-
土壤有效磷缺乏已经成为限制农作物生长的主要因素之一[1]。中国南方土壤多呈酸性或强酸性,活性铁、铝含量较高,易与可溶性磷结合生成难溶的磷酸铁、磷酸铝等螯合物。研究表明,约90%的酸性土壤有效磷低于2 mg·kg−1[2]。尽管南方酸性土壤总磷含量相对丰富,包括磷酸肌醇、核酸、磷脂及少量的磷蛋白、微生物磷等以及铝铁螯合态磷,但这些磷只有被水解和溶解成可溶性无机磷才能被植物吸收利用[3]。
在中国南方酸性土壤环境下,玉米Zea mays的生长普遍受到土壤有效磷缺乏的限制。玉米主要通过以下响应来适应低磷胁迫、促进磷的吸收利用:强化根系特征以提高磷的获取效率,如改变根结构特征,形成簇生根和侧根等,或是改变根的形态特征,形成更细长的根和更多的根毛来扩大根系的吸收范围和吸收面积[4];分泌更多的根系分泌物,激发微生物的活性,促进有机质分解和养分释放[5],其中,分泌物中的羧化物能够活化土壤中被固持的磷,磷酸酶可水解有机磷;与真菌形成菌根共生体,其中丛枝菌根(arbuscular mycorrhiza,AM)是内生菌根最主要的类型,丛枝菌根真菌可与70%~80%的陆地植物形成菌根共生体[6]。丛枝菌根真菌定殖根系后可形成菌丝、泡囊和“丛枝”状结构等[7]。根外菌丝在土壤中形成广泛的菌丝网络作为根系的延伸,进入根系自身无法进入的小孔隙中,增加了植物吸收土壤磷的面积,吸收范围可扩大至17 cm外[8]。
植物、土壤、微生物的碳-磷循环是相互耦合的,土壤有效磷含量势必会影响土壤碳动态,特别是在根际热点微区域[9]。土壤有效磷含量一方面影响玉米植株生长和光合产物的地下分配,从而直接影响土壤碳循环[10];另一方面通过影响根系分泌物的组成和数量,改变根际微生物数量、活性、群落结构和酶活性等,进而影响土壤碳动态[11]。但是土壤有效磷含量对碳动态的影响程度和方向仍未得到一致性的结论。土壤有效磷缺乏会增加玉米光合产物地下分配的比例,例如增加根冠比和根系分泌物量。但严重的磷匮乏会使玉米的正常生长发育受阻,植株生长缓慢、个体矮小,进而减少光合产物地下分配的绝对量[12]。根系分泌物对微生物群落结构和活性的影响也具有不确定性[13],一方面根系分泌物为根际微生物提供丰富的碳源和其他能源物质,能够有效提高土壤微生物的数量和活性,进而促进土壤有机质降解[14];另一方面微生物优先利用根系分泌物以及植物与微生物的养分竞争,抑制了土壤有机质的降解[15]。此外,菌根真菌对土壤碳循环的影响也具有争议性,一方面菌根可通过菌丝分泌物激发其他土壤微生物的活性,促进有机质的分解;另一方面丛枝菌根分泌的球囊霉素蛋白(glomalin-related soil protein,GRSP)能够提高土壤结构稳定性和减慢有机质的分解速度[16]。总之,土壤有效磷含量对玉米根际碳动态的影响取决于植物生理生态反应、植物与微生物互作及微生物间互作等复杂的过程。本研究以玉米为对象,探究土壤有效磷对玉米生长、光合产物的地下分配规律以及根际微生物群落结构的响应机制,揭示并阐明玉米对低磷胁迫的响应机制,评估这些响应对根际土壤水解酶活性和碳动态的影响。
-
本研究采用根箱栽培试验[17],地点位于浙江农林大学玻璃温室大棚。根箱为35 cm×18 cm×2 cm的亚克力盒子,其中18 cm×2 cm的一面为开口,35 cm×18 cm的一面为活面。每个根箱装土1.1 kg。根箱在大棚内倾斜,活面朝下放置在架子上,种子放置在贴近活面、靠近开口侧的土壤中。
试验土壤是先将粒径为0.5 mm与1.0~2.0 mm的石英砂以2∶1的质量比混合配制为专用石英砂,再将过2 mm筛的贫瘠风干土与专用石英砂以7∶3的质量比混合优化土壤结构所得,以避免土壤板结。风干土采自浙江农林大学碳汇楼后山,根据联合国粮食及农业组织的土壤分类系统(WRB,2006年),该土壤被分类为铁铝土。土壤理化性质:pH 5.21,有效氮51.0 mg·kg−1,总磷288.0 mg·kg−1,有效磷2.6 mg·kg−1,有效钾193.0 mg·kg−1,有机碳28.5 mg·g−1,土壤中存在孢子菌丝等侵染结构。试验土壤混合后加入溶解好的肥料:氮200.0 mg·kg−1、钾200.0 mg·kg−1、镁100.0 mg·kg−1、铁2.0 mg·kg−1、锌2.6 mg·kg−1、铜1.0 mg·kg−1。在以上肥料的基础上,依据不同质量分数磷的有效性,32.0 mg·kg−1代表低磷条件,140.0 mg·kg−1为正常或充足的供磷水平,本研究设置2种磷处理:低磷(32.0 mg·kg−1)和高磷(140.0 mg·kg−1)。
玉米种子选用‘郑单958’,先将种子用质量分数为10%的过氧化氢溶液浸泡30 min进行表面消毒后,洗净,再用蒸馏水浸泡8 h,然后捞出种子,放置在2张湿润的滤纸之间,在直径为20 cm的玻璃培养皿中,恒温24 ℃,置于避光的气候箱内培养3 d以发芽。发芽的种子于2023年夏季分批种植,每处理设置6个重复,每个根箱种1株植物。
-
种植6周后,用抖根法[18]采集根际土,过2 mm筛后平均分为3份,一份立即测定鲜土指标,另两份分别于−20 ℃、−80 ℃保存。
称取1.00 g鲜土于离心管中,加入100 mL醋酸钠缓冲液,充分震荡30 min (温度25 ℃,转速180 r·min−1)后,用磁力搅拌器搅拌1 min (转速125 r·min−1),使用移液枪在96孔酶标板中分别加入50 μL土壤悬浮液、50 μL醋酸钠缓冲液、100 μL底物(分别用4-MUB-β-D-葡萄糖苷、4-MUB-β-D-纤维二糖苷、4-MUB-N-acetyl-β-D-葡萄糖胺苷、4-MUB-磷酸盐作为β-葡萄糖苷酶、纤维素二糖水解酶、β-N-乙酰基氨基葡萄糖苷酶、酸性磷酸酶的反应底物)。置于25 ℃恒温培养箱中培养4 h后,用多功能酶标仪(SpectraMax M5,Molecular Devices)测定其荧光度[19]。
取1.00 g风干土样于离心管中,采用考马斯亮蓝法,测定并计算易提取态球囊霉素蛋白(easily extracted GRSP,EE-GRSP)和总球囊霉素蛋白(total GRSP,T-GRSP)质量分数[20]。
取适量−80 ℃土壤样本,土壤DNA提取、PCR扩增和高通量测序由上海美吉生物医药科技有限公司完成,方法参照张骏达等[21]。使用该公司提供的Illumina MiSeq 300平台进行扩增测序,基于97%的相似度,对序列进行操作分类单位(operational taxonomic unit,OTU)聚类。
-
将收集完根际土的植株根系用蒸馏水冲洗干净后,把植株根部浸泡在50 mL的蒸馏水中120 min,然后收集含有根系分泌物的液体[22]。根系分泌物中的可溶性碳(dissolved organic carbon,DOC)分泌速率使用总有机碳分析仪测定[23]。
-
将收集完根系分泌物植株的根剪下,放入装有质量分数为70%的酒精的塑料瓶中保存。将根在根盘中平铺、分离开,尽量保证根系分散无重叠和交叉,利用根系扫描仪(GT-X980,爱普生)扫描根系,WinRHIZO软件分析根系属性特征[24];将扫描后的样品根系在70 ℃条件下烘48 h,称其干质重;取直径为1 mm的新鲜根,用蒸馏水冲洗干净,剪成1 cm长的根段,放在质量分数为10%的氢氧化钾溶液中水浴加热(1 h,90 ℃)至透明,在质量分数为1%的盐酸溶液中酸化,用蓝墨水染色法进行染色,光学显微镜下观察并统计菌根侵染率[25]。
-
将收集完根系分泌物的植株的根剪下后,留下的植株地上部分在烘箱里105 ℃杀青30 min,再60 ℃烘干6 h至恒量后,称量每个植株地上部分干质量[26];将烘干后的植株研磨后,用HNO3-H2O2消煮,采用电感耦合等离子体原子发射光谱仪(ICAP-7000,赛默飞)测定叶片磷质量分数[27]。
-
采用Excel 2019和SPSS 22.0进行数据分析。采用独立样本T检验进行方差分析,对不同磷质量分数处理下的结果进行Duncan’s法差异显著性检验,显著性水平为0.05。对得到的所有OTU进行α多样性分析、β多样性分析(非度量多维尺度分析)和相对丰度组间差异显著性检验。采用线性回归法分析酶活性与根际微生物物种相对丰度的相关关系。采用Origin 2022绘图。数据结果以平均值±标准差表示。
-
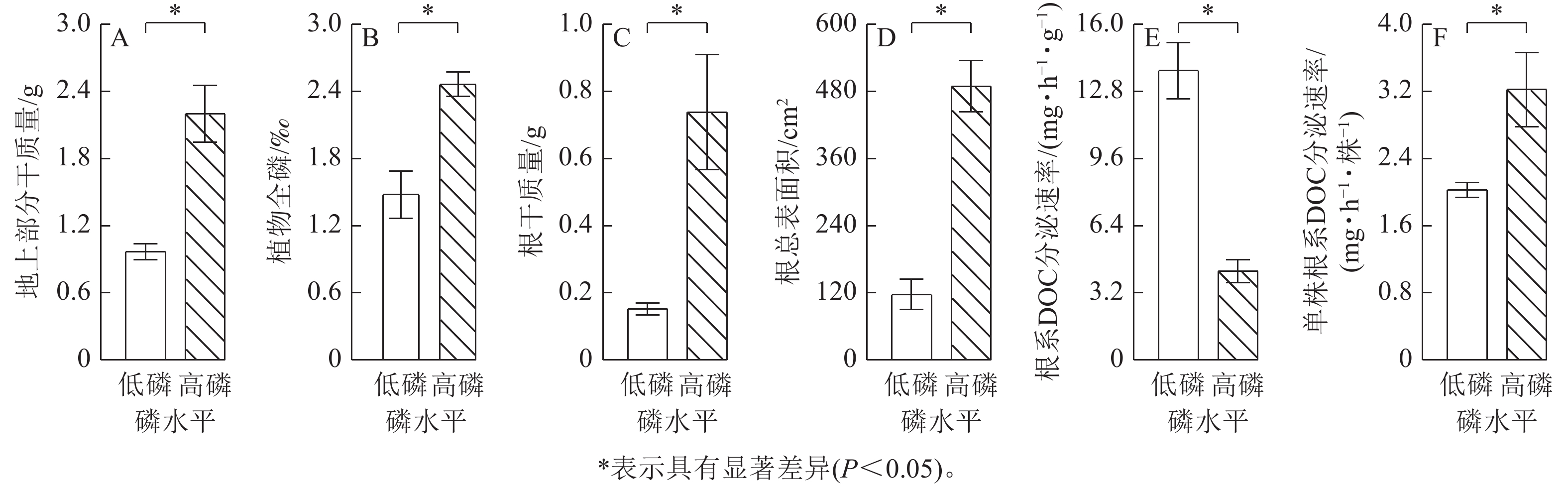
由图1可见:高磷条件下的玉米地上部分干质量、植物全磷、根干质量、根总表面积、单株根系分泌物可溶性有机碳分泌速率比低磷条件下显著增加60%~390% (P<0.05)。相反,低磷条件下玉米根系分泌物可溶性有机碳速率比高磷条件下增加了2.2倍(P<0.05)。
-
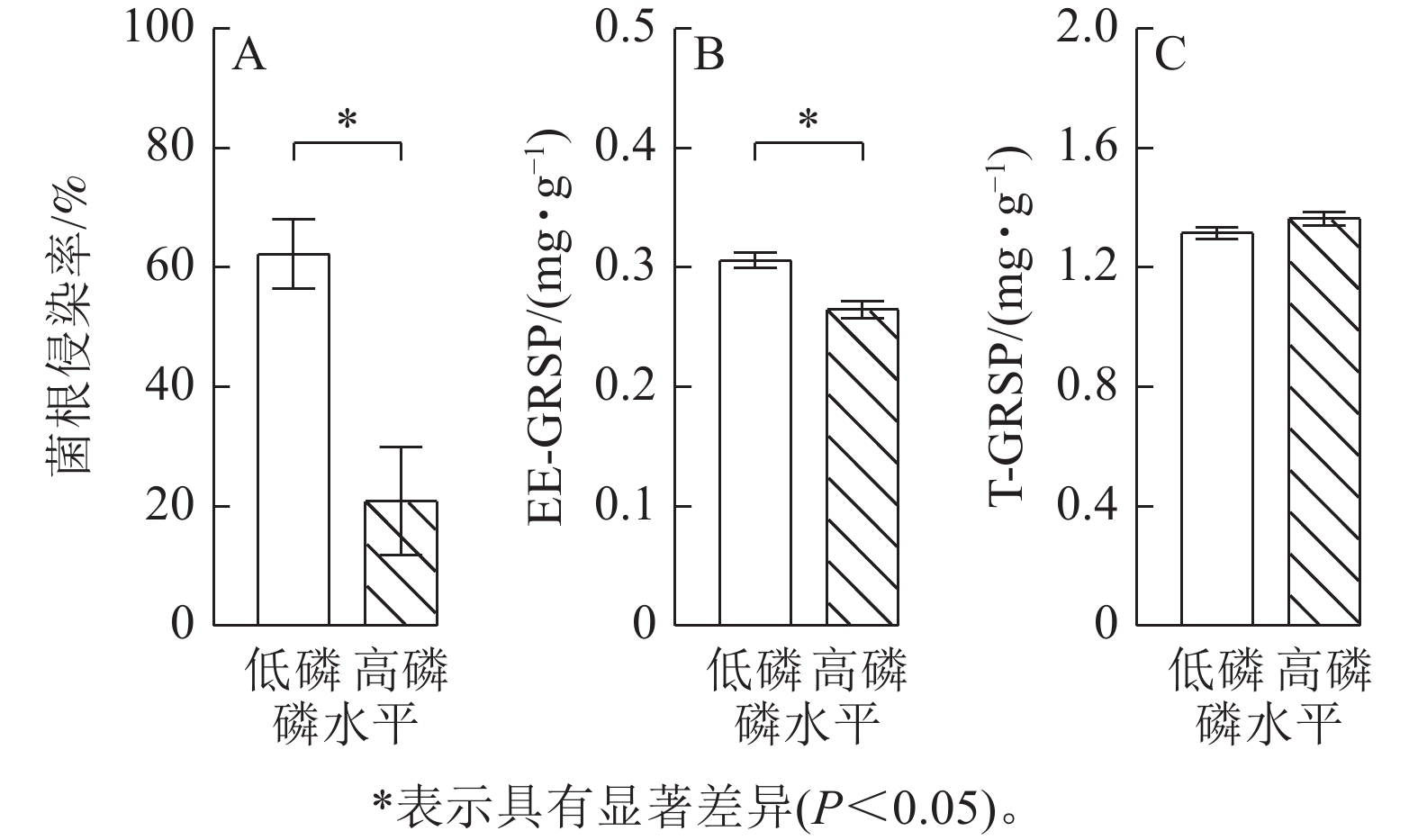
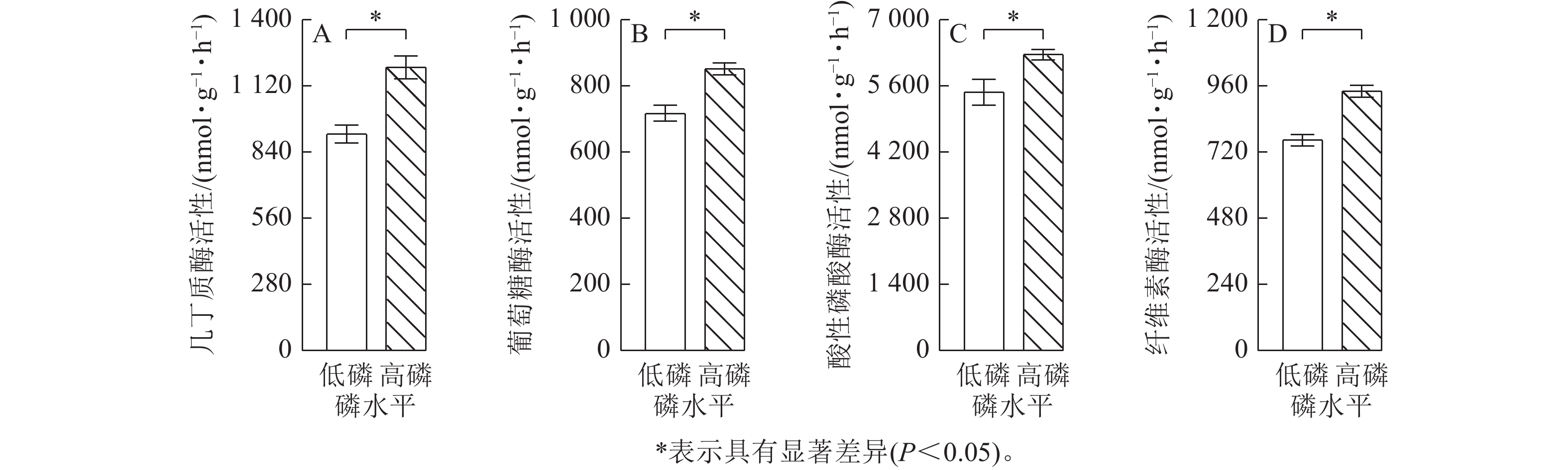
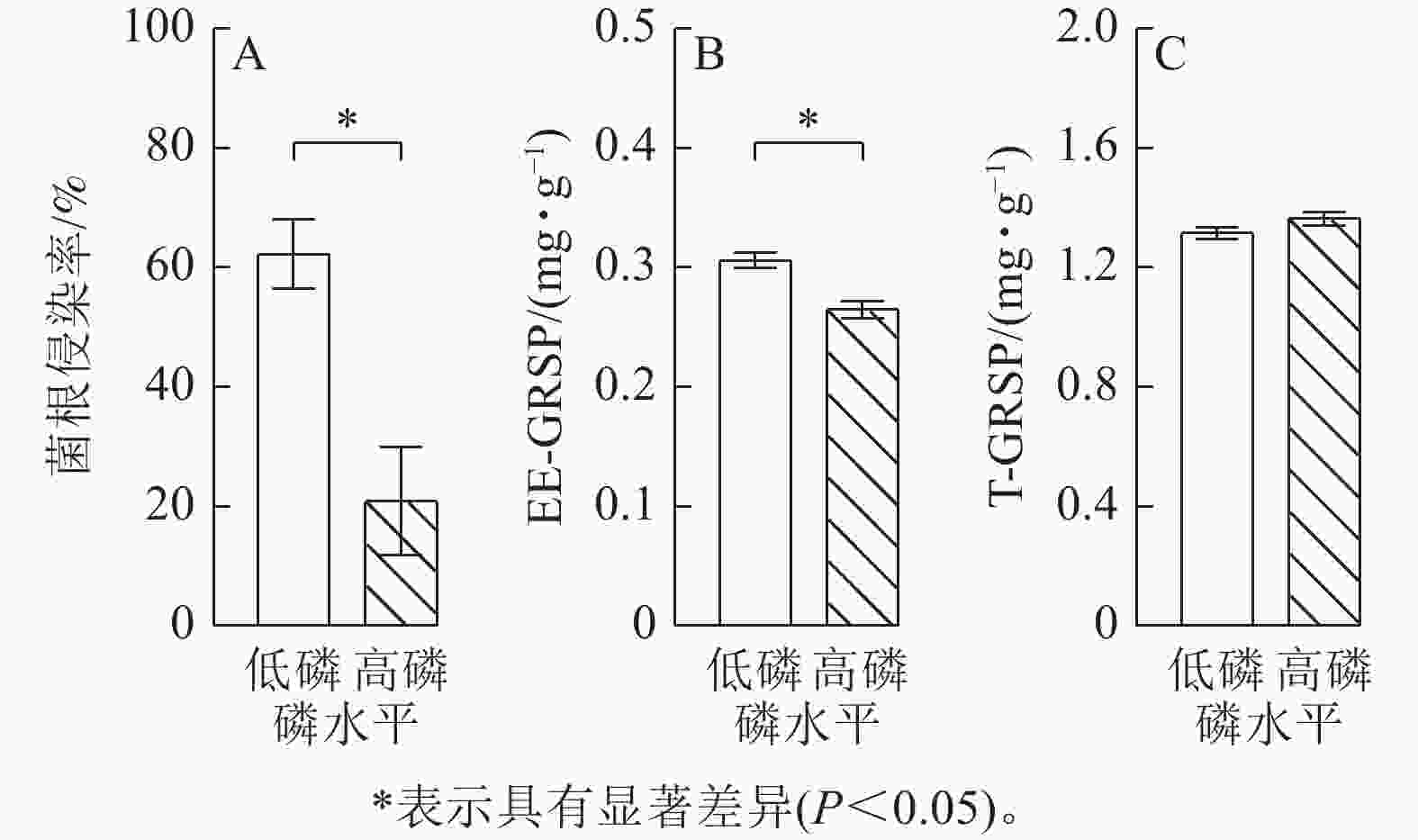
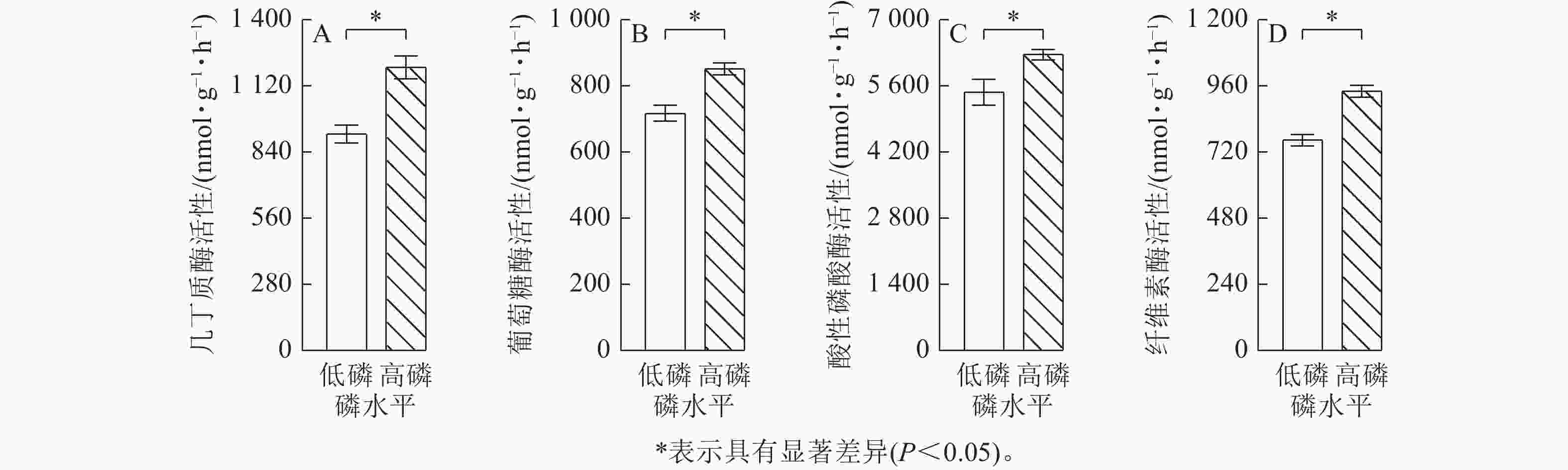
由图2可见:低磷条件下的玉米菌根侵染率比高磷条件下显著增加了2.0倍(P<0.05),易提取态球囊霉素蛋白质量分数比高磷条件下显著增加了16% (P<0.05)。总球囊霉素蛋白质量分数在低磷、高磷条件下没有显著差异。玉米在高磷条件下的几丁质酶活性、葡萄糖酶活性、酸性磷酸酶活性、纤维素酶活性比低磷条件下显著增加了10%~30% (P<0.05)(图3)。
-
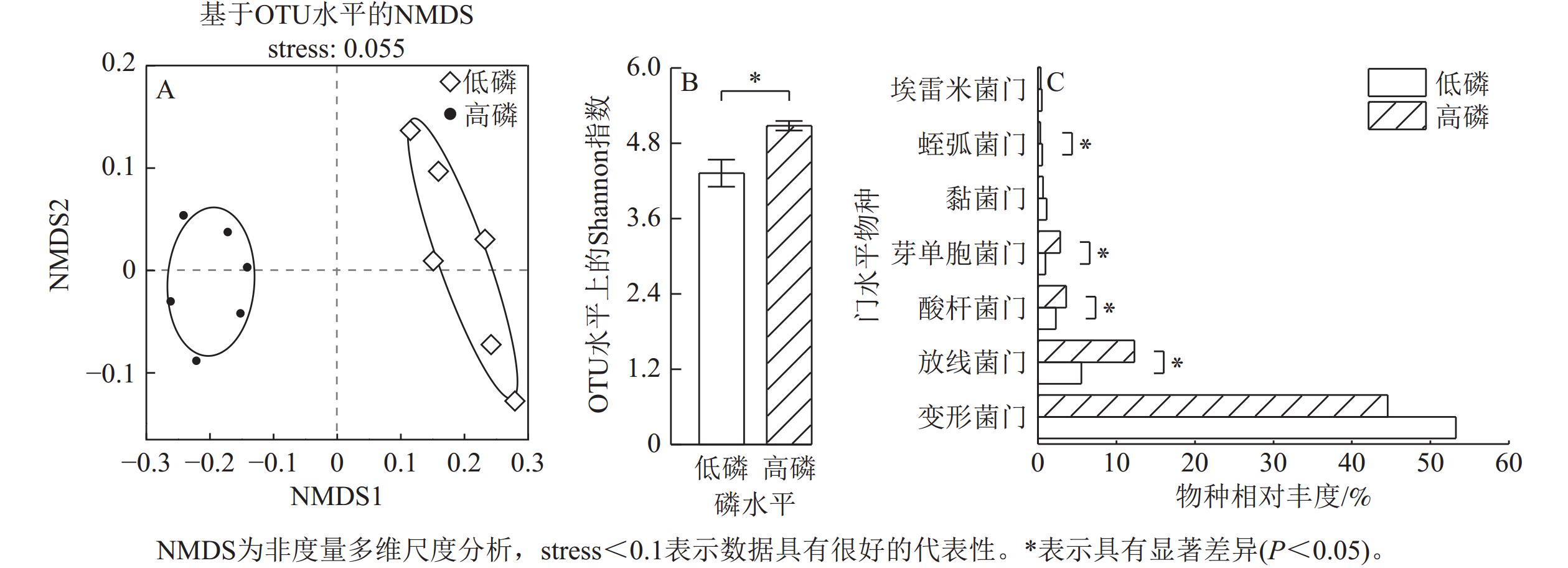
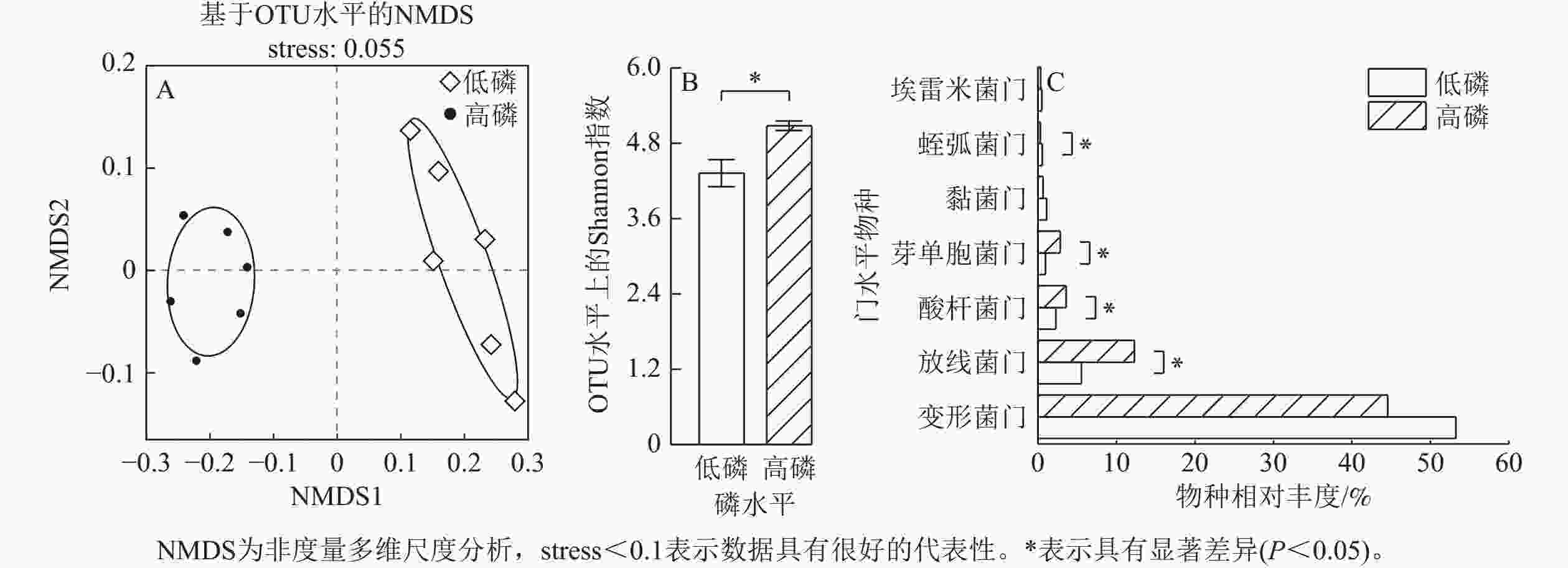
由图4A可见:高磷、低磷条件下的玉米根际群落构成组间差异具有显著性。高磷条件下的玉米根际细菌α多样性指数显著高于低磷条件(P<0.05)(图4B)。高磷条件下的玉米根际微生物群落中酸杆菌门Acidobacteriota、放线菌门Actinobacteriota、芽单胞菌门Gemmatimonadota的物种相对丰度比低磷条件下显著增加50%~200% (P<0.05)。高磷条件下的蛭弧菌门Bdellovibrionota物种相对丰度比低磷条件下显著减少43% (P<0.05)。埃雷米菌门Candidatus Eremiobacterota、黏菌门Myxococcota、变形菌门Proteobacteria的物种相对丰度则在低磷、高磷条件下没有显著差异(图4C)。
-
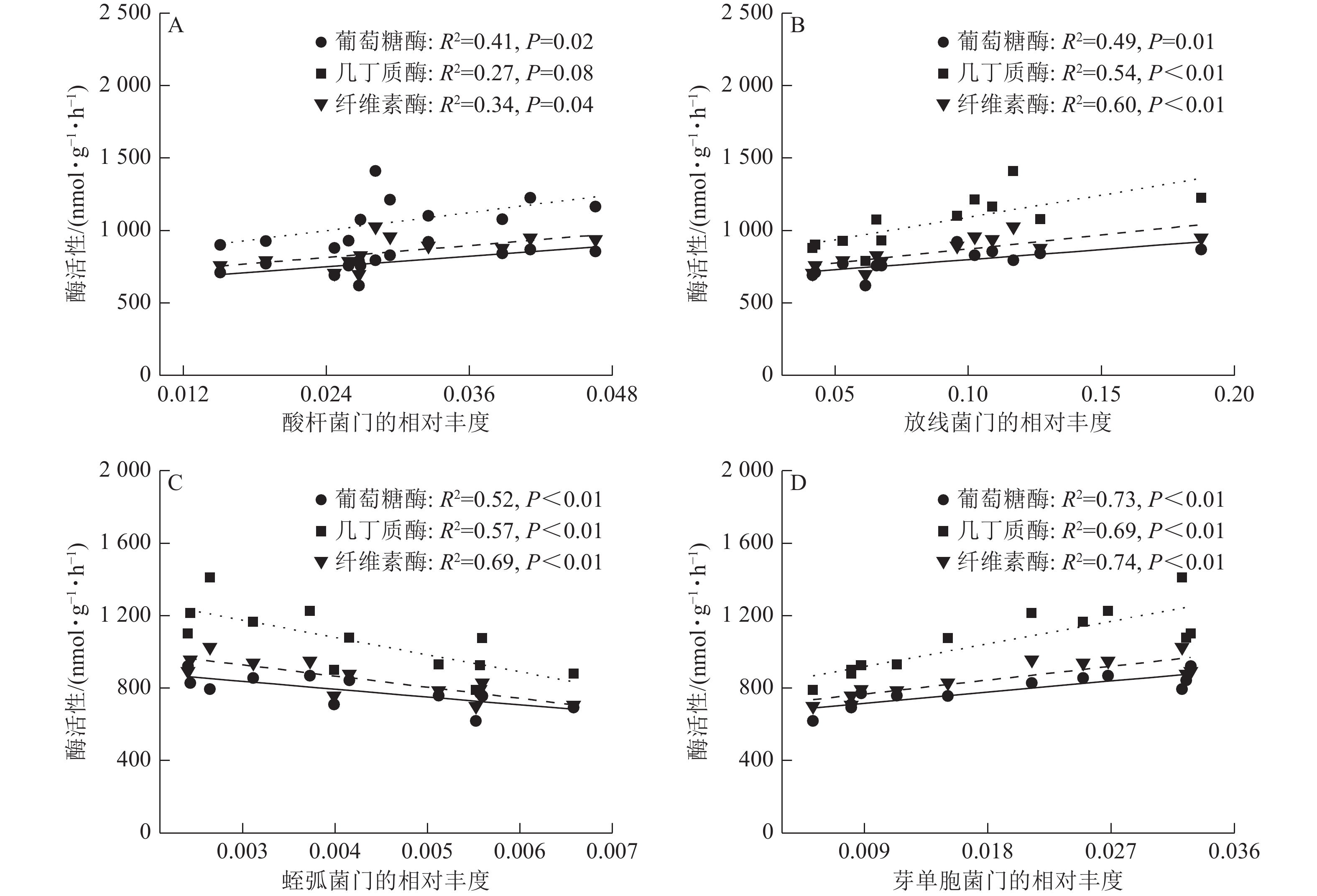
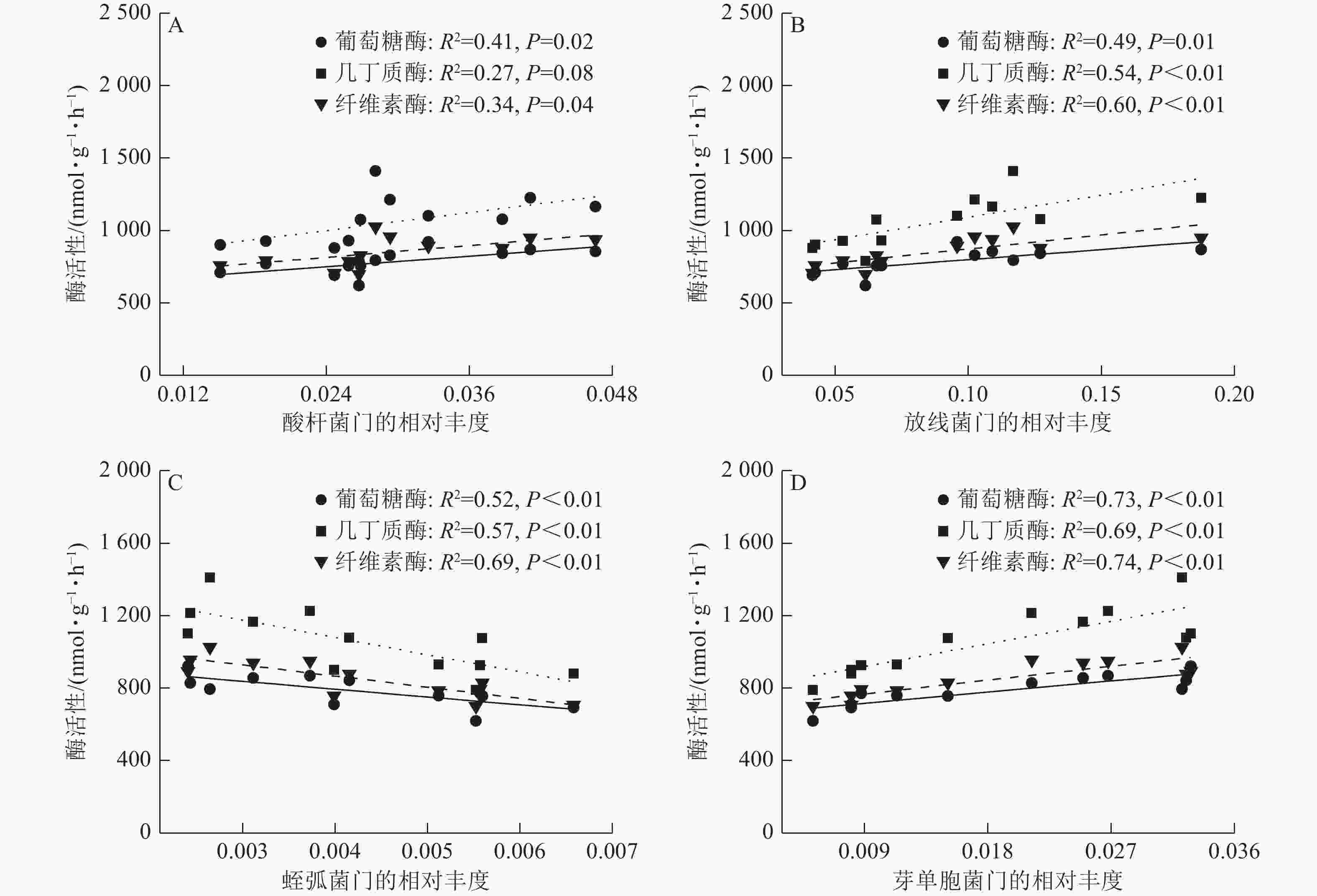
由图5可见:酸杆菌门的相对丰度与葡萄糖酶活性、纤维素酶活性具有较强的正相关线性关系。酸杆菌门、芽单胞菌门的相对丰度与葡萄糖酶活性、几丁质酶活性和纤维素酶活性具有较强的正相关线性关系。蛭弧菌门的相对丰度与葡萄糖酶活性、几丁质酶活性和纤维素酶活性具有较强的负相关线性关系。
-
本研究中,高磷增加了玉米叶片磷含量,促进了玉米地上和地下部分的生长,并促进了总根系分泌物可溶性有机碳的分泌。但低磷条件下根系分泌物可溶性有机碳速率是高磷的2.2倍,表明玉米通过提高根系分泌物的地下分配以应对低磷胁迫。低磷和高磷玉米根际微生物群落的β多样性存在显著差异,这是由于根系分泌物可为根际微生物提供丰富的能源物质,根际微生物群落结构和组成对根系分泌物的组成和含量变化非常敏感。研究结果进一步表明,高磷条件显著提高了玉米根际酸杆菌门、放线菌门、芽单胞菌门的相对丰度;低磷条件显著提高了玉米根际蛭弧菌门的相对丰度。酸杆菌门、放线菌门、芽单胞菌门属于K策略微生物,具有生长缓慢,更适应营养贫乏环境的能力[28]。蛭弧菌门属于R策略微生物,具有在营养丰富的环境中(如根系分泌物和其他低分子物质或水溶性有机碳源)快速生长、优先获取资源的能力。这一现象与本研究结果一致,低磷条件下植物根具有更高的根系分泌物,促进了R策略微生物的生长;相反,高磷条件下根系分泌物减少,有利于K策略微生物的生长。低磷条件下玉米根际细菌的Shannon指数显著低于高磷条件下,表明土壤磷质量分数不仅可以改变细菌的群落组成,还可以改变其群落的多样性。玉米在低磷条件下,根系分泌速率增加,一些快速繁殖的R策略微生物会迅速占领生态位,从而抑制其他微生物的生长。这一现象在根际和非根际中得到普遍验证,根际中的根系分泌物能为微生物提供丰富的营养,土壤中的微生物通过趋化感应向富含根系分泌物的区域靠近并繁殖,导致根际的微生物数量远远高于非根际[29]。本研究进一步表明,同样是根际,根系分泌物的多少同样会影响细菌的多样性,而细菌多样性会随着底物有效性的增加而减少。总之,土壤有效磷质量分数可以影响玉米植物生长和根系分泌物的分泌,从而改变根际微生物的群落结构和多样性。
低磷条件下,丛枝菌根的侵染率是高磷下的3.0倍,表明玉米不仅采用增加根系分泌物策略,还通过与菌根真菌共生策略来增加对磷的吸收。丛枝菌根真菌的根外菌丝可向根外磷素充足的区域延伸,扩大了与土壤的接触范围。丛枝菌根真菌通过表达高亲和力磷转运蛋白、提高土壤磷酸酶活性、分泌有机酸和糖类物质等多种方式,直接或间接作用于土壤和根际微生物,提高磷的有效性和吸收效率。丛枝菌根真菌具有诱导根系提高特异性磷酸酶活性,加快有机酸产生的能力。此外,丛枝菌根真菌菌丝体存在高亲和力磷酸盐转运系统,并具有诱导植物体内产生高亲和力磷转运蛋白的能力[30]。总之,玉米在低磷条件下提高菌根的侵染率,可通过多种机制增强植物对磷的吸收能力,包括扩大吸收范围、提高磷的有效性、通过特定的磷转运蛋白促进磷的吸收和运输,以及通过菌丝根际微生物组的功能增强来提高磷的利用效率。低磷条件下易提取态球囊霉素蛋白比高磷条件下多16%,表明丛枝菌根真菌与植物根系形成共生关系,不仅能够帮助植物吸收磷,还能增加土壤中碳的输入。丛枝菌根真菌产生的球囊霉素蛋白是一种含有碳的糖蛋白,被认为在土壤中稳定且持久。球囊霉素蛋白在土壤中固碳,对陆地生态系统土壤碳固定及循环过程中具有积极作用[31]。此外,丛枝菌根真菌的分泌物和菌丝与土壤中的有机物质相互作用,形成稳定的有机碳复合体[16]。这些菌丝网络还能将植物光合作用固定的碳输送到土壤中,并与土壤矿物结合,形成稳定的有机碳。但因为种植时间仅6周,所以总球囊霉素蛋白在不同磷水平下的玉米根际土壤中没有显著差异,长期缺磷条件下,丛枝菌根真菌通过分泌球囊霉素蛋白对土壤碳的固定有待进一步研究。总之,丛枝菌根真菌通过多种机制影响玉米根际土壤的碳磷循环,包括扩大磷吸收的范围,促进植物生长、增加球囊霉素蛋白的分泌,从而提高对土壤有机碳的固定。
多数情况下,土壤缺磷容易导致根系分泌酸性磷酸酶活性提高[32],而本研究中高磷提高了根际土壤酸性磷酸酶活性。这是因为高磷条件可能改变植物的代谢途径,导致根系分泌物的组成发生变化,从而间接影响酸性磷酸酶的活性。高磷条件下玉米根际几丁质酶、葡萄糖酶、纤维素酶、酸性磷酸酶活性比低磷条件下增加10%~30%。这是因为:①高磷条件玉米具有更多的总根际分泌物可溶性有机碳,这些可溶性碳可以促进微生物的生长繁殖和酶的分泌;②高磷条件下玉米生长得更好,根系可直接分泌更多的酶,但这种解释需要进一步验证,因为目前的研究区分不出根际酶来源于植物还是微生物;③根际微生物群落结构的改变,高磷条件下玉米K策略(酸杆菌门、放线菌门、芽单胞菌门)微生物的相对丰度增加,K策略微生物具有更强的酶分泌能力。这一解释通过相关性分析得以验证,几丁质酶、葡萄糖酶、纤维素酶活性随着酸杆菌门、放线菌门、芽单胞菌门(K策略)的相对丰度增加而增加,随着蛭弧菌门相对丰度的增加而降低。总之,受植物自生长和根际微生物群落结构的影响,玉米高磷条件下具有更高的根际酶活性,特别是碳循环相关的酶活性。
-
低磷条件提高了玉米菌根侵染率和根系分泌物分泌效率,促进了R策略微生物的生长及根际球囊霉素蛋白的积累;而高磷条件下,根际几丁质酶、葡萄糖酶、纤维素酶和酸性磷酸酶活性显著增强,且与K策略微生物的相对丰度呈正相关。综上,土壤磷通过调控根系分泌物和微生物群落结构,影响根际碳循环过程。
Plant-microbe interaction mechanism of soil available phosphorus in regulating carbon dynamics in maize rhizosphere
doi: 10.11833/j.issn.2095-0756.20250112
- Received Date: 2025-01-08
- Accepted Date: 2025-05-06
- Rev Recd Date: 2025-04-12
- Available Online: 2025-06-23
- Publish Date: 2026-02-20
-
Key words:
- soil available phosphorus /
- Zea mays /
- rhizosphere carbon dynamics /
- phosphorus strategy
Abstract:
| Citation: | ZENG Lisha, YANG Guangya, FANG Huixuan, et al. Plant-microbe interaction mechanism of soil available phosphorus in regulating carbon dynamics in maize rhizosphere[J]. Journal of Zhejiang A&F University, 2026, 43(1): 133−141 doi: 10.11833/j.issn.2095-0756.20250112 |










 DownLoad:
DownLoad: