-
昆虫在生长发育过程中,体内许多酶发挥着重要作用,如昆虫中肠上皮细胞能形成和分泌消化酶,将食物中的糖类、蛋白质和脂肪等营养物质消化分解成小分子物质供生长发育需要[1−3]。乳酸脱氢酶(LDH)是参与糖酵解的重要酶类,可将丙酮酸催化成乳酸并释放腺嘌呤核苷三磷酸(ATP),从而完成葡萄糖的酵解过程。丙酮酸激酶(PK)可调节细胞中腺嘌呤核苷三磷酸、腺嘌呤核苷二磷酸(ADP)和糖酵解的中间产物,对糖酵解速率的控制起关键作用[4]。超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和过氧化物酶(POD)是昆虫体内重要的保护酶,SOD能够清除超氧阴离子自由基(${\mathrm{O}}_2^{\;\cdot -} $),形成过氧化氢( H2O2 ),而过氧化物酶、过氧化氢酶又具有分解H2O2的作用。在这3种酶的共同作用下,细胞内的自由基维持较低水平,保护昆虫不受伤害[5−6]。羧酸酯酶(CarE)、酸性磷酸酶(ACP)、谷胱甘肽-S-转移酶(GSTs)是昆虫体内重要的解毒酶,能分解代谢农药、植物次生代谢产物等外源毒物,维持昆虫正常生理生化活动[7−9]。
昆虫是变温动物,温度的变化能直接影响其体内酶的活性[10],如在不同温度处理下,桃小食心虫Carposina niponensis体内超氧化物歧化酶活性差异均达到显著水平,在22 ℃时活性最低,28 ℃时活性达最高,34 ℃时活性急剧下降。过氧化物酶和过氧化氢酶活性均低于超氧化物歧化酶活性,整体呈先上升后下降趋势,在28 ℃时活性最高[11]。经过34、36、38 ℃高温处理后,褐飞虱Nilaparvata lugens若虫体内过氧化氢酶活性有随温度升高而升高的趋势,但以36 ℃处理影响最大,高于36 ℃则过氧化氢酶活性受抑制;若虫体内超氧化物歧化酶活性明显高于对照,超氧化物歧化酶活性随温度升高而缓慢上升[12]。酶活性的高低决定了昆虫生理代谢能力的强弱,从而对昆虫发生数量和消长动态产生重要影响[13−15]。研究环境温度对昆虫酶活性的影响,可以为深入探讨昆虫与环境适应性的生理生化机制提供参考。
金银花尺蠖Heterolocha jinyinhuaphaga属鳞翅目Lepidoptera尺蛾科Geometridae昆虫,别名拱腰虫,是金银花Lonicera japonica主要食叶害虫之一,常将金银花叶片咬成缺刻或孔洞,受害严重的地块金银花叶片被全部吃光,常造成金银花的大面积减产,甚至成片死亡,给金银花生产带来严重损失[16−20]。笔者在前期研究了温度对金银花尺蠖幼虫消化酶活性的影响[21],在此基础上研究温度对金银花尺蠖幼虫、蛹、雌蛾、雄蛾体内乳酸脱氢酶、过氧化物酶、羧酸酯酶、酸性磷酸酶活性的影响,分析金银花尺蠖幼虫、蛹、雌蛾、雄蛾对不同环境温度的生理生化适应性,为深入研究金银花尺蠖对环境适应性的生理生化机制提供科学依据。
-
金银花尺蠖幼虫采集于安徽省明光市三界镇,在人工气候箱(RXZ-288A型)中用新鲜的金银花叶片饲养。人工气候箱的光周期设置为白天14 h/黑暗10 h,温度设置为(25±1) ℃,相对湿度设置为70%±7%。
-
前期研究表明:25 ℃左右是金银花尺蠖取食的最适温度[22]。试验设16、19、22、25、28、31、34 ℃等7个恒温处理,光照周期设置为白天14 h/黑暗10 h,相对湿度设置为70%。取刚孵化第1天的金银花尺蠖幼虫,单头放在各个罐头瓶中,并放入新鲜金银花叶片(用湿棉花包裹叶片基部以保持叶片新鲜,待叶片不新鲜时更换叶片),用纱布罩住瓶口并用橡皮筋扎紧,定期观察,记载各虫态出现的时间,一直饲养到成虫阶段。成虫羽化后,喂质量分数为10%的蔗糖水。每个温度下1次饲养50头幼虫,试验重复6次。
-
每处理挑取大小一致的金银花尺蠖5龄幼虫、蛹(化蛹7 d)、雌蛾(羽化3 d)、雄蛾(羽化3 d)各10头,雌蛾、雄蛾剪去翅膀及附肢。用去离子水将虫体洗干净,滤纸擦干,冰浴中加入预冷的磷酸盐缓冲液(PBS) 5 mL,迅速研磨成匀浆,离心15 min (−4 ℃,12 000 r·min−1),取上清液作酶液。制备乳酸脱氢酶、过氧化物酶、羧酸酯酶、酸性磷酸酶所用的磷酸盐缓冲液浓度分别为0.1 mmol·L−1、0.2 mmol·L−1、0.1 mol·L−1、0.1 mol·L−1,pH分别为7.5、7.0、7.6、7.0。以牛血清蛋白(BSA)为标准蛋白,采用考马斯亮蓝G-250染色法测定样品中酶蛋白质量。
-
采用紫外分光光度法测定乳酸脱氢酶活性。在代谢中磷酸烯醇式丙酮酸和腺嘌呤核苷二磷酸生成腺嘌呤核苷三磷酸和丙酮酸,乳酸脱氢酶进一步催化还原型烟酰胺腺嘌呤二核苷酸(NADH)、丙酮酸生成乳酸和烟酰胺腺嘌呤二核苷酸(NAD+)[23]。由于还原型烟酰胺腺嘌呤二核苷酸在340 nm处有最大吸收峰值,添加一定量的酶液,观察还原型烟酰胺腺嘌呤二核苷酸反应过程中340 nm处吸光度[D(340)]减少量,下降越多表明乳酸脱氢酶的活性越高。因此,将乳酸脱氢酶与丙酮酸以及还原型烟酰胺腺嘌呤二核苷酸反应后,测340 nm处的吸光度变化,便可得到该酶的酶活性。在25 ℃,pH 7.5条件下,以1 min吸光度降低值[ΔD(340)]为1的酶量为1个酶活性单位。通过定量测定酶蛋白质量计算酶活性,酶活性单位为mmol·g−1·min−1。
将丙酮酸溶液(2.5 mg丙酮酸用29 mL 磷酸盐缓冲液溶解)和还原型烟酰胺腺嘌呤二核苷酸液(3.5 mg 纯还原型烟酰胺腺嘌呤二核苷酸用1 mL 磷酸盐缓冲液溶解)放在25 ℃水浴锅中进行预热。取1只比色皿,加入3 mL磷酸盐缓冲液 (0.1 mol·L−1,pH 7.5),置于分光光度计中作对照;另取1只比色皿,加入2.9 mL丙酮酸溶液和0.1 mL还原型烟酰胺腺嘌呤二核苷酸溶液,混匀后测吸光度,再迅速加入稀释20倍的酶液10 μL,开始计时,每30 s记录1次数据,持续测定3 min。用吸光度对时间制作线性反应图,计算1 min吸光度降低值(ΔD(340))。每个处理重复6次,根据公式计算乳酸脱氢酶活性:乳酸脱氢酶活性={[ΔD(340)×稀释倍数] / [加入的酶量×10−3]},酶活性单位为mmol·g−1·min−1。
-
采用愈创木酚比色法测定过氧化物酶活性[9]。在试管中加入1 mL磷酸盐缓冲液(0.2 mmol·L−1,pH 7.0),1 mL过氧化氢(H2O2,质量分数为30%),0.9 mL愈创木酚(30 mmol·L−1)和0.1 mL酶溶液,让混合物反应5 min后,在470 nm波长下测定吸光度[D(470)]。对照组加入等量的蒸馏水代替酶溶液。每个处理重复6次。将1 mg酶引起的每分钟D(470)变化定义为1个酶活性单位。过氧化物酶活性按以下公式计算:过氧化物酶活性= ΔD(470)/ [t×W×(V0/V)]。其中:ΔD(470)是吸光度随反应时间的变化,t是反应时间(min),V是酶溶液体积(mL),V0是反应混合物体积(mL),W是酶溶液中的蛋白质质量浓度(g·L−1),酶活性单位为mmol·g−1·min−1。
-
采用紫外分光光度法测定羧酸酯酶活性[24]。将10 μL酶液、400 μL α-乙酸萘酯(300 μmol·L−1)、30 μL毒扁豆碱(10 μmol·L−1)和560 μL 磷酸盐缓冲液(0.1 mol·L−1,pH 7.6)置于试管中。用振荡器振荡(30 ℃) 30 min后,在反应混合物中加入100 μL显色剂(质量分数为1%的固蓝B盐∶质量分数为5%的十二烷基硫酸钠溶液= 2∶5)以终止反应。15 min后在600 nm波长下测定反应混合物的吸光度[D(600)]。建立α-萘酚的标准曲线,用于计算产量。每分钟1 mg酶催化1 μmol的产物被定义为1个酶活性单位。对照组加入等量的蒸馏水代替酶溶液。每个处理重复6次。羧酸酯酶活性按照以下公式计算:羧酸酯酶活性= P/[t×W×(V0/V)][24] ,其中:P为产物量(μmol),t为反应时间(min),V为酶液体积(mL),V0为反应混合物体积(mL),W为酶溶液中蛋白质质量浓度(g·L−1),酶活性单位为mmol·g−1·min−1。
-
用50 μL酶液测定酸性磷酸酶活性。方法按试剂盒说明书(苏州康明生物技术有限公司)。酶活性单位定义为:在37 ℃下,每分钟1 mg蛋白质催化1 μmol苯酚生成的产物。酶活性单位为mmol·g−1·min−1。每个处理重复6次。
-
采用SPSS 23软件进行方差分析,采用Duncan氏新复极差法在0.05水平上进行差异显著性检验。
-
不同温度下金银花尺蠖幼虫、蛹、雌蛾和雄蛾的乳酸脱氢酶活性均不相同(图1)。温度为16 ℃时乳酸脱氢酶活性分别为12.88、10.37、14.47和13.09 mmol·g−1·min−1。当温度为16~34 ℃时,随着温度的升高,乳酸脱氢酶活性不断上升,在22 ℃时最高,分别为17.93、15.25、19.63和18.81 mmol·g−1·min−1,比16 ℃增加了39.21%、47.06%、35.66%和43.70%。温度进一步升高,乳酸脱氢酶活性则开始下降,34 ℃时下降到最低,为9.87、8.26、12.52和10.66 mmol·g−1·min−1。在幼虫中,除19和25 ℃外,22 ℃时的乳酸脱氢酶活性与其他温度相比差异显著(F6,14=7.807,P=0.001);除19和25 ℃外,蛹在22 ℃时的乳酸脱氢酶活性与其他温度相比差异显著(F6,14=7.96,P=0.001);除25 ℃外,雌蛾在22 ℃时的乳酸脱氢酶活性与其他温度相比差异显著(F6,14=6.18,P=0.002);雄蛾在22 ℃时的乳酸脱氢酶活性与其他温度相比差异显著(F6,14=10.338,P<0.001)。
在相同温度下,金银花尺蠖雌蛾的乳酸脱氢酶活性最高,其次是雄蛾,然后是幼虫,蛹最低。16 ℃ (F3,8 =4.128,P=0.048),19 ℃ (F3,8 =2.256,P=0.039),22 ℃ (F3,8 =2.537,P=0.032)和25 ℃ (F3,8 =2.407,P=0.043)时,雌蛾的乳酸脱氢酶活性与蛹的差异达显著水平,与幼虫和雄蛾差异不显著。28 ℃(F3,8 =5.286,P=0.027)和34 ℃(F3,8 =7.244,P=0.011)时,雌蛾的乳酸脱氢酶活性与幼虫和蛹差异显著,与雄蛾差异不显著。31 ℃时,雌蛾的乳酸脱氢酶活性与幼虫、蛹和雄蛾差异显著(F3,8 =12.223,P=0.002)。
方差分析表明:温度、虫态对金银花尺蠖的乳酸脱氢酶活性均有显著影响(P<0.001),但温度和虫态的交互作用对金银花尺蠖的乳酸脱氢酶活性没有显著影响。
对不同温度下金银花尺蠖幼虫、蛹、雌蛾和雄蛾的乳酸脱氢酶活性的变化趋势进行回归分析,得出乳酸脱氢酶活性与温度的回归模型分别为:y1= 0.004 9x3−0.420 2 x2 +11.263 0x−80.093 0 (R2=0.911 5),y2= 0.005 1x3−0.441 2x2 + 11.886 0x−88.184 0 (R2 = 0.968 8),y3= 0.003 3x3−0.302 0x2 + 8.550 0x−58.898 0 (R2 =0.884 2),y4= 0.005 6x3−0.478 5x2 + 12.823 0x−92. 872 0 (R2 = 0.917 0), y1、y2、y3、y4分别为金银花尺蠖幼虫、蛹、雌蛾和雄蛾体内乳酸脱氢酶活性,x为温度。对模型进行计算,得出金银花尺蠖幼虫、蛹、雌蛾和雄蛾的乳酸脱氢酶活性最高的最适温度分别为21.45、21.44、22.32和21.56 ℃。
-
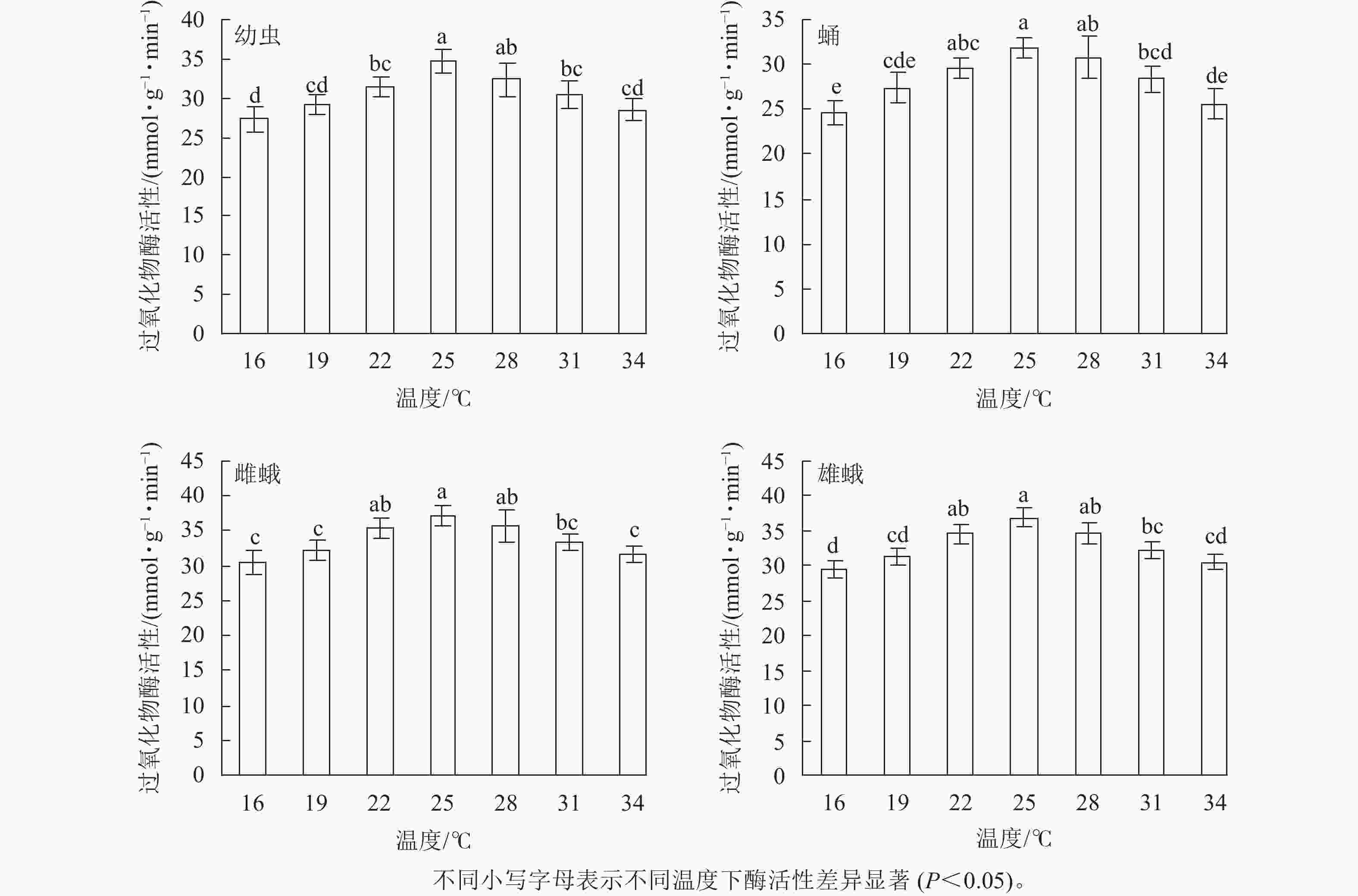
不同温度下金银花尺蠖幼虫、蛹、雌蛾和雄蛾的过氧化物酶活性均不相同(图2)。16 ℃时过氧化物酶活性最低,分别为27.31、24.62、30.49和29.59 mmol·g−1·min−1。温度为16~34 ℃时,随着温度的升高,过氧化物酶活性呈先上升后下降的趋势,在25 ℃时最高,分别为34.63、31.83、37.19和36.87 mmol·g−1·min−1,比16 ℃时增加了26.80%、29.29%、12.68%和24.60%。之后,过氧化物酶活性逐渐下降,34 ℃时下降为28.53、25.58、31.62和30.51 mmol·g−1·min−1。 除28 ℃外,幼虫在25 ℃时的过氧化物酶活性与其他温度相比差异显著(F6,14=7.336,P=0.001);除22和28 ℃外,蛹(F6,14=8.339,P=0.001),雌蛾(F6,14=6.996,P=0.001)和雄蛾(F6,14=12.456,P<0.001)在25 ℃时的过氧化物酶活性与其他温度相比差异显著。
在相同温度下,金银花尺蠖雌蛾的过氧化物酶活性最高,其次是雄蛾,然后是幼虫,蛹最低。16 ℃(F3,8 =9.392,P=0.005),19 ℃ (F3,8 =6.914,P=0.013),22 ℃ (F3,8 =12.877,P=0.002)和34 ℃ (F3,8 =11.565,P=0.003)时,雌蛾的过氧化物酶活性与幼虫和蛹差异达显著水平,与雄蛾差异不显著。25 ℃ (F3,8 =9.962,P=0.004)和28 ℃ (F3,8 =3.358,P=0.046)时,雌蛾的过氧化物酶活性与蛹差异显著,与幼虫和雄蛾差异不显著。31 ℃时,雌蛾的过氧化物酶活性与幼虫和蛹差异显著(F3,8 =7.301,P=0.011),与雄蛾差异不显著。
方差分析表明:温度、虫态对金银花尺蠖的过氧化物酶活性均有显著影响(P<0.001),但温度和虫态的交互作用对金银花尺蠖的过氧化物酶活性没有显著影响。
对不同温度下金银花尺蠖幼虫、蛹、雌蛾和雄蛾的过氧化物酶活性的变化趋势进行回归分析,得出过氧化物酶活性与温度的回归模型分别为:y1=−0.001 1x3 + 0.018 8x2 +1.360 7x+5.033 1 (R2= 0.875 1),y2=−0.001 2x3+ 0.013 5x2 + 1.707 1x−1.454 7 (R2 = 0.970 4),y3=−0.000 3x3−0.044 2x2 + 2.901 1x−3.728 5 (R2 = 0.917 0),y4 =−0.000 3x3−0.049 5x2 + 3.133 4x−7.085 0 (R2 = 0.892 0),y1、y2、y3、y4分别为金银花尺蠖幼虫、蛹、雌蛾和雄蛾体内过氧化物酶活性,x为温度。对模型进行计算,得出金银花尺蠖幼虫、蛹、雌蛾和雄蛾的过氧化物酶活性最高的最适温度分别为26.16、25.94、25.67和25.54 ℃。
-
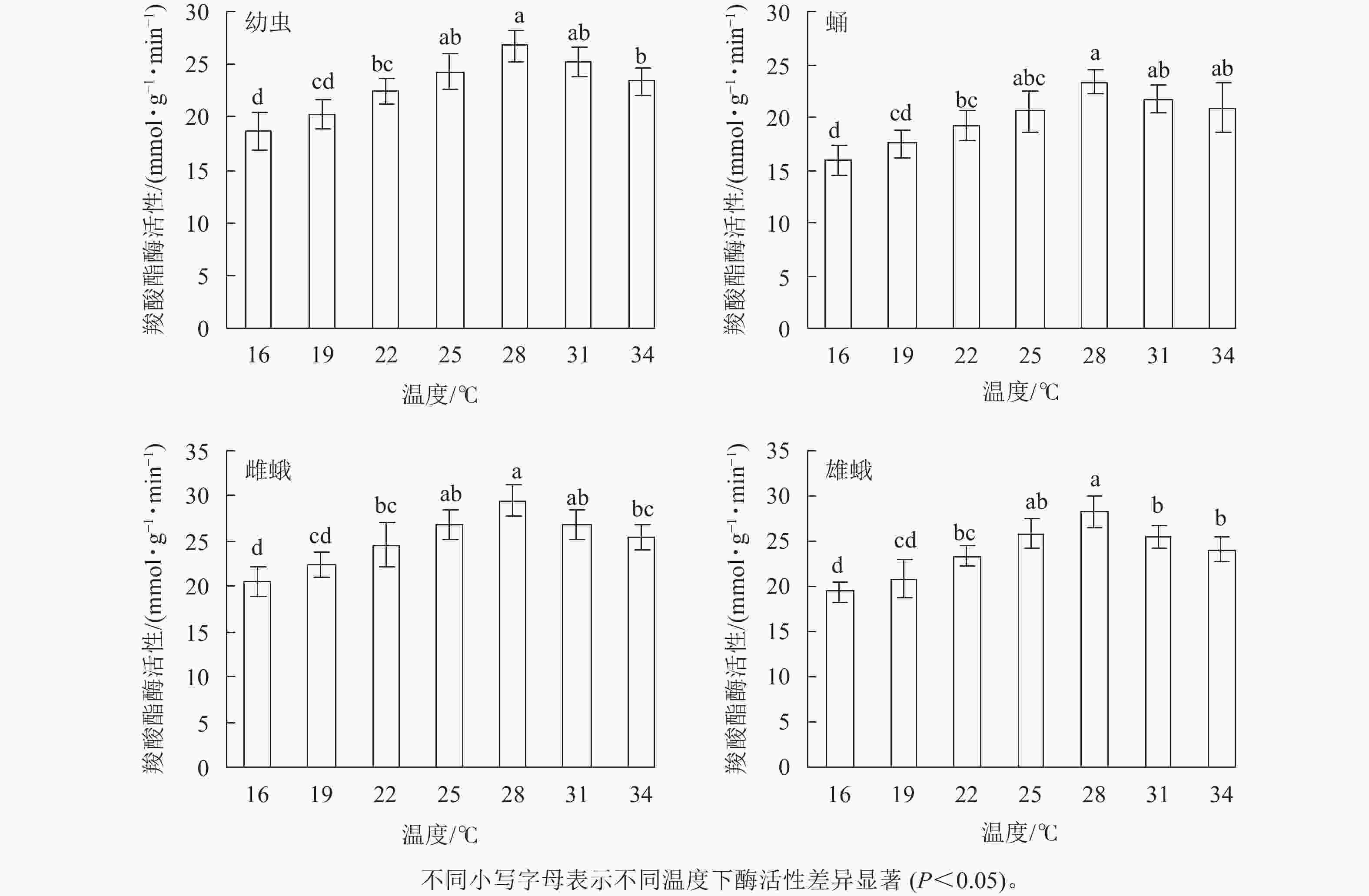
不同温度下金银花尺蠖幼虫、蛹、雌蛾和雄蛾羧酸酯酶活性均不相同(图3)。温度为16℃时最低,羧酸酯酶活性分别为18.64、15.93、20.48和19.42 mmol·g−1·min−1。温度为16~34 ℃时,羧酸酯酶活性呈先上升后下降的趋势,28 ℃时最高,分别为26.78、23.36、29.44和28.32 mmol·g−1·min−1,比16 ℃增加了43.67%、46.64%、43.75%和45.83%。之后,羧酸酯酶活性开始下降,34 ℃下降为23.39、 20.88、 25.37和24.11 mmol·g−1·min−1。 除25和31 ℃外,幼虫(F6,14=11.407,P<0.001)和雌蛾(F6,14=9.11,P<0.001)在28 ℃时的羧酸酯酶活性与其他温度相比差异显著;除25、31和34 ℃外,蛹在28 ℃时的羧酸酯酶活性与其他温度相比差异显著(F6,14=7.397,P=0.001);除25 ℃外,雄蛾在28 ℃时的羧酸酯酶活性与其他温度相比差异显著(F6,14=6.93,P<0.001)。
在相同温度下,金银花尺蠖雌蛾的羧酸酯酶活性最高,其次是雄蛾,然后是幼虫,蛹最低。16 ℃(F3,8 =4.908,P=0.032),19 ℃(F3,8 =4.713,P=0.035),22 ℃(F3,8 =5.981,P=0.019),25 ℃(F3,8 =7.884,P=0.009),28 ℃(F3,8 =8.581,P=0.007),31 ℃(F3,8 =6.842,P=0.013)和34 ℃(F3,8 =3.881,P=0.046)时,雌蛾的羧酸酯酶活性与蛹的差异达显著水平,与幼虫和雄蛾差异不显著。
方差分析表明:温度、虫态对金银花尺蠖的羧酸酯酶活性均有显著影响(P<0.001),但温度和虫态的交互作用对金银花尺蠖的羧酸酯酶活性没有显著影响。
对不同温度下金银花尺蠖幼虫、蛹、雌蛾和雄蛾的羧酸酯酶活性的变化趋势进行回归分析,得出羧酸酯酶活性与温度的回归模型分别为:y1=−0.125 8x3 +1.096 2x2−0.830 8x+18.516 0 (R2=0.978 8),y2=−0.094 7x3+ 0.826 0x2−0.417 2x+ 15.646 0 (R2 = 0.953 5),y3=−0.125 8x3 +1.031 4x2−0.308 5x+ 19.816 0 (R2 =0.951 8),y4=−0.136 4x3−1.151 9x2−0.703 1x+18.957 0 (R2 = 0.942 7),y1、y2、y3、y4分别为金银花尺蠖幼虫、蛹、雌蛾和雄蛾体内羧酸酯酶活性,x为温度。对模型进行计算,得出金银花尺蠖幼虫、蛹、雌蛾和雄蛾的羧酸酯酶活性最高的最适温度分别为29.20、29.65、28.93和28.92 ℃。
-
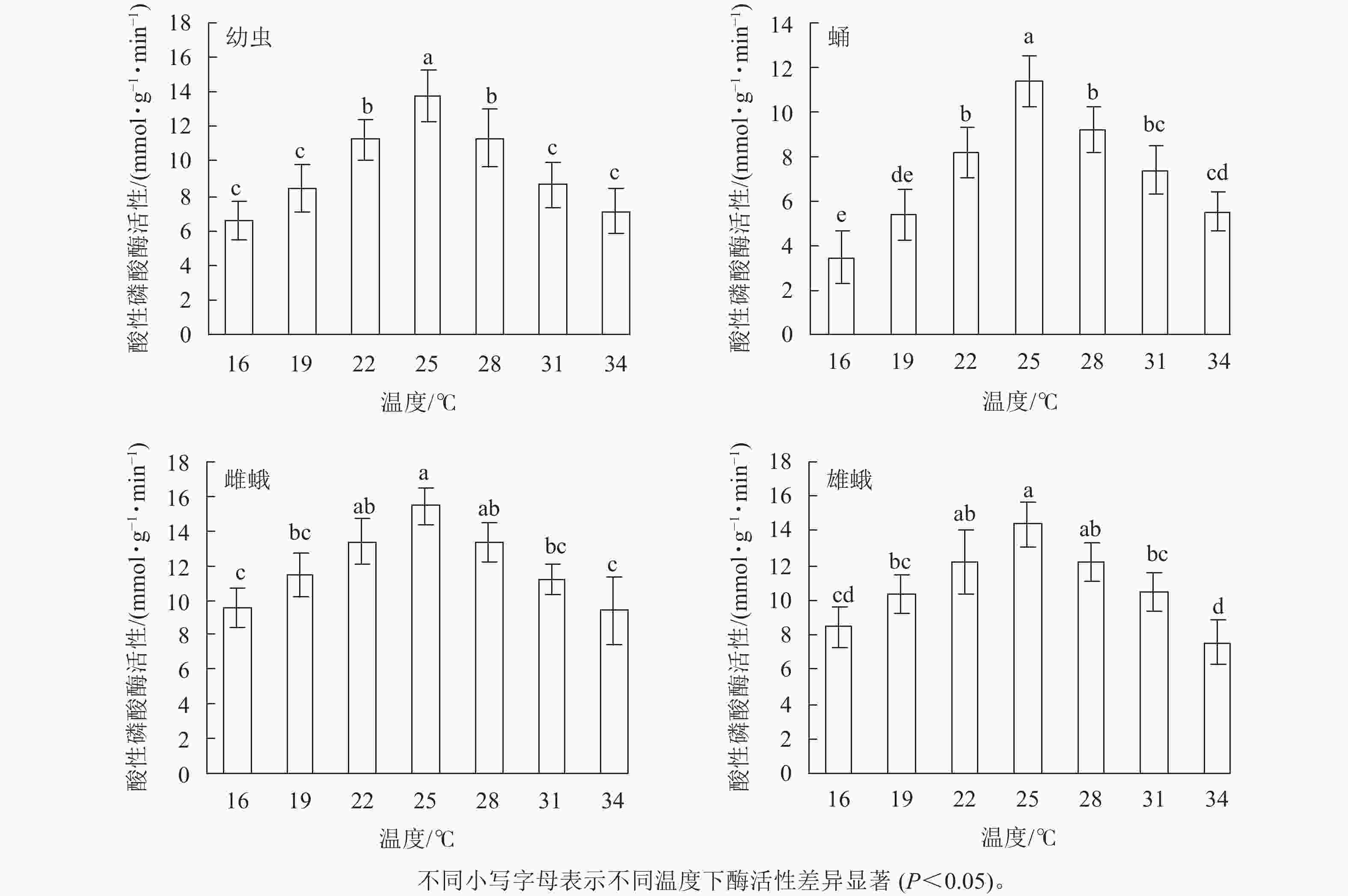
不同温度下金银花尺蠖幼虫、蛹、雌蛾和雄蛾的酸性磷酸酶活性均不相同(图4)。温度为16 ℃时酸性磷酸酶活性分别为6.57、3.46、9.56和8.45 mmol·g−1·min−1。温度为16~34 ℃时,酸性磷酸酶活性随着温度的升高呈先上升后下降的趋势,在25 ℃时最高,分别为13.82、11.37、15.43和14.38 mmol·g−1·min−1,比16 ℃增加了110.35%、228.61%、61.40%和70.18%。之后,酸性磷酸酶活性开始下降,34 ℃下降为7.13、5.54、9.39和7.57 mmol·g−1·min−1。在幼虫(F6,14=11.404,P<0.001)和蛹(F6,14=17.869,P<0.001)中,25 ℃时的酸性磷酸酶活性与其他温度相比差异显著;除22和28 ℃外,雌蛾(F6,14=8.989,P<0.001)和雄蛾(F6,14=9.749,P<0.001)在25℃时的酸性磷酸酶活性与其他温度相比差异显著。
在相同温度下,金银花尺蠖雌蛾的酸性磷酸酶活性最高,其次是雄蛾,然后是幼虫,蛹最低。16 ℃(F3,8 =16.284,P=0.001),19 ℃(F3,8 =14.893,P=0.001),28 ℃(F3,8 =5.641,P=0.023)和31 ℃(F3,8 =7.329,P=0.011)时,雌蛾的酸性磷酸酶活性与幼虫和蛹的差异达显著水平,与雄蛾差异不显著。22 ℃(F3,8 =7.668,P=0.01),25 ℃(F3,8 =5.7,P=0.022)和34 ℃(F3,8 =3.815,P=0.048)时,雌蛾的酸性磷酸酶活性与蛹的差异达显著水平,与幼虫和雄蛾差异不显著。
方差分析表明:温度、虫态对金银花尺蠖的酸性磷酸酶活性均有显著影响(P<0.001),但温度和虫态的交互作用对金银花尺蠖的酸性磷酸酶活性没有显著影响。
对不同温度下金银花尺蠖幼虫、蛹、雌蛾和雄蛾的酸性磷酸酶活性的变化趋势进行回归分析,得出酸性磷酸酶活性与温度的回归模型分别为:y1=0.007 8x3−0.743 8x2 +5.600 6x+1.208 6 (R2 = 0.867 8 ),y2 =−0.027 8x3−0.295 2x2 + 4.294 7x−0.941 4 (R2 = 0.888 1 ),y3 =0.002 2x3−0.588 1x2 + 4.547 5x+ 5.292 9 (R2 = 0.909 6),y4 =−0.026 4x3−0.287 7x2 + 3.665 1x+ 4.860 0 (R2 = 0.937 0), y1、y2、y3、y4分别为金银花尺蠖幼虫、蛹、雌蛾和雄蛾体内酸性磷酸酶活性,x为温度。对模型进行计算,得出金银花尺蠖幼虫、蛹和雌蛾和雄蛾的酸性磷酸酶活性最高的最适温度分别为25.05、26.39、24.86和25.24 ℃。
-
温度是影响昆虫生理代谢过程的重要环境因子,温度的变化能直接影响昆虫体内酶的活性,从而引起体内一系列生理变化。不同温度下金银花尺蠖幼虫、蛹、雌蛾和雄蛾体内乳酸脱氢酶、过氧化物酶、羧酸酯酶和酸性磷酸酶的活性均不一样,温度为16~34 ℃时,这4种酶活性均随着温度的升高表现为先上升后下降的趋势。乳酸脱氢酶活性在22 ℃时最高,34 ℃时最低;过氧化物酶活性在25 ℃时最高,16 ℃最低;羧酸酯酶活性在28 ℃时最高,16 ℃时最低;酸性磷酸酶活性在25 ℃时最高,幼虫和蛹在16 ℃时最低,雌蛾和雄蛾在34 ℃时最低,说明这4种酶对温度的敏感性不同。其他昆虫也类似,如中华真地鳖Eupolyphaga sinensis体内的过氧化物酶活性在30 ℃时最高,在34 ℃时最低[25];桃小食心虫体内,过氧化物酶活性在28 ℃时最高,在34 ℃时最低[11]。但星豹蛛Pardosa astrigera雌成蛛、雄成蛛体内过氧化物酶活性在5 ℃时最低,30 ℃以下随温度的升高变化平缓,且在20 ℃时略有下降,而在35 ℃时急剧升高,均达最大值[26],表明不同生物体内过氧化物酶活性受温度的影响存在差异。
糖酵解途径是昆虫体内重要的能量代谢途径,可以产生大量丙酮酸和少量的腺嘌呤核苷三磷酸,乳酸脱氢酶可催化丙酮酸生成乳酸并释放腺嘌呤核苷三磷酸,从而完成葡萄糖的酵解过程。25 ℃时乳酸脱氢酶活性最高,说明金银花尺蠖在此温度下糖代谢途径速率高,有利于生长发育。过氧化物酶是保护酶,25 ℃时过氧化物酶活性最高,说明金银花尺蠖在此温度下分解H2O2的能力强,保护自身免受氧化伤害。羧酸酯酶和酸性磷酸酶是解毒酶,28 ℃时羧酸酯酶活性最高,25 ℃时酸性磷酸酶活性最高,说明此时金银花尺蠖解毒能力最强,能够清除大量的外源毒物。这与金银花尺蠖的生长气候条件相吻合。金银花在春季和夏初季节生长迅速,尤其在夏季多雨期,而金银花尺蠖体内这些酶活性恰好在22~28 ℃时最高,有利于糖代谢的进行和对外源毒物的清除,加速生长发育,导致其大发生。在夏末高温天气,这些酶活性下降,金银花尺蠖的代谢减弱,生长发育受阻,虫口数量下降。到10月,环境温度下降,低于适宜温度,这些酶活性又开始下降,金银花尺蠖的代谢减弱,开始以蛹越冬。
在同一温度下,金银花尺蠖雌蛾体内的乳酸脱氢酶、过氧化物酶、羧酸酯酶和酸性磷酸酶活性最高,蛹体内这4种酶活性最低。这可能是雌蛾除了完成生长发育外,还要承担产卵和繁殖后代的任务,代谢旺盛,需要更高的酶活性,而金银花尺蠖以蛹越冬,蛹的生存环境相对稳定,耗能少,代谢弱,酶的活性也就最低。
-
测定了金银花尺蠖幼虫、蛹、雌蛾和雄蛾在不同温度下乳酸脱氢酶、过氧化物酶、羧酸酯酶和酸性磷酸酶的活性。结果表明:温度能影响金银花尺蠖幼虫、蛹、雌蛾和雄蛾体内乳酸脱氢酶、过氧化物酶、羧酸酯酶和酸性磷酸酶的活性。
Effect of temperature on the activity of 4 enzymes in Heterolocha jinyinhuaphaga
doi: 10.11833/j.issn.2095-0756.20240471
- Received Date: 2024-08-02
- Accepted Date: 2024-10-08
- Rev Recd Date: 2024-10-03
- Available Online: 2025-05-30
- Publish Date: 2025-05-30
-
Key words:
- Heterolocha jinyinhuaphaga /
- temperature /
- enzyme activity
Abstract:
| Citation: | XIANG Yuyong, ZHANG Yan, TAO Cuiling. Effect of temperature on the activity of 4 enzymes in Heterolocha jinyinhuaphaga[J]. Journal of Zhejiang A&F University, 2025, 42(3): 554−563 doi: 10.11833/j.issn.2095-0756.20240471 |











 DownLoad:
DownLoad:


