-
杨树Populus spp.生长迅速,木材质量优良,对环境的适应能力强,在中国东北地区被大规模栽培,是重要的用材林、生态防护林和城市绿化树种。然而,由于造林树种选择、造林方法不当、栽培区域地理条件和环境因素复杂多变等问题,使得杨树在生长过程中会遭受到多种病虫害威胁,其中以病害尤为突出,已对林业生产和生态建设造成巨大的经济损失[1−3]。杨树病害种类繁多、危害严重,由栅锈菌属Melampsora真菌导致的叶锈病是分布最广、危害最严重的杨树病害之一[4−5]。目前,全球范围内已报道的杨树栅锈菌包括17个种2个专化型和2个杂交种[6]。自从在中国首次发现由栅锈菌属真菌引起的杨树叶锈病以来,中国杨树已报道的栅锈菌共10种[7−14]。其中,北美栅锈菌M. medusae被列为中国重要的对外检疫性有害生物。该物种原产于北美洲东部,其主要寄主之一为美洲黑杨P. deltoides[15]。北美栅锈菌可通过气流传播,适应性强,易在温暖潮湿的环境中繁殖,一旦侵入新的区域,可迅速建立种群,导致杨树叶片早期脱落,生长受阻,木材品质下降,进而影响生态系统和经济收益[16]。该病菌已逐渐传播至全球多个地区,目前已被日本、印度和欧洲等国家和地区列为检疫性有害生物[17]。北美栅锈菌与中国本土流行的松杨栅锈菌M. laricis-populina在形态特征和寄主谱范围等方面具有高度的相似性,危害极为严重,并展现出较强的扩散趋势[18]。20世纪70年代,中国开始引种美洲黑杨,其推广种植为林业发展提供了重要支撑[19]。北美栅锈菌的潜在威胁也随之引起关注。2019年,中国首次报道在陕西、四川和河南等地发现北美栅锈菌,其寄主包括中华红叶杨P. ‘Zhonghua Hongye’、川杨P. szechuanica、小叶杨P. simonii和滇杨P. yunnanensis[14]。
以往栅锈菌属物种的研究主要是以夏孢子的形态特征作为分类依据,但杨树栅锈菌夏孢子结构较为简单,且同属种之间的夏孢子形态高度相似,因此在夏孢子形态特征的基础上结合DNA条形码相互印证,同时借助电子显微镜进一步对显微特征观察以提高鉴定的准确性[6, 12, 20]。蓝燕等[18]通过建立可视化环介导等温扩增-羟基萘酚蓝(LAMP-HNB)检测体系,成功区分并鉴定了北美栅锈菌和松杨栅锈菌,为栅锈菌的快速检测提供了新方法。胥卓尔等[21]通过对河北和北京地区杨树叶锈病的调查,利用分子生物学技术将北美栅锈菌的ITS (internal transcribed spacer)序列与栅锈菌属其他不同种类的ITS序列进行比对分析,以实现精准鉴定。
辽宁省杨树国家林木种质资源基地和辽宁省杨树研究所是中国重要的杨树种质保存与研究基地,收集并保存了丰富的杨树种质资源。两地在杨树叶锈病的监测与防控方面具有典型性,可为病原菌的种类鉴定、传播监测及防控策略研究提供可靠的样本来源和科学依据。本研究采用形态学观察结合分子生物学的方法对在两地采集到的样本进行鉴定,明确杨树叶锈病的具体种类,以期为杨树叶锈病病菌遗传多样性分布以及检疫性有害生物北美栅锈菌的传播检测提供重要的科学依据和基础信息。
-
2024年10月在辽宁省锦州市黑山县新兴镇辽宁省杨树研究所管辖的杨树国家林木种质资源基地共采集到37份叶锈病样本,在盖州市辽宁省杨树研究所院内采集到2份叶锈病标本。所有样本带回实验室进行观察与鉴定。
-
参照纪景欣[22]的方法对采集的叶锈病样本进行光学显微镜的观察并鉴定。取少量感病叶片,挑取夏孢子,以乳酚油为载体制作叶锈病玻片,并在Olympus BX51光学显微镜下观察其形态。同时,制作夏孢子堆和冬孢子堆的纵切片,仍以乳酚油为载体,利用Olympus BX51光学显微镜进行观察。扫描电镜的观察参照ZHAO等[23]的方法对感病叶片制备扫描电镜样本。将样本置于Apreo S HiVac扫描电子显微镜下,观察夏孢子堆、侧丝和夏孢子的微观结构。随机选取每种样本中的100个夏孢子进行拍摄,并通过Photoshop 2023软件测量长度、宽度及刺间距等参数。使用SPSS 27.0进行统计分析,采用单因素方差分析(ANOVA)对数据进行差异显著性检验,显著性水平设为0.05。
-
使用DNA提取试剂盒(Plant Genomic DNA Kit)提取各样品的DNA。以提取的栅锈菌基因组DNA为模板,用ITS引物Y3(5'-CCTGCGGAAGGATCATTATT-3')和Y4(5'-TAAGTTCAGCGGGTAGTCCC-3')[24],以及28S引物NL1(5'-GCATATCAATAAGCGGAGGAAAAG-3')和NL4(5'-GGTCCGTGTTTCAAGACGG-3')进行PCR扩增。PCR反应体系参考文献[22],反应总量为25 μL,包括:2×Taq Plus Master Mix 12 μL、双蒸水 9 μL、正向引物1 μL、反向引物1 μL、模板DNA 2 μL。PCR反应程序为:94 ℃预变性3 min;随后反应35个循环,包括94 ℃变性30 s、退火30 s (ITS为54 ℃,28S为58 ℃)、72 ℃延伸3 min;最后72 ℃延伸10 min,反应结束后4 ℃保温。PCR产物通过质量分数为1%的琼脂糖凝胶电泳检测,筛选出电泳条带清晰,且大小与预期一致的样品进行测序。测序结果首先使用SnapGene软件(GSL Biotech)检查测序峰图质量,随后将序列上传至美国国家生物技术信息中心(NCBI)的GenBank数据库,进行序列搜索和同源性比对,以明确菌株的分类和种类。
-
利用39份样本的ITS与28S序列数据,以及从GenBank数据库和相关文献中搜集的栅锈菌属不同种的ITS与28S序列数据78个[12, 14, 25−28],包括对外检疫性杨树叶锈病菌——北美栅锈菌的序列信息,以杜鹃金锈菌Chrysomyxa rhododendri作为外群,采用MEGA11软件的最大似然法(ML)分别构建ITS与28S序列系统发育树,并通过1 000次Bootstrap重复计算分支支持率,评估系统发育树的可靠性,以明确栅锈菌属不同种之间的亲缘关系。
-
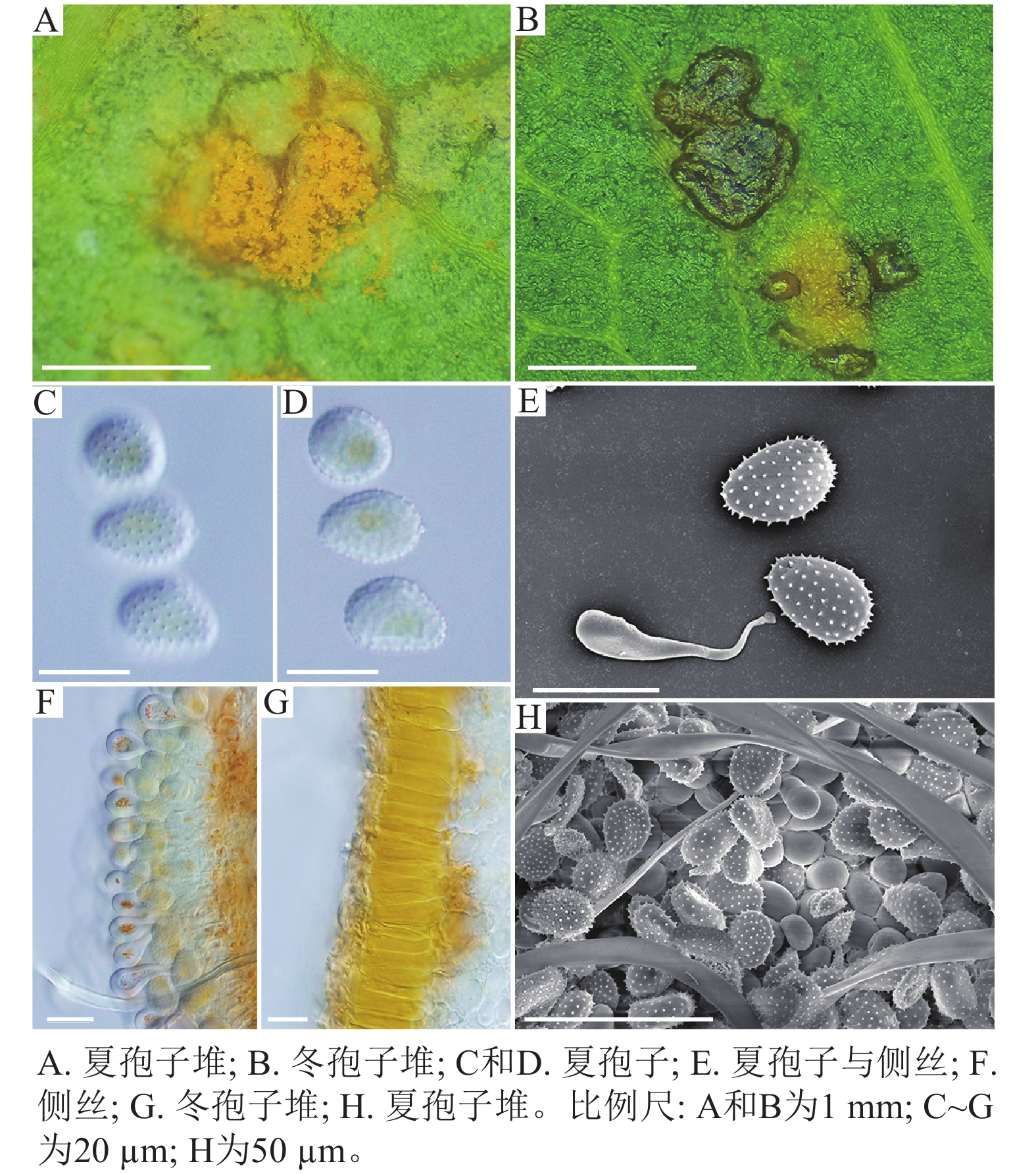
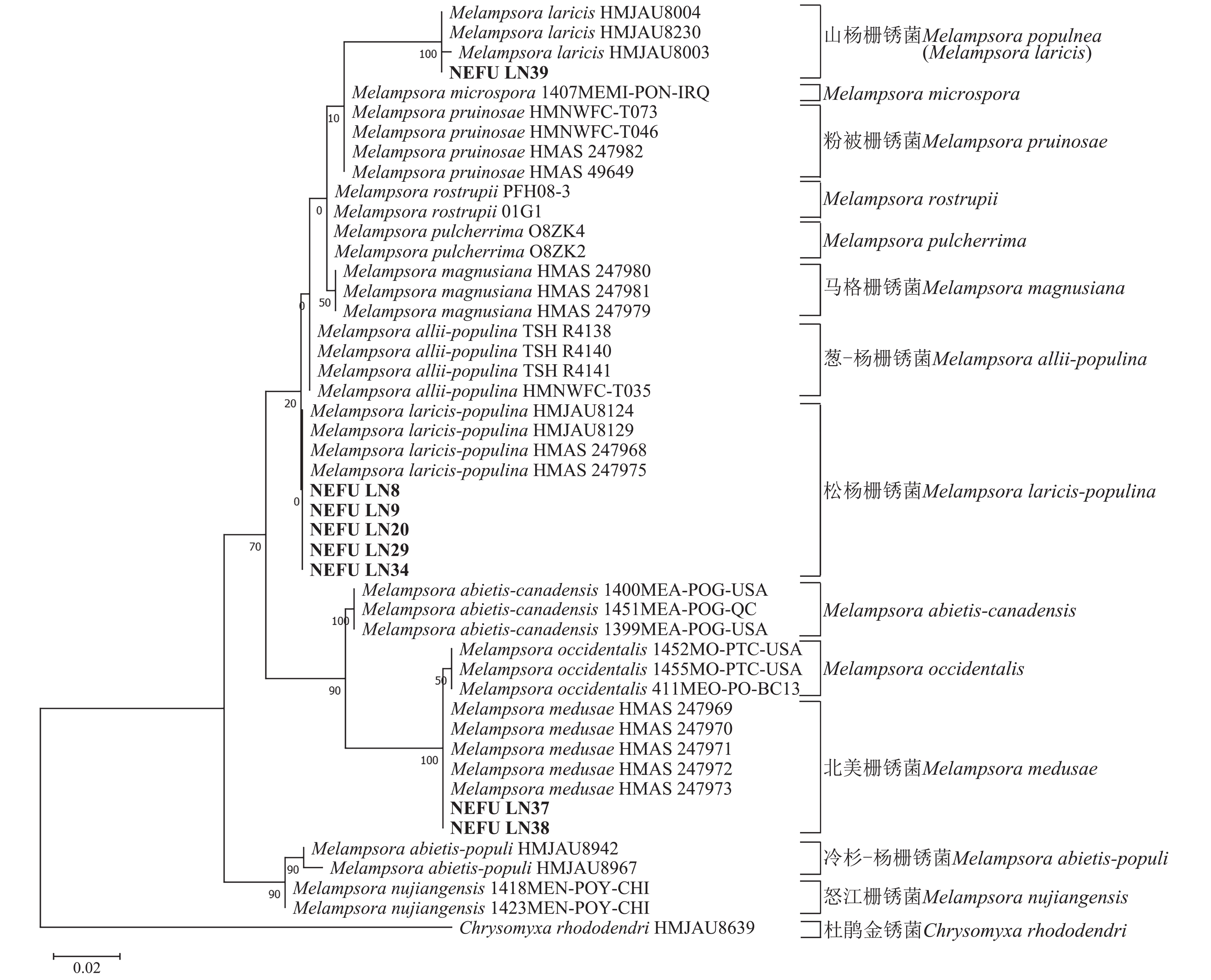
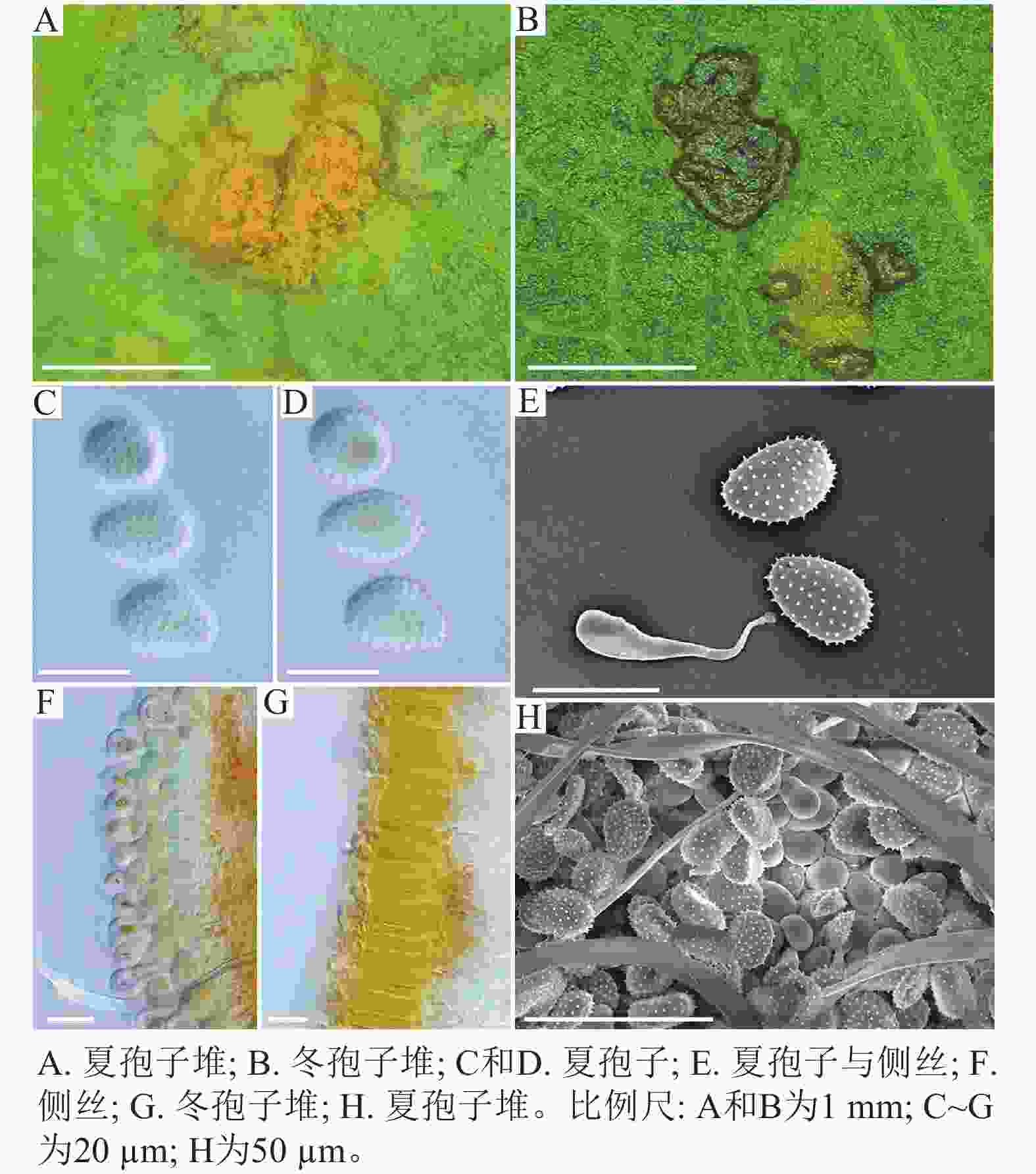
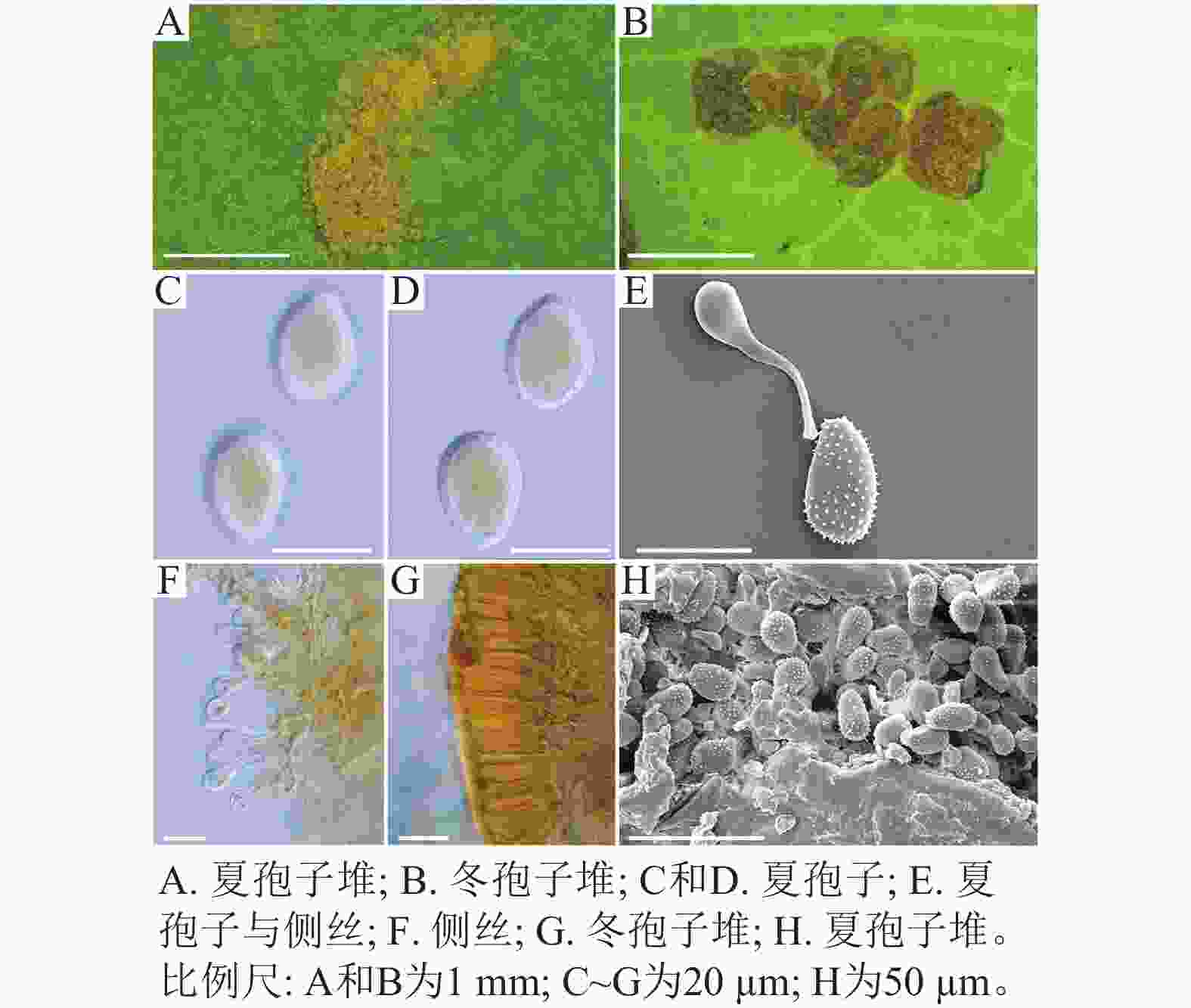
采自黑山县的35份样本与盖州市的1份样本在微观结构上具有相似性。夏孢子堆叶背面生,表皮下生,橘黄色,近圆形,直径0.3~1.0 mm,有粉末状夏孢子附着;夏孢子呈长椭圆形至棍棒状,橘黄色,(18.21~34.98) µm × (11.58~18.36) µm,基部和顶部壁较薄,赤道处壁增厚;夏孢子基部刺大而尖,刺间距较近,越接近顶部刺越小且刺间距越远,顶部刺消失,且光滑,刺间距为1.1~2.4 µm;侧丝呈棍棒状至头状,顶部较厚;冬孢子堆叶正面生,多为散生或聚生,形状不规则;冬孢子圆柱状,橘黄色,以栅栏状排列(图1)。上述特征与纪景欣[22]描述的松杨栅锈菌的形态特征一致,因此初步将这些叶锈病样本鉴定为松杨栅锈菌。
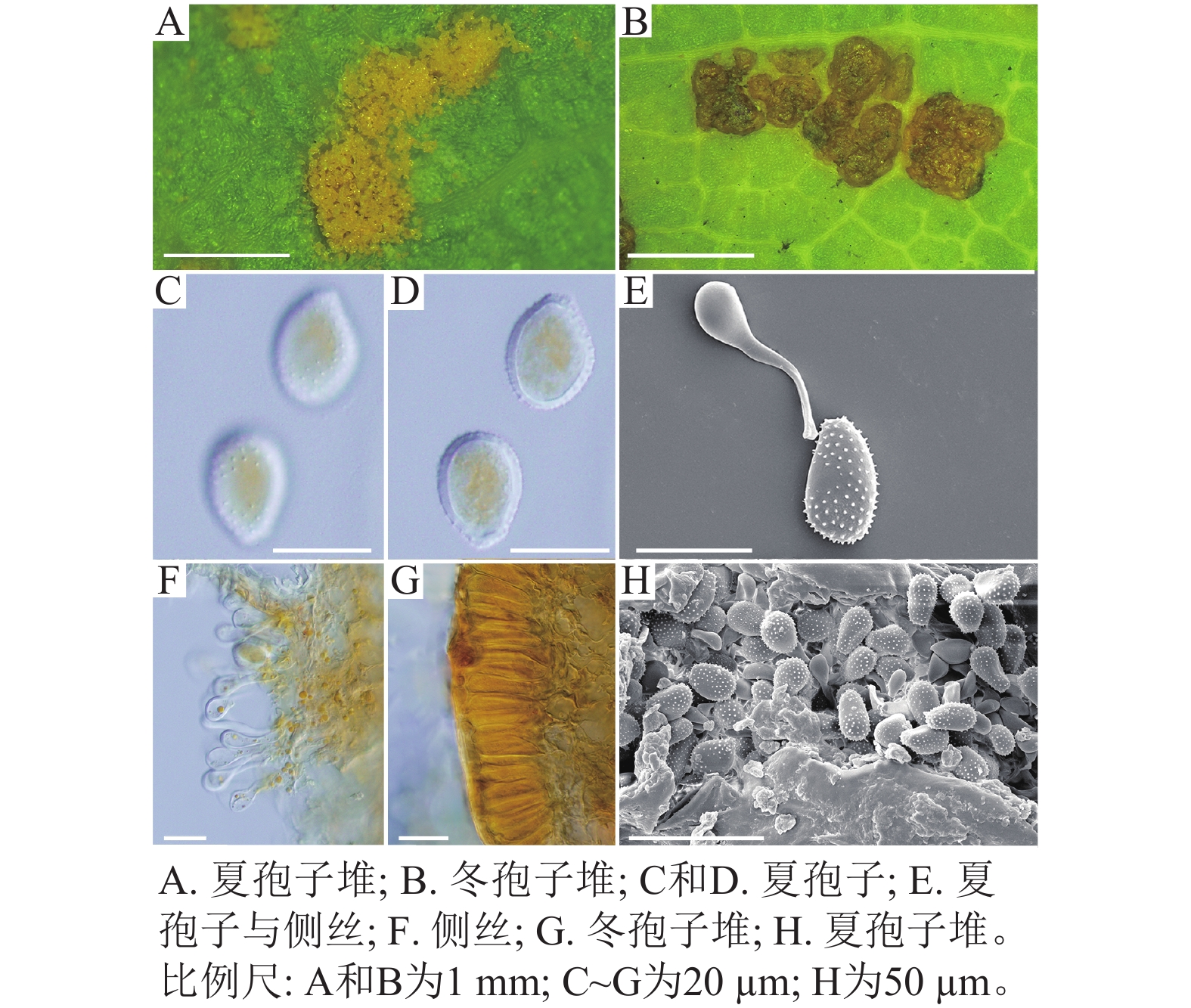
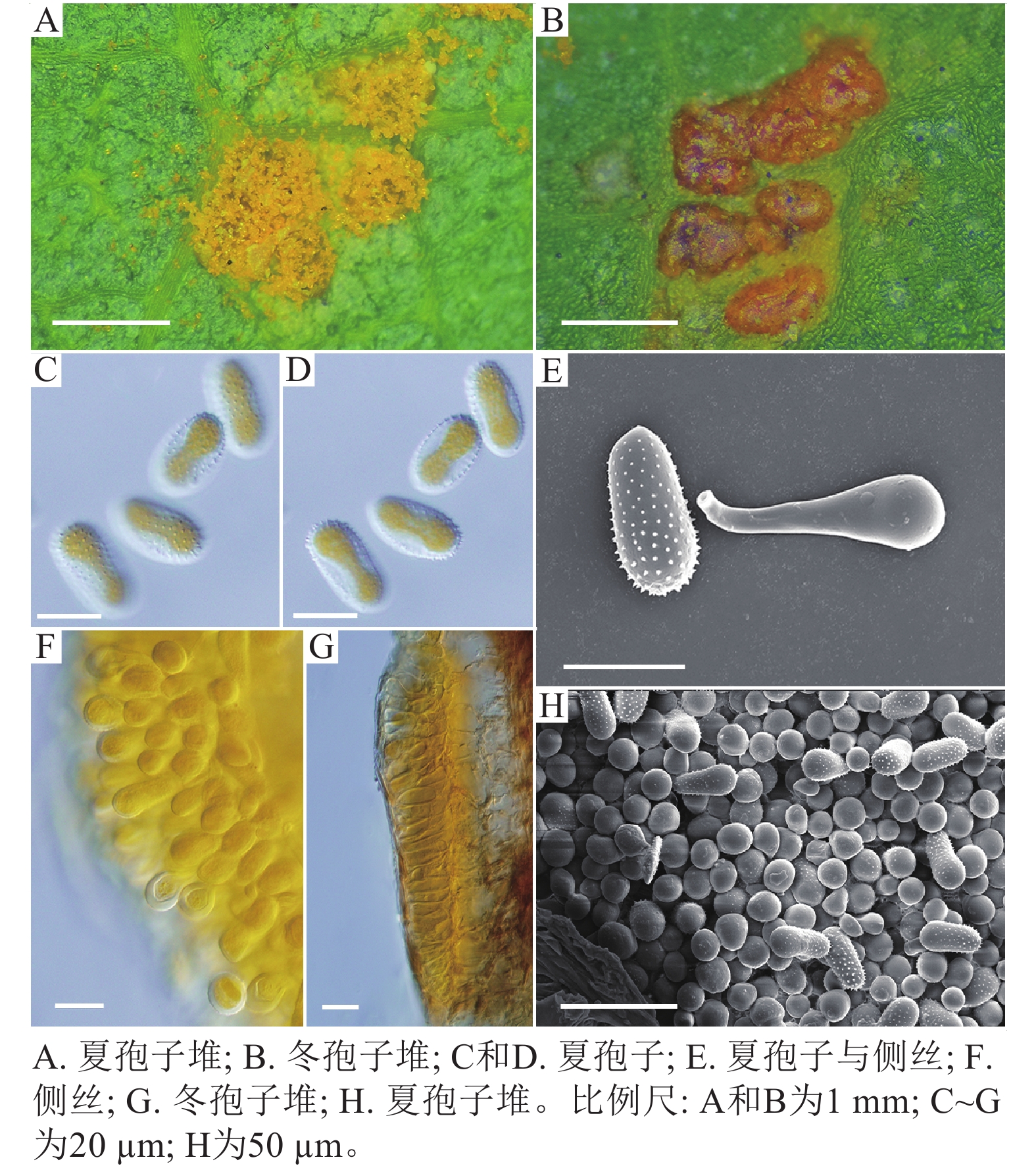
采自盖州市辽宁省杨树研究所院内的另一份样本观察结果发现,夏孢子堆叶背面生,表皮下生,淡黄色或土黄色,直径0.3~0.6 mm;夏孢子呈球形至倒卵形,黄色或浅黄色,(11.23~27.87) µm × (10.82~24.38) µm,壁厚度在基部和顶部相同,仅在赤道部位略微增厚;夏孢子具刺,刺间距为1.2~1.8 µm;侧丝呈纺锤状至头状,壁较厚;冬孢子堆黑褐色,多边形;冬孢子棱柱形,黄色,以栅栏状排列(图2)。以上特征与姜宁等[7]对山杨栅锈菌M. populnea的描述相似,因此将该样本的叶锈病初步鉴定为山杨栅锈菌。
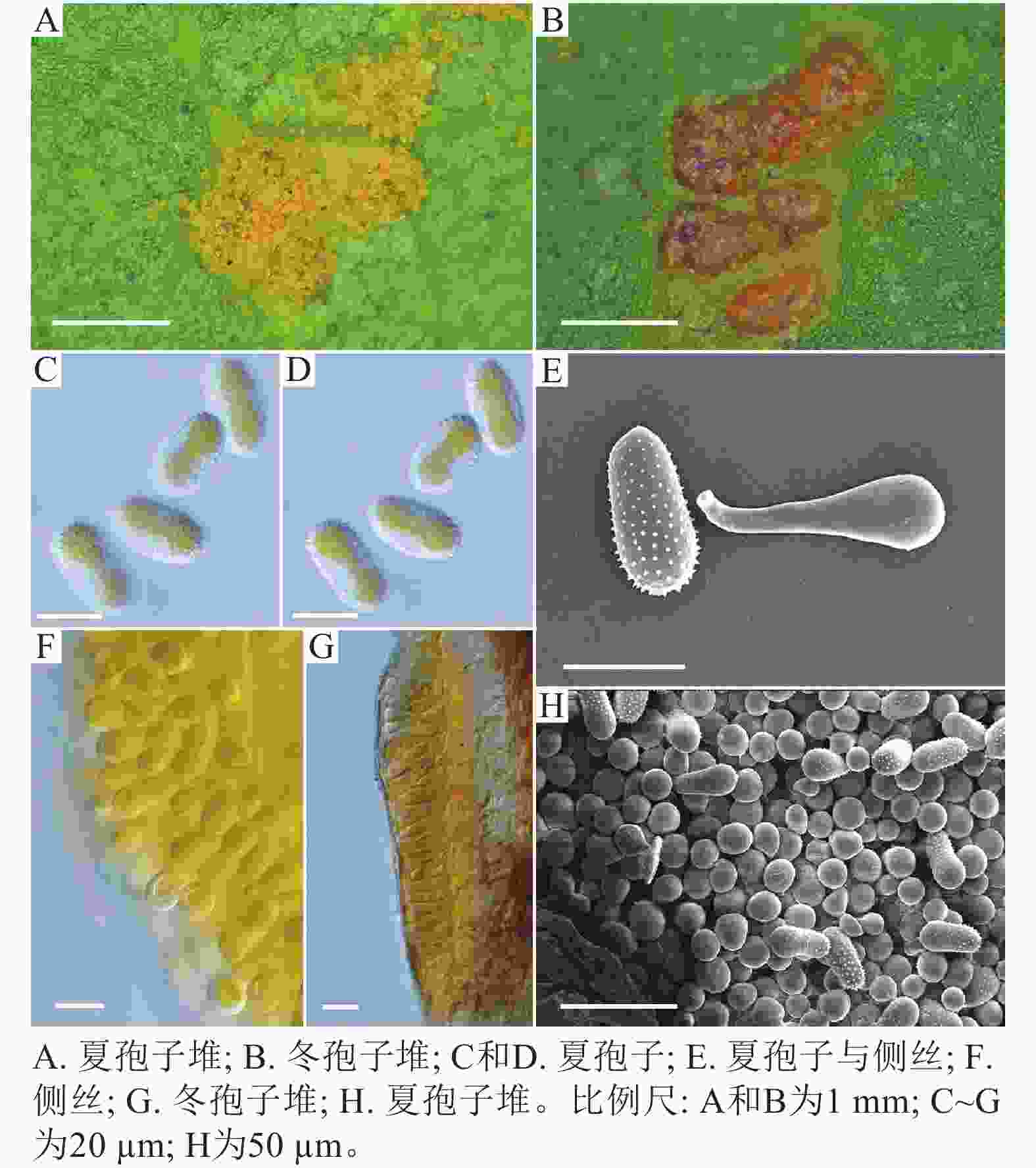
在黑山县采集到的37份样本中,有2份样本与其他的样本的微观结构有着明显的区别,其夏孢子堆叶背面生,金黄色或橘黄色,直径0.6~1.2 mm;夏孢子椭圆形至卵形,黄色或金黄色,(20.11~31.26) µm × (11.29~17.61) µm,壁在赤道部略微增厚;夏孢子具刺,但在赤道处刺消失,形成光滑区,区域通常占赤道区的一半以上,且光滑区附近的刺较小,刺间距为0.9~2.2 µm;侧丝呈头状;冬孢子堆散生,黄褐色,圆形至不规则形;冬孢子圆柱形至角形,黄褐色,并以栅栏状排列(图3)。上述特征与ZHENG等[14]描述的北美栅锈菌的特征一致,因此初步将这2份样本的叶锈病鉴定为北美栅锈菌。
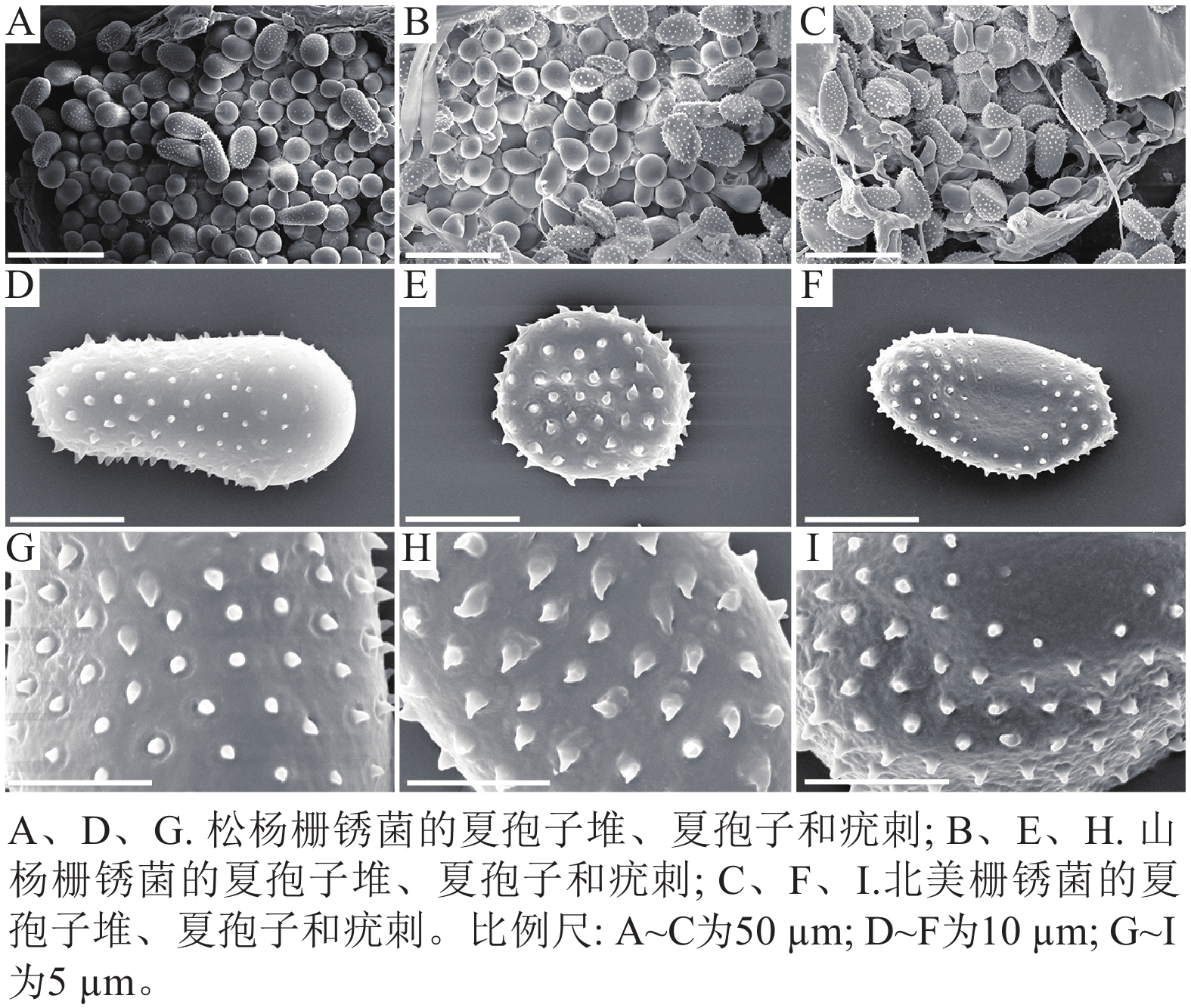
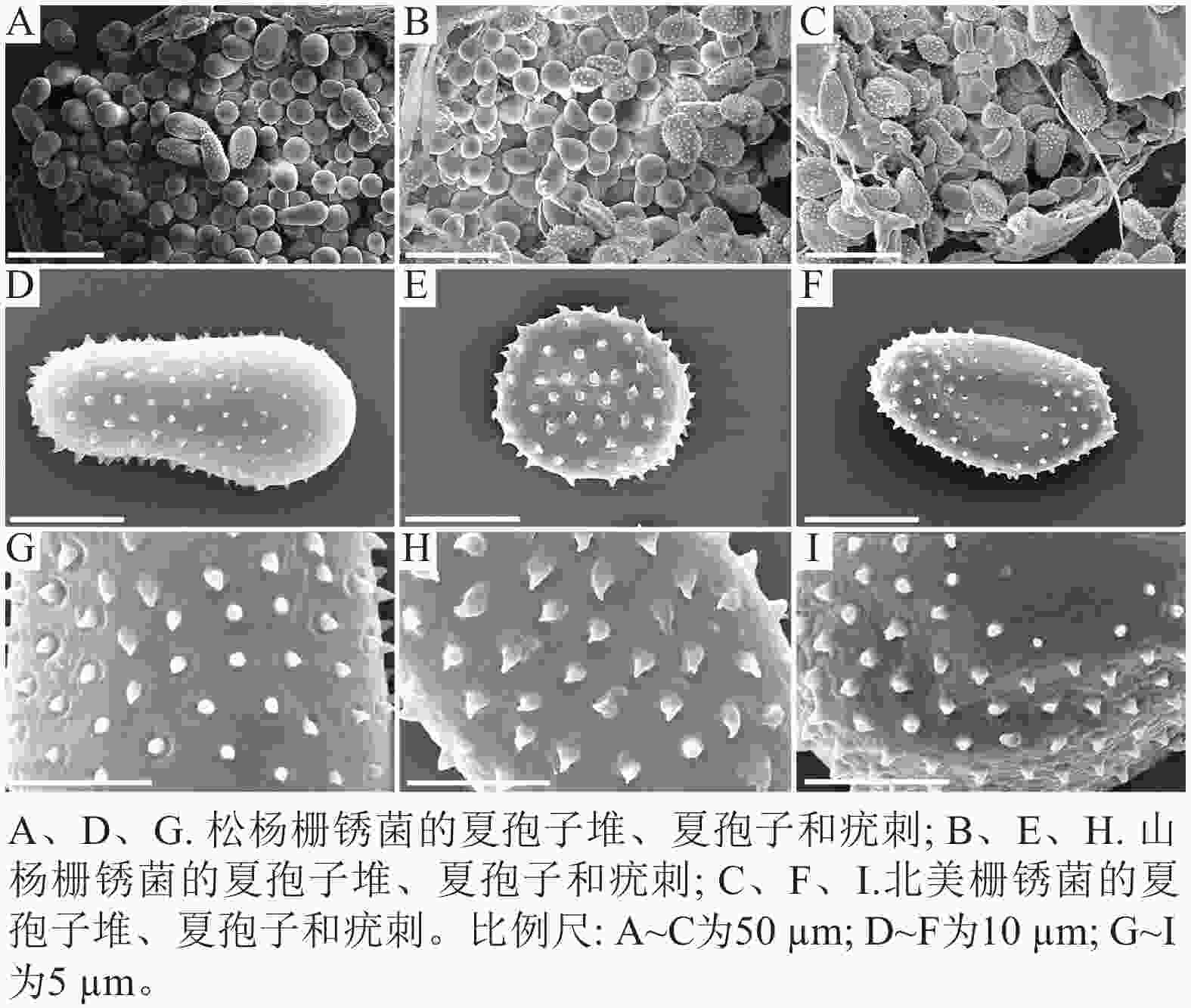
扫描电子显微镜的夏孢子观察结果表明:3种栅锈菌的夏孢子堆从叶片下表皮隆起并破裂,形状以圆形为主,大多数为不规则形,夏孢子与侧丝混合分布于夏孢子堆内,因此仅通过夏孢子堆的外观难以进行种类鉴定(图4 A~C)。但是3种杨树栅锈菌的夏孢子形态、表面疣刺差异均比较明显(图4 D~I,表1~2),因此,夏孢子的形态特征可作为对杨树栅锈菌进行初步分类鉴定的依据。
病原体 夏孢子 长/µm 宽/µm 刺间距/µm 松杨栅锈菌 30.47±3.58 a 15.57±1.27 a 1.46±0.33 a 山杨栅锈菌 17.37+1.61 c 14.14±1.14 b 1.43±0.16 a 北美栅锈菌 23.77+6.00 b 12.99±1.17 c 1.43±0.26 a 说明:数据为平均值±标准差,同列不同小写字母表示差异显著(P<0.05,n=100)。 Table 1. Comparison of urediniospores size of 3 poplar rust pathogens
病原菌 夏孢子 疣刺 松杨栅锈菌 夏孢子基部较小,顶部较大,且在赤道处有明显的缢缩,
顶部具有光滑区疣刺细小而尖锐,靠近顶部刺变稀疏,刺间距
平均值为(1.46±0.33) µm山杨栅锈菌 夏孢子近圆形,赤道处无缢缩,基部和顶部无光滑区 疣刺粗大,在表面均匀分布,刺间距平均值为
(1.43±0.16) µm北美栅锈菌 夏孢子近卵圆形,赤道处无缢缩,但有明显的光滑区,
基部和顶部无光滑区疣刺较短且钝,且越接近赤道光滑区刺越短小,
刺间距平均值为(1.43±0.26) µmTable 2. Comparison of urediniospores morphology and spines of 3 poplar rust pathogens
-
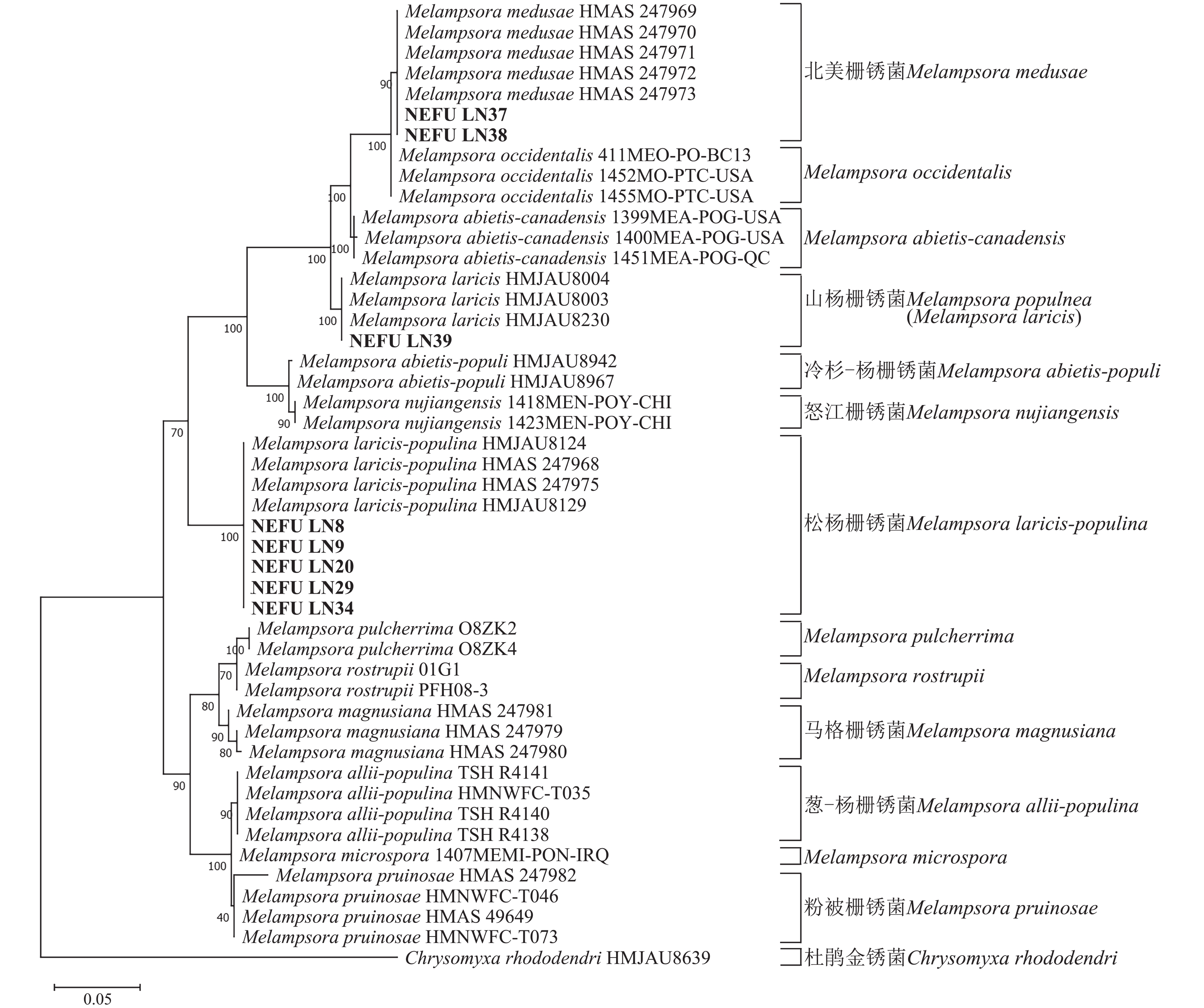
通过测序获得39份样本的ITS和28S序列合格,均可用于后续分析。将39份样本提交到NCBI进行相似性比对后,明确其种类,并将序列上传至NCBI获得相应的登录号。基于寄主分布的广泛性考虑,从采集的松杨栅锈菌样本中选取了寄主为小黑杨P. simonii × P. nigra NEFU-LN8、小叶杨P. simonii NEFU-LN9、小青杨P. pseudosimonii NEFU-LN20、北京杨P. × beijingensis NEFU-LN29和加杨P. × canadensis NEFU-LN34的序列用于构建系统发育树。
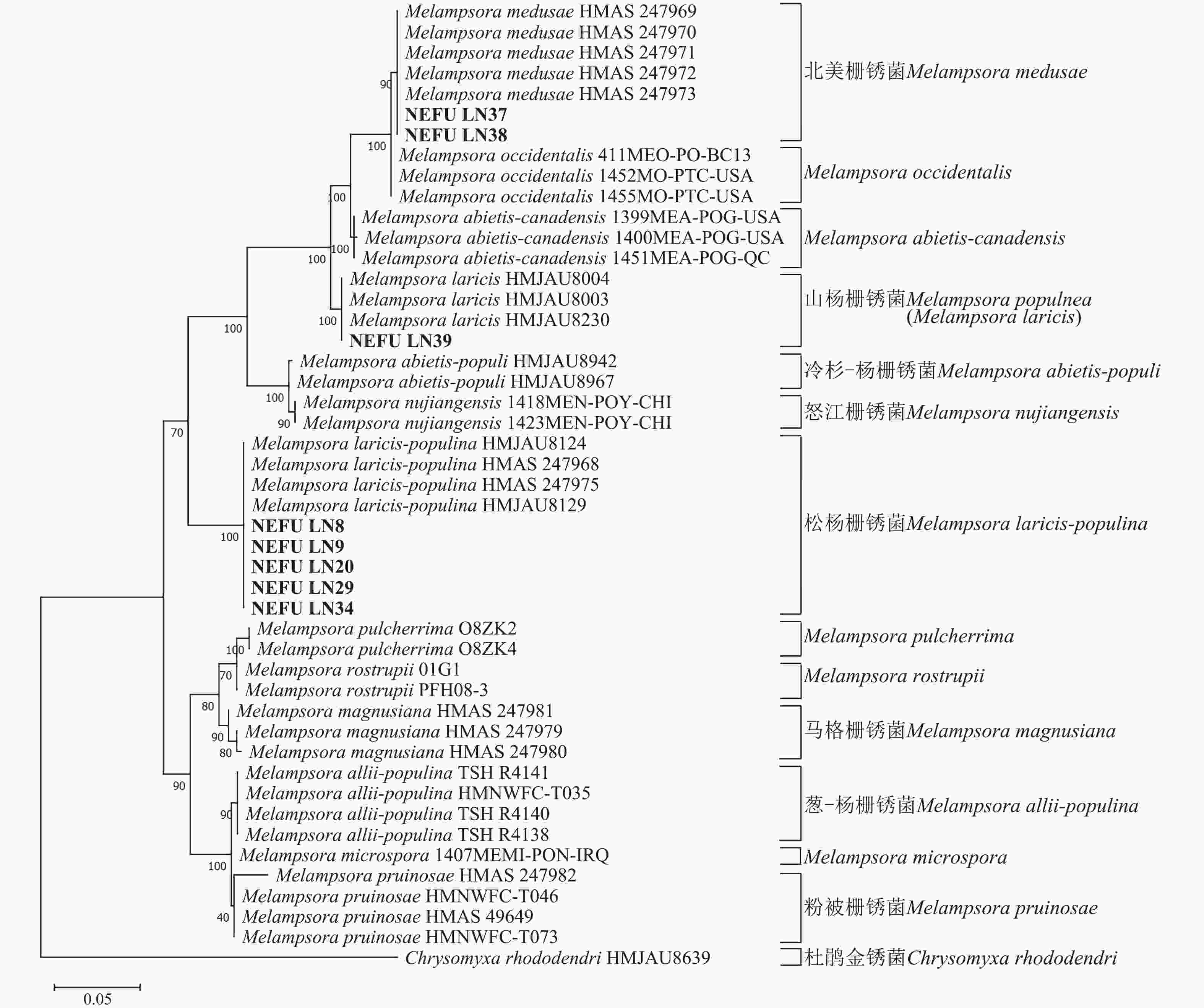
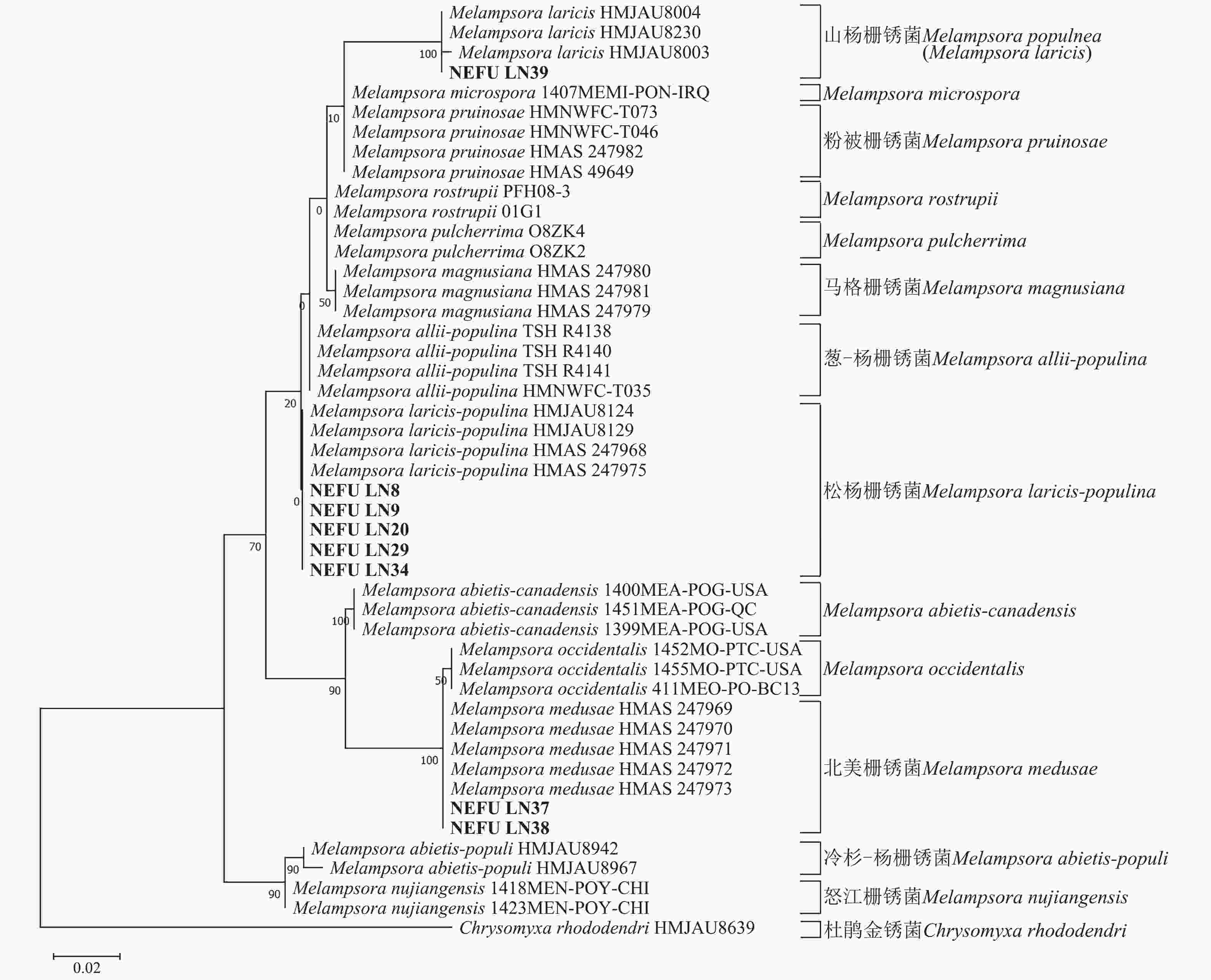
利用最大似然法分别构建ITS与28S系统发育树后发现:ITS序列系统发育树分为13个大分支(图5),本研究选取的NEFU-LN8、NEFU-LN9、NEFU-LN20、NEFU-LN29和NEFU-LN34样本的序列与松杨栅锈菌聚为一支,与形态学鉴定结果一致;而84K杨P. alba × P. glandulosa NEFU-LN39样本的序列与Melampsora laricis聚为一支,根据Index Fungorum (Index Fungorum Home Page)的信息,M. laricis为山杨栅锈菌的同物异名,因此NEFU-LN39样本的栅锈菌被鉴定为山杨栅锈菌,同样与形态学鉴定结果一致;而西丰杨P. deltoides × P. cathayana ‘Xifeng’ NEFU-LN37和辽宁杨P. × liaoningensis NEFU-LN38样本的序列与北美栅锈菌聚为一支,表明该病原菌为北美栅锈菌。利用28S序列构建的系统发育树中(图6),NEFU-LN8、NEFU-LN9、NEFU-LN20、NEFU-LN29和NEFU-LN34样本的序列与松杨栅锈菌聚为一支;NEFU-LN39样本的序列与Melampsora laricis聚为一支;NEFU-LN37和NEFU-LN38样本的序列与北美栅锈菌聚为一支,与ITS序列鉴定结果一致。这也是北美栅锈菌在中国东北地区被首次发现。
-
杨树叶锈病是杨树上发生最为普遍且严重的病害之一,在一些地区可能导致显著的生态问题和经济损失[29−30]。据统计,在中国该病害对杨树幼苗和幼树的危害可造成31%~42%的材积损失,严重制约了杨树用材林及防护林的可持续发展[31]。针对杨树叶锈病病原分类和鉴定开展了大量研究,由于不同种类的锈菌在形态上高度相似,尤其是夏孢子的形态和大小存在重叠,仅凭形态学特征进行鉴定易导致结果偏差,导致鉴定和分类问题一直存在争议。袁毅[9]首次系统调查了中国杨树栅锈菌,通过夏孢子的形态特征将中国发生的杨树栅锈菌分为7类。戴玉成[32]对杨树上发生的栅锈菌通过数量分类学方法,讨论了栅锈菌之间的进化关系,认为杨树栅锈菌有14种。TIAN等[12]通过光学显微镜和扫描电子显微镜观察发现:不同地区采集的杨树叶锈病标本在形态特征上被分为5类,但通过分子鉴定则划分为6类。姜宁等[7]进一步对中国杨树叶锈病进行了形态学与分子数据相结合的系统整理,将杨树栅锈菌划分为8类,成为当前较广泛接受的分类体系。上述研究表明,仅依赖形态学鉴定难以解决杨树叶锈病分类中的难题,形态学与分子技术相结合已成为目前最为流行且可靠的鉴定方法[33]。
本研究结合形态学观察和分子鉴定技术手段,对辽宁省采集的39份样本进行鉴定,共鉴定到松杨栅锈菌、山杨栅锈菌和北美栅锈菌3种栅锈菌。通过对这3种栅锈菌夏孢子的大小及刺间距的比较分析发现,松杨栅锈菌的夏孢子显著大于XIE等[34]在长春采集的样本;山杨栅锈菌的夏孢子的大小与姜宁等[7]和刘铁志[35]的描述基本一致;北美栅锈菌的夏孢子小于ZHENG等[14]在陕西和四川采集的样本,但与周妍等[36]在湖北和江苏采集的样本基本一致。这些结果进一步验证了不同地理区域内,由于气候和寄主等因素,导致同种病原菌在形态学特征出现差异,同时表明结合形态学与分子技术的鉴定方法在明确病原菌种类上的必要性和科学性。
GB/T 33124—2016《杨树叶锈病菌检疫鉴定方法》[37]以形态学特征为基础规定了栅锈菌的鉴定方法,适用于包括白杨P. balsamifera、黑棉杨P. balsamifera subsp. trichocarpa、美洲黑杨以及落叶松属Larix、松属Pinus、美国花旗松Pseudotsuga menziesii上的锈病菌的检疫和鉴定。然而,仅依赖形态学特征并不足以区分。例如M. abietis-canadensis的夏孢子在赤道处也具有光滑区,易与北美栅锈菌混淆[14]。因此,结合分子生物学技术是提高鉴定准确性的关键手段。
本研究中,在西丰杨和辽宁杨上首次发现了北美栅锈菌。西丰杨为美洲黑杨与青杨P. cathayana的杂交品种,美洲黑杨已知是北美栅锈菌的主要寄主之一[38]。但辽宁杨并非美洲黑杨的杂交品种,也成为了北美栅锈菌的寄主。此外,在辽宁省的杨树国家林木种质资源基地中,虽然存在许多美洲黑杨的杂交品种,如‘西雄1号’P. × euramericana ‘Xixiong1’、‘白皮箭’P. × euramericana ‘Baipijian’和‘帝国杨’P. deltoides ‘Imperial’等,以及美洲黑杨与青杨的杂交品种‘中金杨’P. deltoides × P. cathayana ‘Zhongjin’,但这些品种并未被北美栅锈菌感染。这种寄主选择性的差异值得进一步研究。
与大多数栅锈菌类似,北美栅锈菌需要通过转主寄生完成生活史,已知的转主寄主包括落叶松L. gmelinii和美国花旗松[15]。然而在中国,北美栅锈菌的转主寄主尚未明确,可能涉及落叶松属和黄杉属Pseudotsuga,这一假设需进一步验证。此外,目前北美栅锈菌仅在辽宁省的杨树国家林木种质资源基地被发现,东北其他地区尚未开展此类调查研究。因此,北美栅锈菌在东北地区其他区域是否存在,其潜在的传入途径和传播机制亟待更进一步调查并采取相应的措施。
-
本研究结合形态学观察和分子鉴定技术,对辽宁省采集的39份杨树叶锈病样本进行了系统分析,明确其物种组成与分布特征。结果显示:36份样本为松杨栅锈菌,1份为山杨栅锈菌,2份为北美栅锈菌。其中,北美栅锈菌首次在辽宁省被发现,标志它在东北地区的首次记录。北美栅锈菌的发现为杨树叶锈病的防控及生物安全检疫提供了新线索,为进一步研究其传播机制及寄主范围奠定了基础。未来需明确北美栅锈菌在中国的转主寄主及传播途径,并加强东北地区的病害监测,为杨树资源的可持续发展提供保障。
HTML
1.1. 材料
1.2. 形态学观察与鉴定
1.3. DNA提取及PCR扩增测序
1.4. 系统发育树构建
2.1. 杨树叶锈病症状与形态学观察
2.2. DNA系统发育分析
 2025-0118Fubiao_print.pdf
2025-0118Fubiao_print.pdf
|

|









 DownLoad:
DownLoad: