段)、中部茎段(中间3~5个节)及底部茎段(第6个节及以下茎段)。经液氮研磨成粉末,用于分析金线莲苷及其差向异构体、内源激素含量。用100 mg·L−1 TIBA处理[23]‘尖叶’品系,分别在0、1、2、3、4 、5 d取样,经液氮研磨成粉末,用于分析内源激素和金线莲苷积累模式的变化。所有的植物材料重复3次取样。
-
金线莲苷(CAS:HS21117B2)、斑叶兰苷(CAS:HS22302B1)购自宝鸡市辰光生物有限公司,生长素(CAS:B21810)、脱落酸(CAS:B27484)、水杨酸(CAS:B21197)、2, 3, 5-三碘苯甲酸(CAS:S30709)、色谱级乙腈和甲醇购自上海源叶生物科技有限公司。
-
岛津高效液相色谱仪(LC-2030 Plus)购自日本岛津公司;超声仪(KQ-300GDV)购自绍兴谱尔仪器设备有限公司。
-
金线莲苷及差向异构体分离、提取参照WANG等[24]方法并有所改动,准确称取样品粉末0.2 g,加入1 mL 体积分数为90%的甲醇,30 ℃超声处理30 min称量,用体积分数为90%的甲醇补足挥发溶剂。5 000 r·min−1离心10 min,取上清液用0.22 µm滤膜过滤。HPLC-ELSD测定金线莲苷及差向异构体,检测方法参照张闻婷等[21]测定金线莲苷的方法并有所改动,利用岛津高效液相色谱仪(LC-2030 Plus,日本岛津),分别采用NH2、XSelect HSS T3色谱柱,柱温为30 ℃,流动相为水∶乙腈=92∶8,流速为1 mL·min−1,等度洗脱。PDA检测波长为210 nm,ELSD检测条件:载气为氮气,雾化室温度为50 ℃,进样量为10 µL。以峰面积(x)和物质质量浓度(y)构建线性回归方程。金线莲苷线性回归方程为:y=1E+06x−126 593,R2=0.999,线性范围为0.10~1.25 g·L−1;斑叶甘兰线性回归方程为:y=591 928x−118 70,R2=0.993,线性范围为0.02~0.16 g·L−1。
-
准确称取样品粉末0.2 g,加1 mL体积分数为90%的甲醇,超声提取30 min,称量,用90%甲醇补足损失的溶剂,5 000 r·min−1离心10 min,取上清液用0.22 μm滤膜过滤,每个样品重复3次。利用岛津高效液相色谱仪,采用C18色谱柱,以体积分数为0.05%的甲酸水溶液(A)-0.05%的甲酸乙腈(B)为流动相梯度洗脱(B:0~1.5 min,10%→5%;1.5~24.0 min,5%→95%;24.0~27.0 min,95%→95%;27.0~27.3 min,95%→5%;27.3~36.0 min,5%→10%),柱温为30 ℃,流速为0.6 mL·min−1,进样量为10 μL, IAA、ABA和SA检测波长分别为 254、254和304 nm。以峰面积(x)和物质质量浓度(y)构建线性回归方程。IAA线性回归方程为:y=15 492 788.47x+137.74,R2=0.999,线性范围为
0.0001 ~0.010 0 g·L−1;ABA线性回归方程为:y=87 093 692.12x−536.04,R2=0.999, 线性范围为0.000 1~0.010 0 g·L−1;SA线性回归方程为:y=20 994 444.05x+10.92,R2=0.999,线性范围为0.000 1~0.0100 g·L−1。 -
金线莲苷及其差向异构体、IAA、ABA、SA质量分数(鲜质量)用平均值±标准差表示,利用SPSS 22.0分析样品间的差异显著性,所有样品重复3次。
-
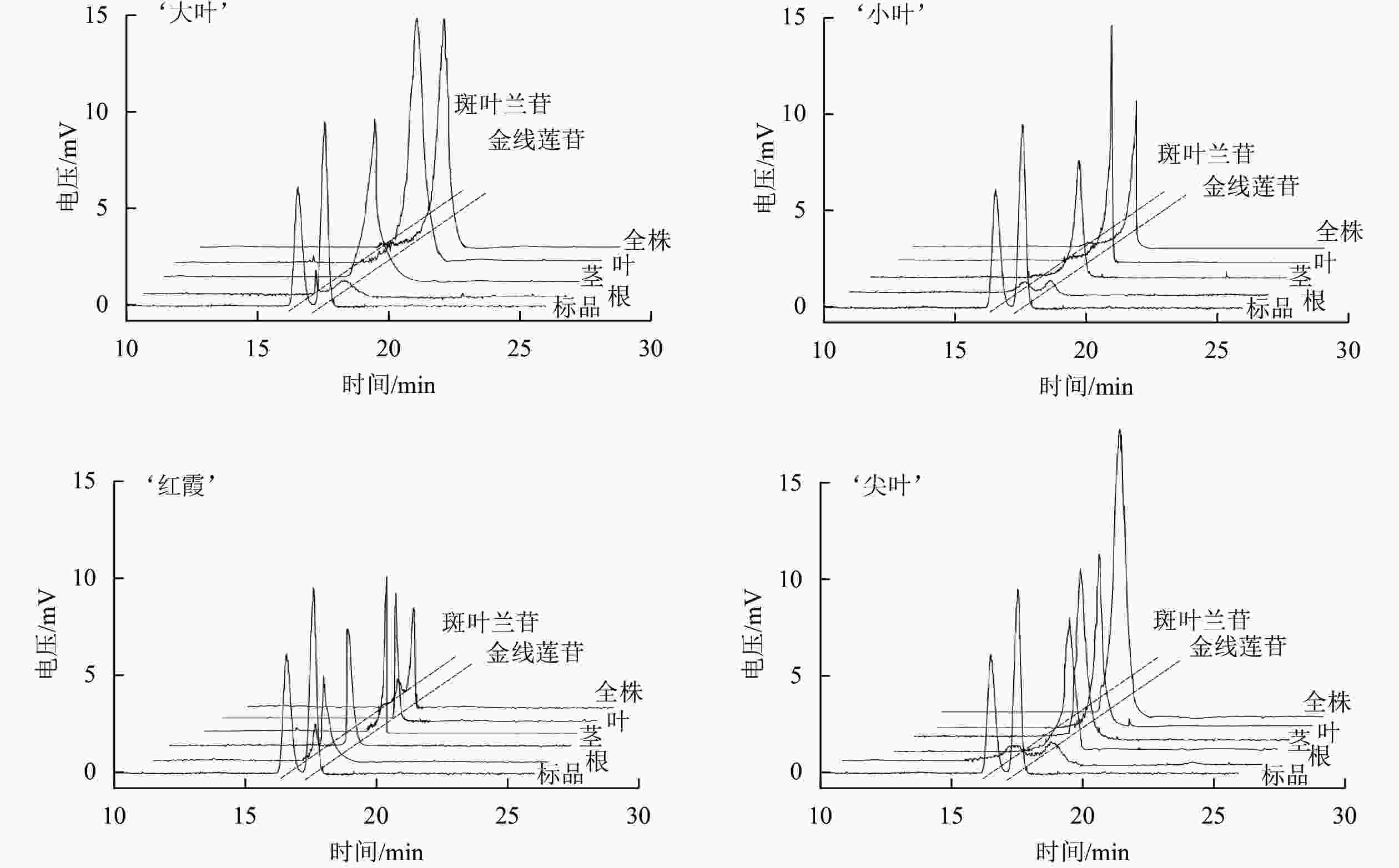
如表1和图1所示:4个品系金线兰中金线莲苷质量分数为2.84~3.81 mg·g−1,‘大叶’品系中金线莲苷质量分数最高,‘红霞’品系中金线莲苷质量分数最低,‘大叶’品系金线莲苷质量分数显著高于‘尖叶’‘红霞’品系(P<0.05),与‘小叶’品系无显著差异;4个品系根、茎和叶中金线莲苷质量分数分别为1.35~1.62、2.27~3.72和3.69~5.34 mg·g−1,叶中金线莲苷质量分数最高,茎中次之,根中最低,并且金线兰地上部分金线莲苷质量分数远高于根;4个品系金线兰中金线莲苷差向异构体质量分数极低(0.14~0.50 mg·g−1),并且特异性地积累于根部,在金线兰的地上部分中没有检测到金线莲苷差向异构体。分别以‘尖叶’和‘红霞’品系茎段为外植体诱导类原球茎,‘尖叶’品系类原球茎中金线莲苷质量分数(4.25 mg·g−1)高于全草, 并且没有检测到金线莲苷差向异构体;‘红霞’品系类原球茎中金线莲苷质量分数为2.56 mg·g−1,金线莲苷差向异构体质量分数为0.52 mg·g−1。上述结果表明金线兰地上部分、‘尖叶’类原球茎有望用作低成本、高质量工业化生产金线莲苷的药源。
品系 金线莲苷/(mg·g−1) 斑叶兰苷/(mg·g−1) 全株 根 茎 叶 类原球茎 全株 根 茎 叶 类原球茎 ‘大叶’ 3.81±0.02 a 1.62±0.06 a 3.55±0.04 ab 3.69±0.26 b \ − 0.24±0.01 d − − \ ‘小叶’ 3.68±0.13 a 1.35±0.02 c 3.15±0.04 b 4.78±0.37 ab \ − 0.43±0.01 c − − \ ‘红霞’ 2.84±0.01 c 1.45±0.07 bc 2.27±0.10 c 5.34±0.68 a 2.56±0.53 b 0.22±0.01 0.50±0.07 a − − 0.52±0.13 ‘尖叶’ 3.47±0.02 b 1.51±0.05 ab 3.72±0.27 a 3.76±0.41 b 4.25±0.46 a − 0.46±0.14 b − − − 说明:同列不同小写字母表示差异显著(P<0.05)。“−”表示没有检测到;“\”表示未检测。 Table 1. Contents of kinsenoside and it’s isomer in A. roxburghii
-
由表2可见:4个品系金线兰中金线莲苷在不同部位叶中积累模式不同。‘大叶’品系中金线莲苷积累模式为:中间部位叶中金线莲苷质量分数最高,显著高于顶端叶中金线莲苷(P<0.05),与底部叶中金线莲苷无显著差异;‘小叶’品系中金线莲苷质量分数积累模式为:顶端叶中金线莲苷质量分数最高,中间部位叶中金线莲苷质量分数最低,顶端叶中金线莲苷质量分数显著高于中部和底部叶(P<0.05);‘红霞’品系中金线莲苷质量分数积累模式为:金线莲苷质量分数随着叶位置降低而逐渐升高,底部叶片中金线莲苷质量分数显著高于中部和顶部叶(P<0.05);‘尖叶’品系中金线莲苷积累模式为:中间部位叶中金线莲苷质量分数最高,显著高于顶端和底部叶中金线莲苷质量分数(P<0.05)。在所有叶中没有检测到金线莲苷差向异构体。
组织
部位金线莲苷/(mg·g−1) ‘大叶’ ‘小叶’ ‘红霞’ ‘尖叶’ 叶 茎段 叶 茎段 叶 茎段 叶 茎段 顶端 2.65±0.17 b 4.36±0.37 a 9.74±0.40 a 4.81±0.16 a 2.91±0.09 c 3.44±0.41 a 3.90±0.28 b 3.65±0.30 a 中部 4.14±0.17 a 4.32±0.26 a 6.68±0.05 c 4.71±0.06 a 3.39±0.13 b 2.97±0.16 a 5.33±0.59 a 3.03±0.81 ab 底部 3.71±0.26 a 4.13±0.21 a 8.35±0.03 b 4.55±0.13 a 4.13±0.07 a 2.64±0.33 a 3.10±0.55 b 2.52±0.02 b 说明:未检测到金线莲苷异构体,因此未在表中列出。同列不同小写字母表示差异显著(P<0.05)。 Table 2. Contents of kinsenoside and it’s isomer in leaves and stems of in A. roxburghii
金线兰不同部位茎段中,‘大叶’‘小叶’‘红霞’‘尖叶’品系中金线莲苷质量分数分别为4.13~4.36,4.55~4.81,2.64~3.44和2.52~3.65 mg·g−1,表现为顶端茎段中金线莲苷质量分数最高,中间部位茎段中金线莲苷质量分数次之,底部茎段中金线莲苷质量分数最低,呈现极性积累模式,且在不同部位茎段中金线莲苷质量分数均无显著差异。在所有茎段中没有检测到金线莲苷差向异构体。上述研究结果进一步证实,金线兰地上部分可以作为工业化生产金线莲苷的优质药源。
-
如表3所示:‘大叶’‘小叶’‘红霞’‘尖叶’品系茎段中ABA质量分数分别为0.020~0.030、0.002~0.005、0.002~0.003和0.010~0.012 mg·g−1,SA质量分数分别为0.009~0.013、0.085~0.107、0.074~0.108、0.001~0.005 mg·g−1,IAA质量分数分别为0.020~0.030、0.120~0.140、0.010~0.030和0.003~0.006 mg·g−1。在所有品系中,顶端茎段中IAA质量分数最高,中间部位茎段中IAA质量分数次之,底部茎段中IAA质量分数最低,IAA积累模式与金线莲苷积累模式相似,相关性系数为0.874~0.937 (P<0.01)(图2Ⅰ);ABA在4个品系茎段中积累模式有所不同,在‘大叶’品系中,底部茎段中ABA质量分数显著高于中部茎段中ABA质量分数(P<0.05),与顶端茎段中ABA质量分数无显著差异;在‘尖叶’品系茎段中ABA质量分数无显著差异;在‘小叶’‘红霞’品系茎段中与金线莲苷积累模式一致,相关性系数分别为0.835、0.898 (P<0.01)(图2Ⅱ);SA在4个品系茎段中积累模式有所不同,在‘大叶’品系中,底部茎段中ABA质量分数显著高于顶端茎段中ABA质量分数(P<0.05),与中部茎段中ABA质量分数无显著差异;在‘小叶’‘红霞’‘尖叶’品系茎段中与金线莲苷积累模式一致,相关性系数分别为0.958、0.895、0.838 (P<0.01)(图2Ⅲ)。上述结果表明:在所研究的金线兰中金线莲苷与内源IAA积累模式相关性不受品种基因型影响,金线莲苷与ABA和SA积累模式相关性一定程度受品种基因型影响。
茎段不
同部位IAA/(mg·g−1) ABA/(mg·g−1) SA/(mg·g−1) ‘大叶’ ‘小叶’ ‘红霞’ ‘尖叶’ ‘大叶’ ‘小叶’ ‘红霞’ ‘尖叶’ ‘大叶’ ‘小叶’ ‘红霞’ ‘尖叶’ 顶端 0.027±
0.003 a0.142±
0.005 a0.032±
0.003 a0.006±
0.001 a0.022±
0.003 ab0.005±
0.001 a0.003±
0.001 a0.012±
0.001 a0.009±
0.002 b0.107±
0.008 a0.108±
0.005 a0.005±
0.001 a中部 0.026±
0.004 a0.133±
0.008 ab0.020±
0.003 b0.005±
0.001 a0.019±
0.001 b0.003±
0.001 b0.003±
0.001 a0.013±
0.001 a0.011±
0.001 ab0.098±
0.006 ab0.098±
0.007 a0.004±
0.001 b底部 0.025±
0.004 a0.122±
0.011 b0.015±
0.001 b0.003±
0.001 b0.024±
0.003 a0.002±
0.001 b0.002±
0.001 a0.010±
0.001 a0.013±
0.001 a0.085±
0.005 b0.074±
0.005b0.001±
0.000 c说明:同列不同小写字母表示差异显著(P<0.05)。 Table 3. Content distribution of IAA, ABA and SA in stems of A. roxburghii
-
由表4可见:喷施TIBA 1 d后,不同部位IAA及金线莲苷质量分数均有少量下降,但积累模式无变化,顶端茎段中IAA和金线莲苷质量分数显著高于中部和底部(P<0.05);喷施TIBA 2 d后,IAA和金线莲苷积累模式发生变化,中部和底部IAA质量分数显著高于顶部(P<0.05),中部金线莲苷质量分数与底部无显著差异,两者均显著高于顶部(P<0.05);喷施TIBA 3 d后,中部IAA质量分数显著高于顶端和底部(P<0.05),金线莲苷在中部最高,但与其他部位差异不显著。喷施TIBA 4~5 d后,顶端IAA和金线莲苷质量分数均显著高于中部和底部(P<0.05)。喷施TIBA后,顶端IAA质量分数在处理第4天达到最高,显著高于其他天数(P<0.05),中部IAA质量分数在处理第3天达到最高,底部IAA质量分数在第2天达到最高,且显著高于其他天数(P<0.05);顶端金线莲苷质量分数在处理第4天达到最高,与第5天无显著差异,并显著高于其他天数(P<0.05),中部金线莲苷质量分数在处理第2天达到最高,底部金线莲苷质量分数也在第2天达到最高,且显著高于其他天数(P<0.05)。喷施TIBA处理前后IAA与金线莲苷积累极显著相关(R=0.714,P<0.01)(图3)。以上结果说明:喷施TIBA改变了内源IAA和金线莲苷分布模式,内源IAA积累可能调控金线莲苷合成。
茎段不
同部位IAA/(mg·g−1) 金线莲苷/(mg·g−1) 0 1 2 3 4 5 0 1 2 3 4 5 d 顶端 0.006±
0.001 Ba0.005±
0.001 CDa0.004±
0.000 Cb0.003±
0.001 Eb0.008±
0.001 Aa0.005±
0.000 BCa3.65±
0.30 Ba3.58±
0.07 Ba2.77±
0.27 Cb2.87±
0.09 Ca4.21±
0.01 Aa3.98±
0.07 Aa中部 0.005±
0.001 ABa0.003±
0.000 Bb0.006±
0.001 ABa0.007±
0.002 Aa0.004±
0.003 Bb0.003±
0.000 Bb3.03±
0.81 Bab2.71±
0.34 Bb3.98±
0.28 Aa3.37±
0.34 ABa3.24±
0.43 ABb2.93±
0.24 Bb底部 0.003±
0.001 Bb0.002±
0.001 Bc0.006±
0.001 Aa0.003±
0.003 Bb0.003±
0.019 Bc0.003±
0.001 Bb2.52±
0.02 Bb2.52±
0.11 Bb3.89±
0.08 Aa2.87±
0.31 Ba2.87±
0.21 Bb2.77±
0.70 Bb说明:不同大写字母表示同部位同一激素不同处理时间差异显著(P<0.05);不同小写字母表示相同时间不同部位间差异显著(P<0.05)。 Table 4. Contents of endogenous IAA and kinsenoside before and after TIBA treatment
-
本研究利用HPLC-ELSD尝试用NH2柱和T3色谱柱分离金线莲苷及其差向异构体,NH2柱无法分开这2种物质。利用T3色谱柱,移动相中水的比例为98%~100%,可以分开金线莲苷及其差向异构体,结果与WEI等[25]报道的结果一致。
金线兰4个品系中金线莲苷及其差向异构体质量分数各不相同,说明金线兰品种基因型会影响金线兰中金线莲苷[18−20]及其差向异构体积累,品种基因型是筛选工业化生产金线莲苷药源要考虑的重要因素。在所研究的金线兰中,金线莲苷主要积累于地上部分,根部含量很低,研究结果与金线莲苷组织特异性表达的报道一致[26],金线莲苷差向异构体特异性积累于根部,组织部位是影响金线莲苷[26]及其差向异构体分布模式的另一重要因素。WANG等[24]前期研究发现:金线兰类原球茎金线莲苷质量分数低于3个月全草(大棚苗),本研究中类原球茎中金线莲苷质量分数高于5个月全草(大棚苗),可能的原因是金线兰类原球茎和全草中金线莲苷随着生长发育时期积累模式不同[22]。类原球茎增殖周期短、增殖快,有望代替金线兰用于工业化生产金线莲苷[24]。
在所研究的4个品系茎段中内源IAA与金线莲苷积累模式呈正相关,几乎不受品种基因型影响。茎段中ABA、SA[27]与金线莲苷[20]积累模式在一定程度上受品种基因型影响。内源激素与金线莲苷积累模式正相关说明金线莲苷在金线兰生长防御过程中或发挥一定的作用[28]。TIBA处理改变了内源IAA和金线莲苷积累模式,这说明内源IAA积累拟调控金线莲苷的合成。结果与王林等[29]、于海涛等[30]、麦翠珊等[31]等报道生长素能促进糖苷类物质合成一致。
-
本研究建立金线兰中金线莲苷及其差向异构体质量分数的HPLC-ELSD分析方法,测定了全草、根、茎和叶中金线莲苷及其差向异构体质量分数,金线莲苷特异性积累于地上部分,其差向异构体特异性积累于根部,金线兰地上部分可作为工业化生产金线莲苷的药源。‘尖叶’品系类原球茎中不含金线兰苷差向异构体,并且金线莲苷质量分数高于全草,类原球茎有望代替原植物用于工业化生产金线莲苷。TIBA处理前后,金线兰茎段中内源IAA和金线莲苷积累模式正相关,内源IAA积累可能调控金线莲苷合成。本研究结果可为金线兰质量评价提供基础数据,为工业化生产金线莲苷药源筛选提供方法和思路。
-
华中科技大学药学院周渊对本研究进行了指导,浙江农林大学胡润淮对金线兰品系进行了鉴定,匿名审稿人仔细审阅了手稿,并提出许多宝贵的意见。谨致谢意。
Detection of kinsenoside and it’s isomer, endogenous hormones in Anoectochilus roxburghii
doi: 10.11833/j.issn.2095-0756.20240435
- Received Date: 2024-07-11
- Accepted Date: 2024-12-20
- Rev Recd Date: 2024-12-10
- Available Online: 2025-04-01
- Publish Date: 2025-04-01
-
Key words:
- Anoectochilus roxburghii /
- kinsenoside and it’s isomer /
- distribution pattern /
- endogenous hormone /
- TIBA treatment
Abstract:
| Citation: | LI Lulu, ZHU Zhibin, LI Shuikun, et al. Detection of kinsenoside and it’s isomer, endogenous hormones in Anoectochilus roxburghii[J]. Journal of Zhejiang A&F University, 2025, 42(2): 230−238 doi: 10.11833/j.issn.2095-0756.20240435 |












 DownLoad:
DownLoad:

