-
象鼻兰Phalaenopsis zhejiangensis是兰科Orchidaceae蝴蝶兰属Phalaenopsis植物[1],是中国特有兰科植物,主要分布在浙江省杭州市临安、淳安等地,在陕西、安徽、甘肃等地有少量分布。象鼻兰首次发表时为新属,即象鼻兰属Nothodoritis[2],后重新分类,归蝴蝶兰属[3]。象鼻兰喜通风、潮湿的环境,原生境多近溪流小河,附生在树龄较大且青苔较多的山核桃Carya cathayensis、银杏Ginkgo biloba、枫杨Pterocarya stenoptera等的树枝和树干上。象鼻兰是单轴类兰花,茎极短小,具2~5片叶,在冬季掉落。花期6—7月,总状花序,1~2个花序,花较多,花朵为白或浅绿色底并带有深紫色条纹,蕊喙狭长,似象鼻。果实为蒴果[1],授粉后60 d左右种子成熟。象鼻兰被列入《濒危野生动植物种国际贸易公约》(CITES)附录Ⅱ、《全国极小种群野生植物拯救保护工程规划》、《世界自然保护联盟濒危物种红色名录》(UNCN)。2021年9月公布的《国家重点保护野生植物名录》将象鼻兰列为一级保护植物。目前,已对象鼻兰野外资源进行了调查,并对象鼻兰种子萌发率进行了相关研究[4]。
植物种质资源保存有原生境保存、迁地保存和离体保存3种方式[5]。原生境保存能使植物在原生态环境中成长繁衍,维持自然演化和遗传多样性,但气候变化与病虫害威胁导致种质资源损失的可能性较大,因此需利用其他保存形式补充[6]。迁地保护是对栖息地破坏严重,种群生存受到严重威胁的珍稀植物进行抢救性保护的有效途径,需要良好的生境和人工养护,但有些野生兰花对生长环境依赖性强,不适于资源长期保存[7]。离体保存是目前兰科植物最有效的资源保存方法,主要包括离体限制生长保存和超低温保存[8]。组培苗的离体限制生长保存仍需多次继代,耗费大量的人力、物力,且植物材料存在一定遗传变异的风险[5]。
超低温保存技术是基于组织培养技术,将植物外植体、愈伤组织、细胞等经冷冻保护剂处理后,在超低温条件(一般指−196 ℃的液氮低温)下冻存的一种方法。该方法使植物体内生理生化活动几乎停止,可降低在植物储藏过程中遗传变异的风险[9],是植物种质资源长期保存的有效方法之一。王军晖等[10]指出:玻璃化超低温保存法适用于园艺作物茎尖和分生组织的冻存,效果优于其他方法。在兰科植物中,该技术已经成功用于多个种质资源不同部位材料的保存,包括种子、原球茎[11]、类原球茎[12]、花粉[13]等。在超低温条件下,植物细胞快速降温后,细胞质溶液玻璃化,使细胞不受直接伤害[14]。小液滴玻璃化法(droplet-vitrification)是将植物材料经玻璃化处理后,在铝箔上滴成小液滴,直接投入液氮中迅速冷冻,可实现材料的快速降温,以提高保存后的活力[15]。对Brassidium(长萼兰属Brassia× 文心兰属Oncidium)的流星兰 ‘Shooting Star’ 进行小液滴玻璃化处理,得到高再生率[16]。ZARGAR AZAD等[17]对Cephalanthera rubra茎尖进行液氮超低温保存,得到83.66%的再生率。由于茎尖遗传稳定性较高,可分化出与原生苗基因型几乎一致的再生植株,适宜于长期保存。目前对蝴蝶兰属植物茎尖采用小液滴玻璃化法超低温保存的研究仍较少,本研究以象鼻兰茎尖为试验材料,优化预培养基蔗糖浓度、装载时间和玻璃化时间,建立小液滴玻璃化超低温保存体系。通过组织学观察,了解材料在超低温保存中组织结构的变化,达到象鼻兰种质资源的长期保存目的,为其他兰科珍稀濒危种质资源的超低温保存研究提供参考。
-
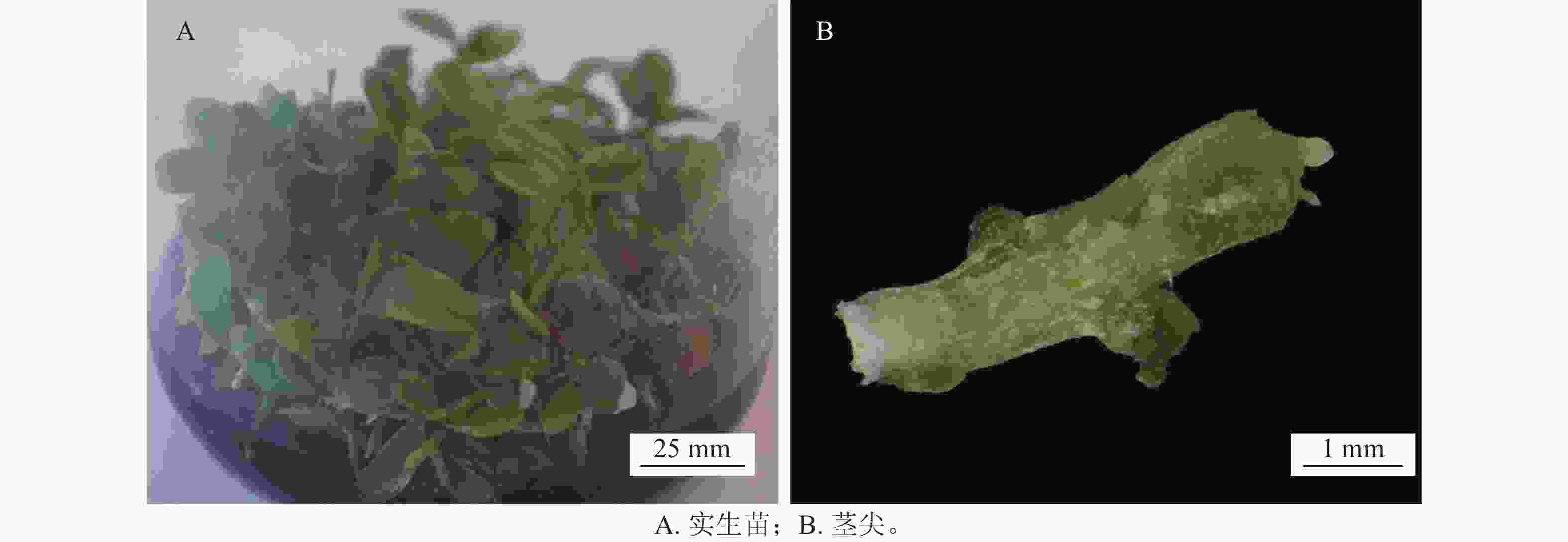
实验在中国农业科学院蔬菜花卉研究所开展。象鼻兰种子于2021年8月采自浙江省杭州市临安区,无菌播种萌发后生长10个月的实生苗用于茎尖的分离(图1A)。将实生苗用镊子剥去外层叶片仅剩茎尖(图1B),作为超低温保存的材料。
-
参照文献[18]的方法并适当改进。①预培养:将分离的5 mm象鼻兰茎尖接种到浓度为0.5 mol·L−1蔗糖的预培养基(1/2 MS+0.5 mol·L−1蔗糖+100.0 mL·L−1椰汁+6 g·L−1琼脂)上,4 ℃条件下黑暗培养2 d。②装载处理:将预培养后的象鼻兰茎尖放入含有装载溶液(1/2 MS+0.5 mol·L−1蔗糖+146.0 mL·L−1丙三醇)的小烧杯中,在室温下处理20 min。③玻璃化溶液(PVS2)处理:将装载溶液吸出,加入4 ℃的PVS2溶液(1/2 MS+0.4 mol·L−1蔗糖+300.0 mL·L−1丙三醇+150.0 mL·L−1乙二醇+150.0 mL·L−1二甲基亚砜),冰上处理90 min。④小液滴处理:将预先制备的长宽为3 cm×1 cm的锡纸条上均匀滴加新鲜的PVS2溶液5 μL,将玻璃化后的茎尖放于该小滴溶液中。⑤液氮冷冻处理:将放有象鼻兰茎尖的锡纸条迅速转移至预装液氮的冻存管中,随后将该冻存管浸入液氮中冷冻保存1 h。⑥卸载处理:将含有茎尖组织的锡纸从液氮中取出后,置于装满卸载溶液(1/2 MS+1.2 mol·L−1蔗糖)的小烧杯中,卸载处理20 min。⑦再生培养:将茎尖从卸载溶液中取出,用滤纸吸干后置于恢复培养基(1/2 MS+20.0 g·L−1蔗糖+100.0 mL·L−1椰汁+6.0 g·L−1琼脂+1.5 g·L−1活性炭),暗培养7 d后置于正常光照下继续培养。
-
采用单因素实验方法对上述已设置的小液滴玻璃化超低温保存程序进行优化。①预培养基蔗糖浓度筛选:设置0、0.1、0.3、0.5、0.7 mol·L−1共5个处理,其余步骤同1.2.1。②装载溶液处理时间筛选:设置0、10、20、30、40、50 min共6个处理,预培养蔗糖浓度为步骤①筛选出的最佳结果,其余步骤同1.2.1。③PVS2溶液处理时间筛选:设置0、30、60、90、120、150 min共6个处理。预培养蔗糖浓度、装载处理时间为步骤①和步骤②筛选出的最佳结果,其余步骤同1.2.1。每个处理取10个茎尖,重复3次,以上实验均在超净台中完成。
-
本研究采用2,3,5-三苯基氯化四氮唑 (TTC)染色法[19]评估象鼻兰茎尖细胞的活力。具体操作为:将经卸载溶液处理的茎尖转移到含有1 mL TTC染色液的离心管中,确保样本完全浸没于染色液,置于室温避光条件下染色24 h。将去除卸载溶液的象鼻兰茎尖接种至恢复培养基,暗培养1周后,转移到正常光照条件下继续培养以促进进一步的恢复生长。观察到茎尖颜色由白色逐渐转变为绿色,并在7 d后对茎尖的再生率进行统计分析。存活率=(被染成红色的茎尖数/在TTC溶液中处理的总茎尖数)×100%。再生率=(再生茎尖数/去装载后接种茎尖总数)×100%。
-
为了解在超低温保存过程中茎尖组织结构的受损情况,对8种不同阶段的茎尖进行了石蜡切片观察,即预培养2 d、装载后、PVS2后、经历冻存及解冻过程、卸载后、恢复再生、未经任何处理直接液氮冷冻的茎尖及正常生长未经任何处理的对照组茎尖。茎尖经甲醛-乙酸-乙醇(FAA)固定24 h,梯度乙醇脱水1 h,用环保浸蜡脱蜡透明液处理,最后45 ℃浸蜡;65 ℃处理24 h完成包埋。包埋后的茎尖经过切片、烘干、脱蜡处理,用质量分数为0.1%的甲苯胺蓝染色,使用显微镜对染色后的组织进行观察并拍照记录。
-
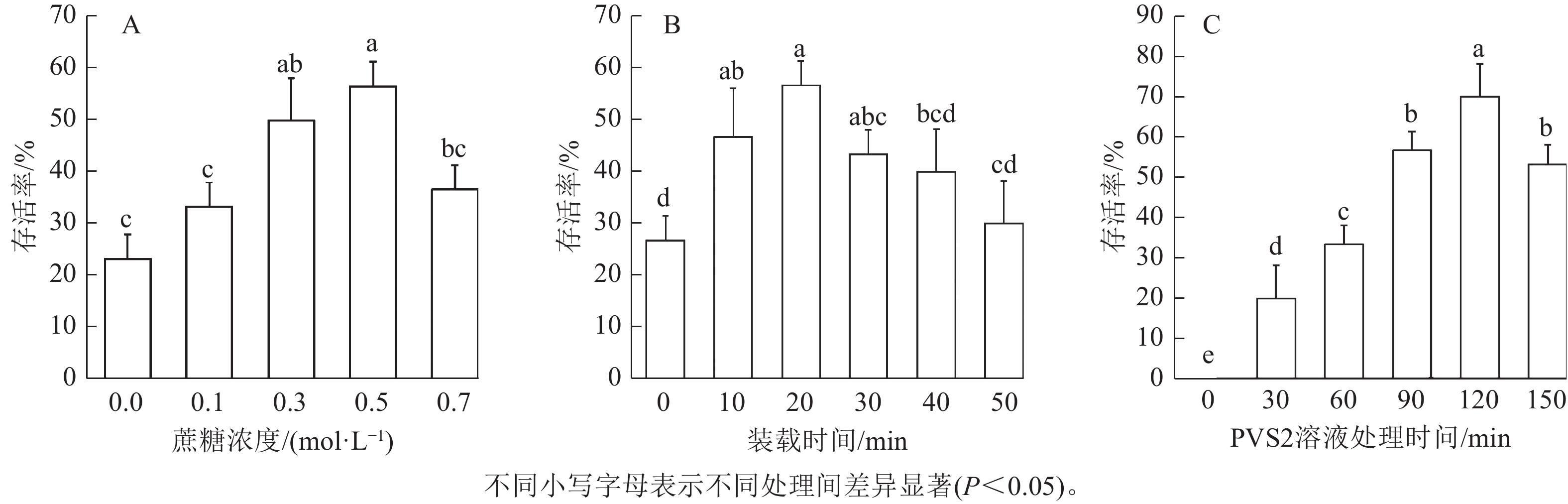
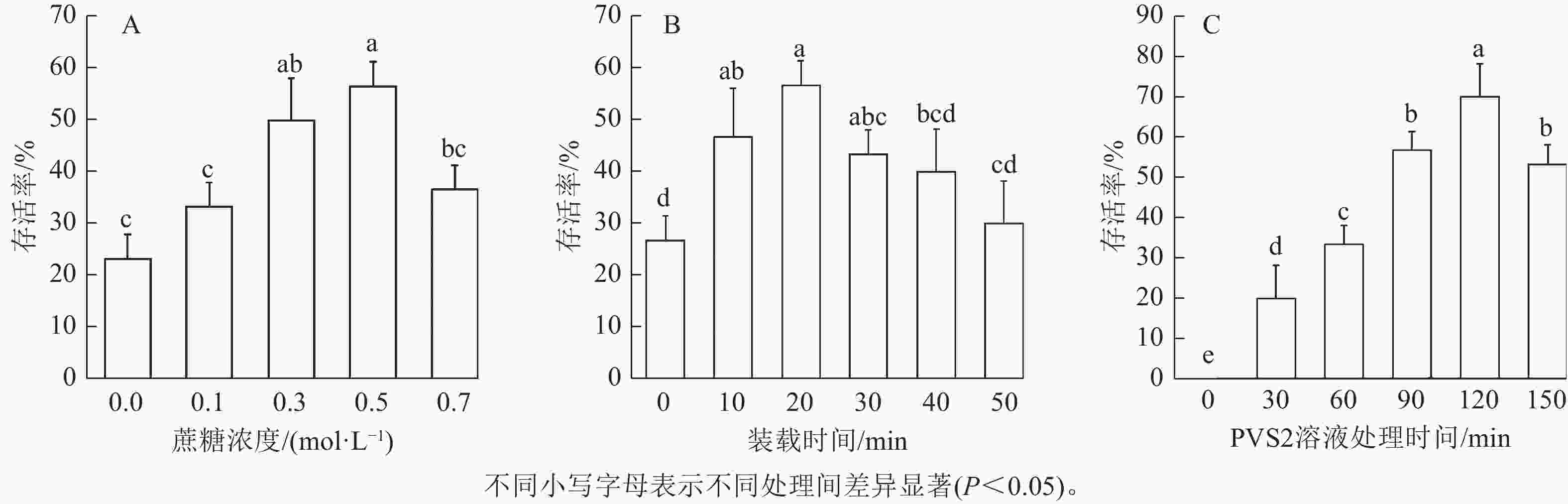
在其他条件(装载时间20 min、PVS2处理90 min、卸载20 min)不变时,茎尖用不同蔗糖浓度预培养基冷驯化2 d,存活率如图2。由图2A可知:蔗糖浓度为0.1~0.5 mol·L−1时,存活率呈逐渐上升趋势。蔗糖浓度为0.5 mol·L−1时,茎尖存活率最高,达56.7%。当蔗糖浓度上升到0.7 mol·L−1时,存活率下降,与蔗糖浓度为0.5 mol·L−1的存活率存在显著差异(P<0.05)。因此,选用0.5 mol·L−1蔗糖浓度的预培养基进行后续实验。
-
如图2B所示:其他条件 (0.5 mol·L−1预培养基冷驯化2 d、PVS2处理 90 min、卸载20 min)不变时,存活率随装载时间增加呈先上升后下降的趋势,装载时间为20 min时,存活率达到最大值56.7%,与装载时间为40和50 min的存活率存在显著差异(P<0.05)。随着处理时间的延长,象鼻兰茎尖存活率逐渐下降。因此,后续实验选用20 min为装载时间的处理。
-
由图2C可知:其他条件(0.5 mol·L−1预培养基冷驯化2 d、装载时间20 min、卸载20 min)不变时,在未经PVS2溶液处理时,其茎尖存活率降至0,表明玻璃化溶液处理在超低温保存中对茎尖存活至关重要,且随处理时间延长,茎尖的存活率逐渐提升;处理120 min时,茎尖存活率达到峰值,为70.0%,与其他处理时间的存活率存在显著差异(P<0.05),之后呈现下降的趋势。结果表明:象鼻兰茎尖的超低温保存时,PVS2处理的最佳时间为120 min。
-
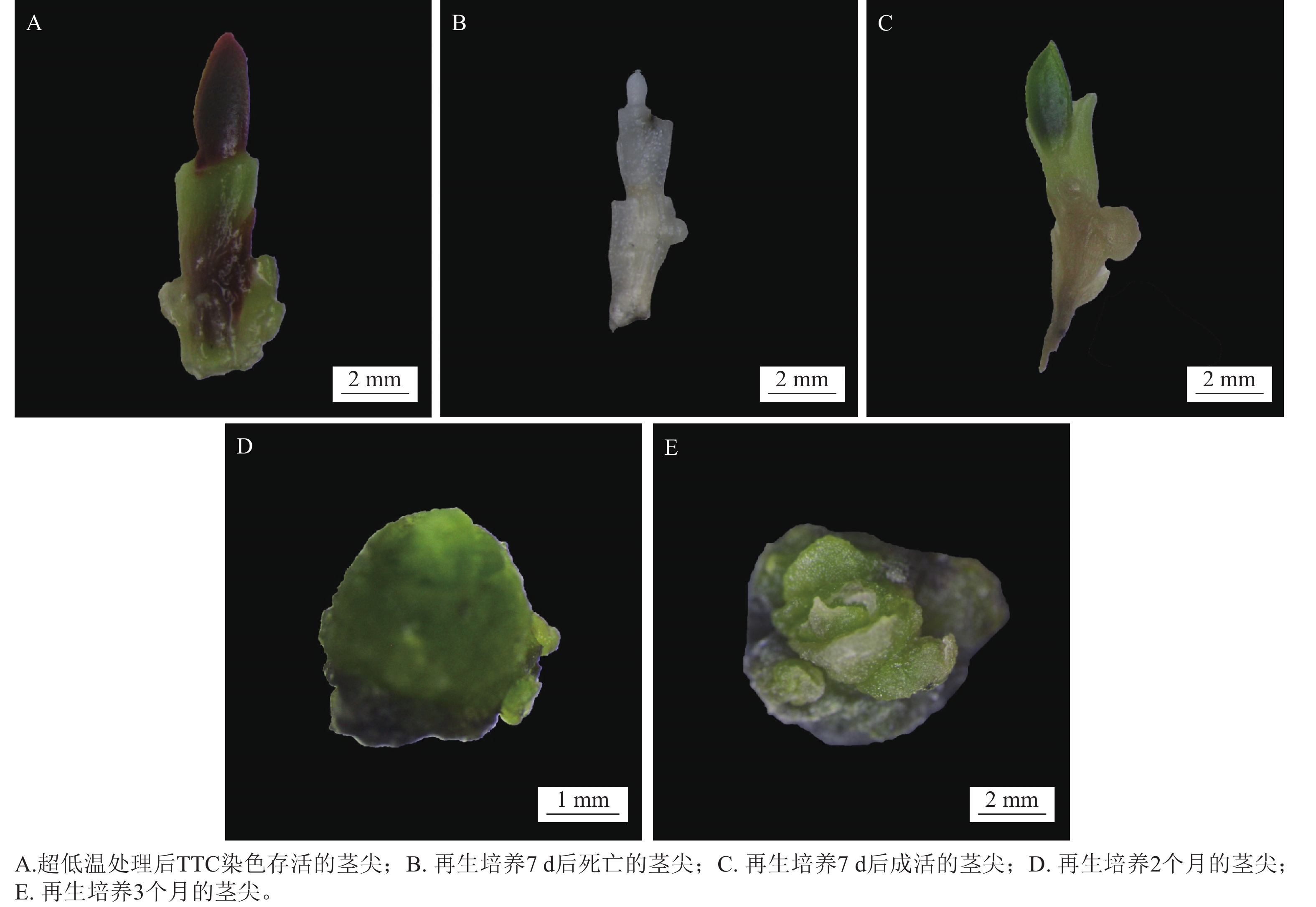
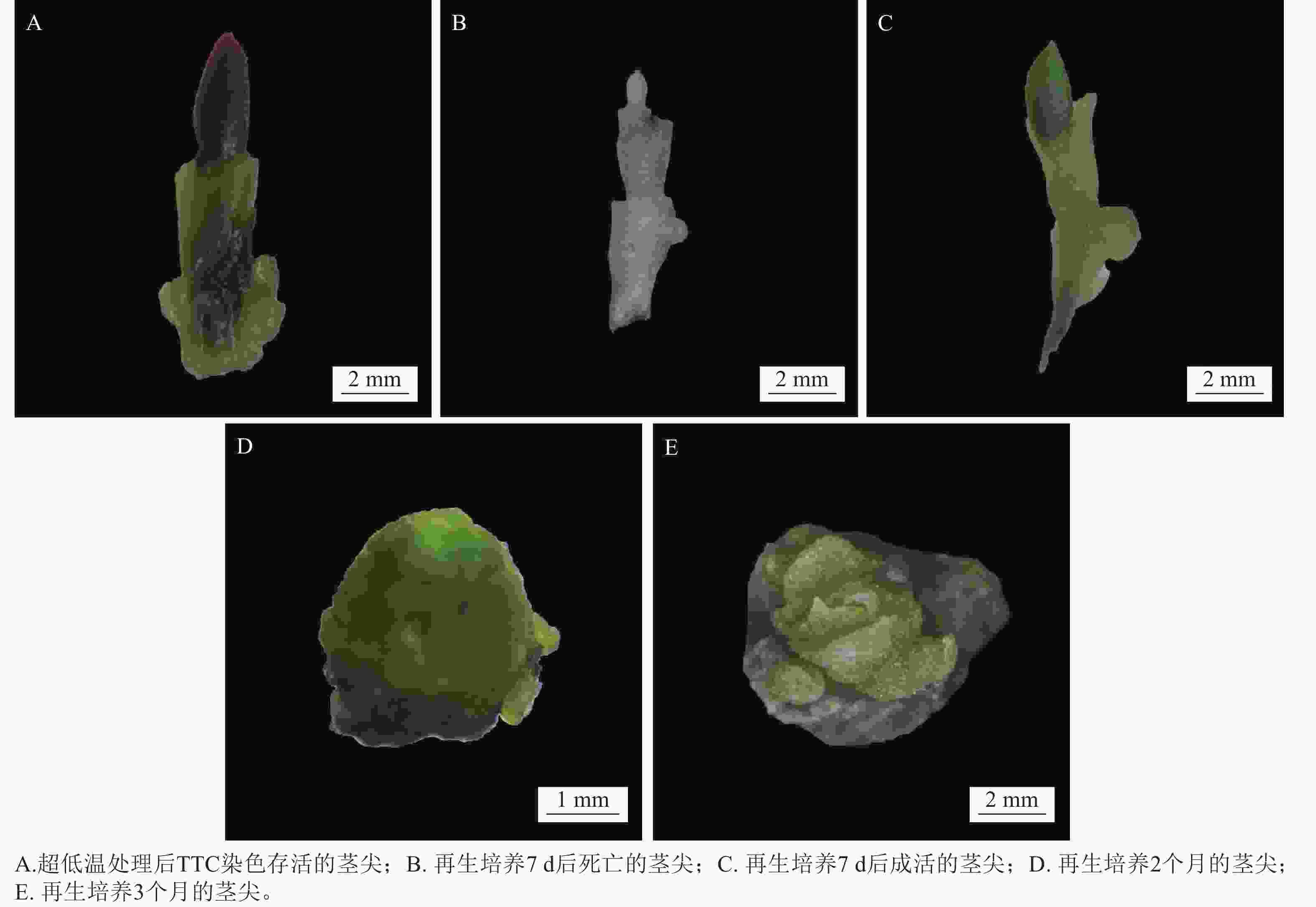
经超低温处理的茎尖卸载,以及TTC染色后存活茎尖如图3A所示。将其接种至恢复培养基中,暗培养7 d后,茎尖转移到新培养基中,正常光照条件促进茎尖生长。培养7 d后,象鼻兰茎尖的再生率为43.33%。通过形态观察,死亡的茎尖组织逐渐变为白色(图3B),未能恢复绿色。存活的茎尖组织(图3C)则表现出持续的体积膨胀现象,并最终脱分化为愈伤组织(图3D)。再生培养2个月的茎尖形成类原球茎,再生培养3个月后分化出芽原基(图3E)。可见愈伤组织的生成与茎尖组织的存活能力密切相关。
-
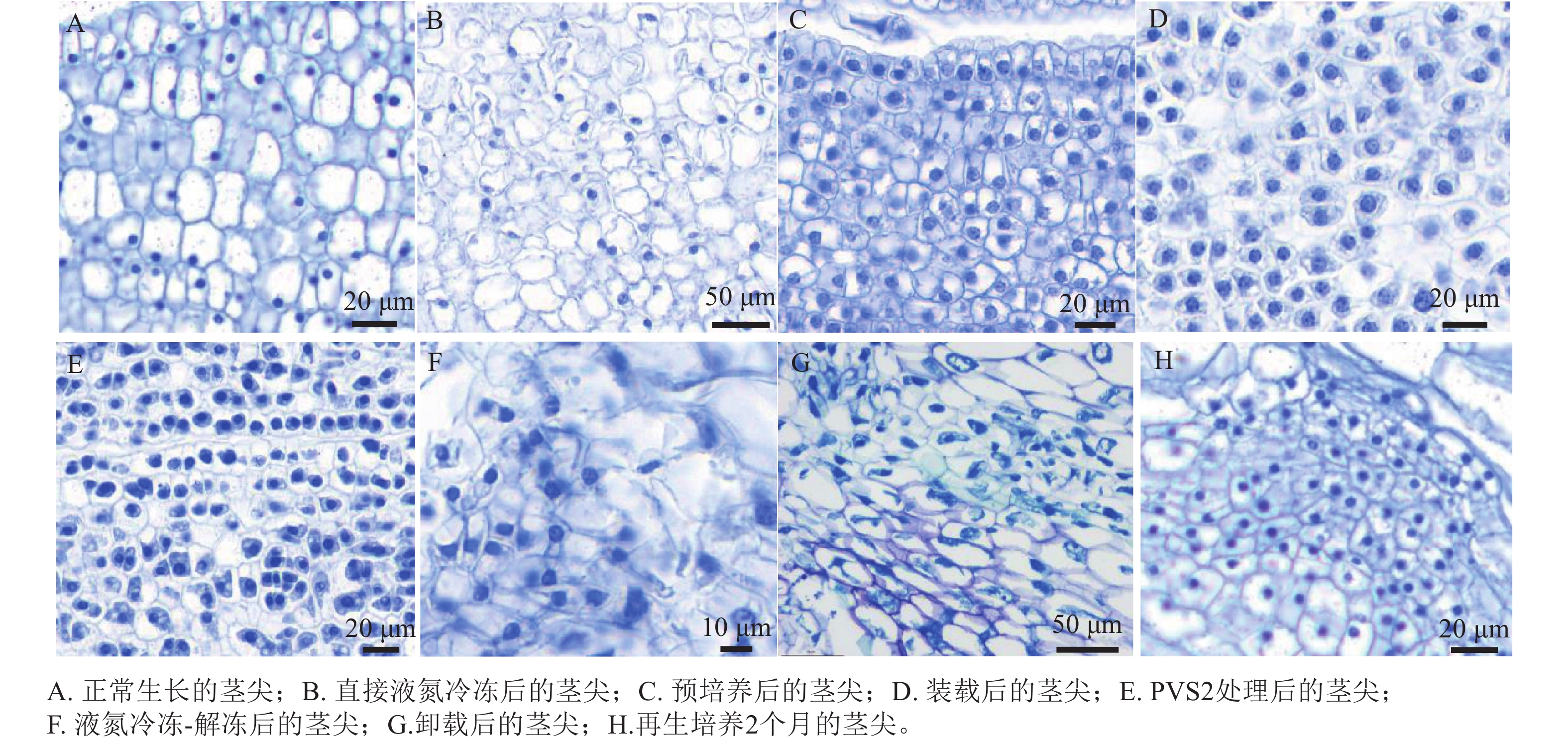
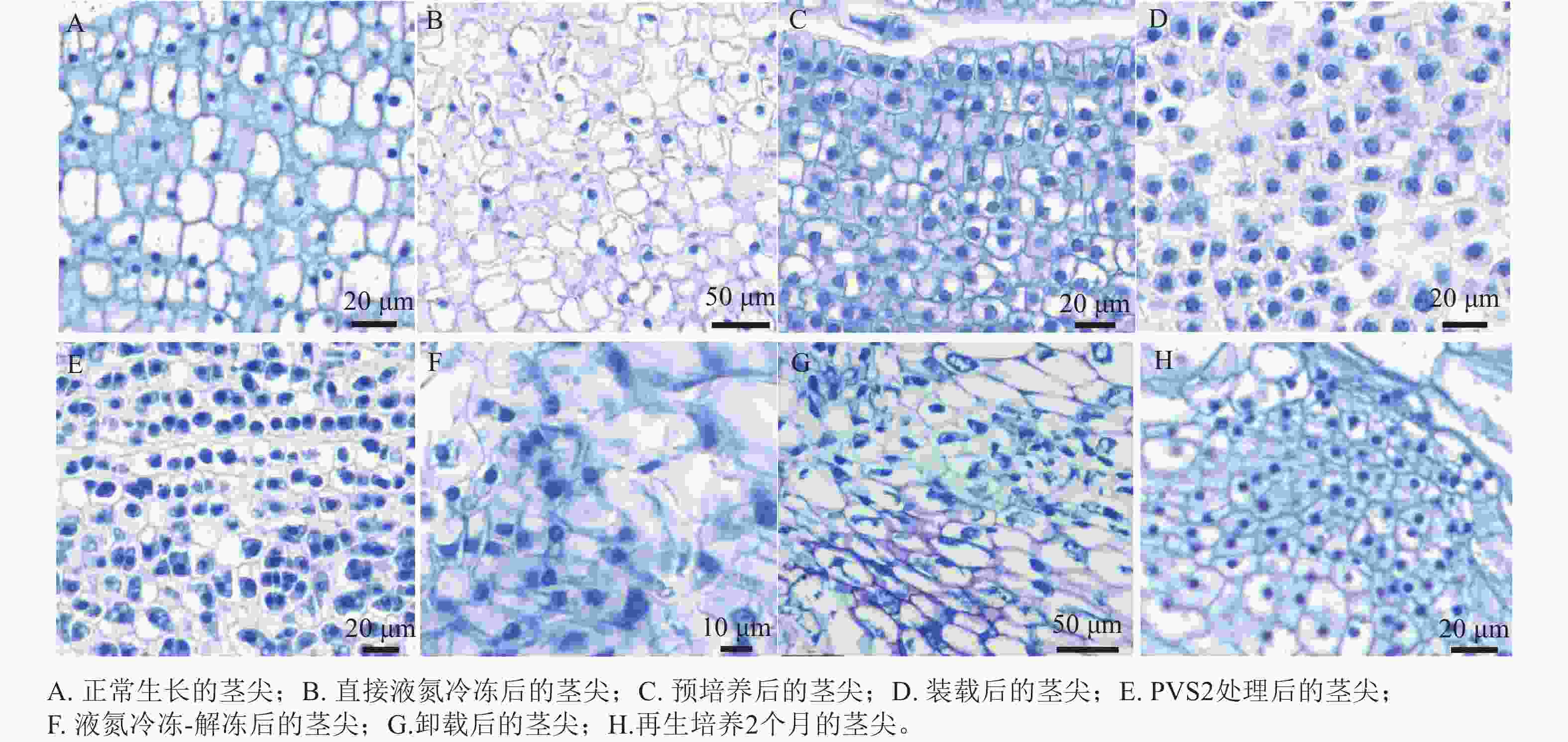
由石蜡切片可见:未经任何处理正常生长的象鼻兰茎尖,细胞排列疏松,体积较大,呈现椭圆形或长椭圆形,细胞核清晰可见,并进行旺盛的分裂活动(图4A)。未经任何处理直接投入液氮冻存的茎尖,发生细胞破裂,细胞壁被破坏,胞质外流,细胞形态不规则,散乱排列,细胞核消失,并且出现较为严重的质壁分离现象(图4B)。预培养后茎尖细胞小而排列紧密,细胞质及细胞核等染色加深,但与正常生长的茎尖无明显差别(图4C)。装载之后的细胞排列紧密,开始出现轻微的质壁分离现象(图4D)。在玻璃化溶液处理后茎尖细胞质壁分离现象加重,细胞核变得不清晰(图4E)。经冷冻卸载之后的细胞排列散乱,细胞壁破裂,结构不完整,细胞质等内容物散出,出现严重的质壁分离现象,细胞核染色加深(图4F,G)。恢复再生的茎尖细胞分裂旺盛(图4H)。
-
超低温保存技术应用日益广泛,小液滴玻璃化法具有适应性广、存活率和再生率高、样品处理量大、操作简易等优点,极大提高了植物种质资源的再生率[20]。FOLGADO等[21]采用小液滴玻璃化技术对白玉兰Magnolia heptapeta茎尖进行处理得到较高再生率。对菊花Chrysanthemum × morifolium小液滴玻璃化法超低温保存后的平均再生率为68.00%[22]。杨晶文等[23]对红豆越橘Vaccinium vitisidaea苗茎尖进行小液滴玻璃化处理,经再生培养,实现76.00%的高再生率。此外,小液滴玻璃化法在樱桃Prunus pseudocerasus [24]、苹果Malus pumila [25]、鳄梨Persea americana [26]、香葱Allium cepiforme [27]等多种植物保存上也有成功报道。本研究采用小液滴玻璃化法进行象鼻兰茎尖超低温保存探索。
通过优化预处理条件,可以显著提高植物材料的抗冻性,提高存活率[28]。LURSWIJIDJARUSA等[29]发现:大苞鞘石斛Dendrobium wardianum茎尖在0.5 mol·L−1蔗糖预培养后,超低温保存再生率较高。在象鼻兰茎尖预培养时,不同蔗糖浓度处理结果也存在显著差异,尤其是在0.5 mol·L−1蔗糖预培养基中暗培养48 h后,茎尖存活率显著提升。
装载溶液通过渗透进入细胞内部,进而保护细胞膜及细胞内部结构的完整性,防止其在超低温保存过程中遭受损伤[30]。在对珍贵种质蓝莓Vaccinium uliginosum进行小液滴玻璃化预培养处理后,用含有1.0 mol·L−1蔗糖和2.0 mol·L−1甘油的装载液处理茎尖30 min,获得较高的存活率[31]。在本研究中,茎尖预处理后,装载处理20 min得到较高存活率。玻璃化保护溶液可降低细胞内水分冰点,减少冰晶形成,降低细胞损伤风险。由于二甲基亚砜有较强毒性,因此,需控制PVS2处理时间。有研究对蔷薇Rosa茎尖进行冻存技术研究时,对比3种玻璃化溶液(PVS2、PVS3及PVS4)对存活率的影响,发现PVS2溶液处理的茎尖展现出最高的存活率[32]。在本研究中所采用的玻璃化溶液为PVS2。百合Lilium经液滴-玻璃化处理,于0 ℃下使用PVS2溶液浸泡90~120 min,其存活率显著提高[33]。铁皮石斛Dendrobium officinale经PVS2溶液0 ℃处理150 min后的存活率达89.4%[34]。在本研究中,0 ℃下用PVS2溶液处理象鼻兰茎尖120 min的存活率最高,未经玻璃化处理的茎尖存活率极低。因此,玻璃化溶液的处理被认为是关键步骤。
在本研究中,将茎尖放入浓度为1.2 mol·L−1蔗糖卸载液中处理20 min,可有效清除茎尖表面残留的PVS2溶液,避免PVS2溶液对茎尖的潜在二次毒性作用从而减少其对茎尖毒性影响,并显著提高存活率。
在对文心兰类原球茎进行组织结构观察,发现在玻璃化、液氮冻存-解冻阶段对类原球茎造成的损害较大[12]。本研究发现:预培养和装载处理对细胞结构的影响较小,经过PVS2处理、液氮冷冻及随后的卸载步骤后,观察到细胞破裂现象显著增强。这可能是导致材料活性下降的关键因素,表明在关键阶段,细胞完整性受到了较大的影响。未经任何处理直接投入液氮的茎尖遭受了较严重的损伤,这一发现强调了在进行液氮冷冻保存前,采用适当浓度的预培养及装载溶液处理的必要性。
-
本研究采用小液滴玻璃化法成功实现了象鼻兰组培苗茎尖的超低温保存,为珍稀植物种质资源的长期保存提供了有效途径。通过优化预培养、PVS2处理及卸载等关键步骤,茎尖存活率达到70.0%,再生率为43.33%,证实了该方法在兰科植物中的适用性。通过石蜡切片观察发现:液氮冻存前的预处理和装载步骤对茎尖细胞结构影响较小,但在液氮冻融过程中,部分细胞结构发生显著变化甚至损伤。本研究建立了适用于象鼻兰茎尖的小液滴玻璃化法超低温保存体系。未来可进一步探索不同植物材料的适应性,优化冷冻保护剂配方及再生培养条件,提高保存效率和再生率,推动该技术在植物种质资源保护中的广泛应用。
Establishment of droplet vitrification cryopreservation system for Phalaenopsis zhejiangensis shoot tips
doi: 10.11833/j.issn.2095-0756.20250172
- Received Date: 2025-03-04
- Accepted Date: 2025-07-10
- Rev Recd Date: 2025-07-08
- Available Online: 2025-10-29
- Publish Date: 2025-10-20
-
Key words:
- Phalaenopsis zhejiangensis /
- shoot tips /
- droplet vitrification /
- cryopreservation
Abstract:
| Citation: | ZHAO Yue, SHAN Lu, CHEN Zhiguang, et al. Establishment of droplet vitrification cryopreservation system for Phalaenopsis zhejiangensis shoot tips[J]. Journal of Zhejiang A&F University, 2025, 42(5): 1102−1109 doi: 10.11833/j.issn.2095-0756.20250172 |










 DownLoad:
DownLoad: