-
种子萌发是植物生命周期的关键起点,直接关系到种群的自然更新与繁衍,而种子休眠则是植物在长期进化中形成的一种适应性策略,能够通过调控种子萌发的时间和空间,使植物避开不利环境,提高后代存活概率[1−2]。然而,过度或难以解除的休眠往往会导致种子萌发率低下,成为制约其自然更新的瓶颈。不同植物种子的休眠原因存在差异,这与其生存环境及自身特性密切相关[3]。根据植物种子不同的休眠特性,将种子休眠划分为形态休眠、物理休眠、生理休眠、形态生理休眠、复合休眠5种类型,其中形态生理休眠包括2种类别和9种类型[4]。
铁线莲属Clematis是毛茛科Ranunculaceae中的一个大属,其绝大多数的种为木质藤本植物,全世界约有355种(含亚种、变种等种下级单位,下同)[5]。中国有自然野生分布约147种,西南地区的种类较多,而浙江有32种[6]。根据国内外研究,大多数铁线莲属植物的种子休眠属于形态休眠和形态生理休眠,也存在无休眠的种类。C. microphyiia的种子存在生理休眠的特性[7]。女萎C. apiifolia、大叶铁线莲C. heracleifolia、齿叶铁线莲C. serratifolia、C. heracleifolia var. urticifolia的种子属于形态休眠类型[8]。圆锥铁线莲C. terniflora、C. trichotoma、C. taeguensis的种子具有形态生理休眠特性[9]。棉团铁线莲C. hexapetala的种子存在深度复杂的形态生理休眠类型,只需冷层积就可以萌发[10]。灌木铁线莲C. fruticosa的种子,在室温下发芽率可达到75.33%,不存在休眠现象[11]。
舟柄铁线莲是浙江的特有种之一,属于极小种群,其分布范围狭窄,主要分布于浙江丽水各县,生于山坡林中或山谷溪边[6, 12]。舟柄铁线莲为常绿型木质藤本,花白色,圆锥花序,花量大且可以二次开花,花期为5—6月,果期为6—7月,具有极佳的园林应用潜力,其种子在自然环境下长期不萌发,存在休眠现象,现已被列入《世界自然保护联盟濒危物种红色名录》,属于近危级植物。目前,关于舟柄铁线莲种子繁育的相关研究尚未见报道。本研究通过对舟柄铁线莲种子的形态观察、种皮透水性、内含抑制物、各类解除休眠方法的研究,旨在解析种子的休眠原因,寻找有效破眠方法,为该植物的保护和开发利用提供依据。研究成果不仅可为其人工繁育、种群恢复提供关键技术支撑,还有助于推动该近危物种的迁地保护与可持续利用,对丰富植物多样性及生态修复实践具有重要的应用价值。
-
舟柄铁线莲种子于2024年8月采自浙江省丽水市景宁畲族自治县(东坑村、九龙山、标溪乡),经有效氯浓度为7.5 g·L−1的次氯酸钠溶液浸泡20 min进行表面灭菌,再用无菌水冲洗5次,置于干燥阴凉处自然晾干备用。内源抑制物质测定所用材料为白菜Brassica rapa var. glabra种子,购自安徽九七种苗科技有限公司,净度≥98.00%,发芽率≥98.00%,含水量≤8.00%。
-
随机选取30粒种子,拍摄种子的平面和立体图像后导入AutoCAD 2024,测量长、宽和厚度;随机选取
1000 粒种子,用电子天平(精确至0.001 g)测定种子千粒重。使用体视显微镜(SMZ745)对种子的种皮和横切面结构进行观测,并测定胚长和宽。均重复测定3次。 -
随机选取100粒种子称鲜质量,后放于60 ℃烘箱中,每隔1 h取出称量,直至恒量,得种子干质量。按照相对含水量=[(种子鲜质量−种子干质量)/种子鲜质量]×100%进行计算,重复测定3次。
随机选取200粒种子均分为2组,其中1组进行刻皮处理(用手术刀在种皮非胚部位划1~2道浅痕,不伤及种胚),另1组作为对照,种子不做刻皮处理[13]。种子称量后,分别放入蒸馏水中浸泡48 h,其中前12 h每2 h取出称量,后36 h每6 h取出称量,直至种子恒量,每次称量前擦干种子表面水分,按照吸水率=[(吸水后质量−吸水前质量)/吸水前质量]×100%进行计算,重复测定3次。
-
将种皮和胚乳分离后粉碎,分别称取粉碎物1.0 g于锥形瓶中,加入蒸馏水10 mL,于室温下无光密封浸提36 h,每隔2 h震荡摇匀一次;收集浸提液,
4000 r·min−1离心10 min,取上清液定容至10 mL,得到0.10 g·mL−1的粗提液,置于4 ℃冰箱密闭保存备用[14]。选取饱满且大小形态相近的白菜种子,用5 g·L−1高锰酸钾溶液浸泡30 min消毒,用蒸馏水冲洗后浸泡,选择能沉在水底的种子风干备用。用去离子水将种皮、胚乳粗提液分别配制成浓度为0、0.05和0.10 g·mL−1的浸提液。分别选取50粒白菜种子浸泡在上述浸提液中24 h,随后在直径为9 cm的培养皿中铺入2层滤纸,将浸泡后的白菜种子均匀撒播在滤纸上,沿培养皿的内壁滴入相应的浸提液润湿滤纸,用保鲜膜密封,置于恒温为25 ℃、光周期为光照16 h/黑暗8 h,光强为2 500 lx的培养箱中,24 h后统计种子发芽率(以突破种皮为发芽标准),48 h后测定胚根长度,72 h后测定下胚轴长度。重复测定3次。 -
激素处理:随机选取400粒种子,均分成4份,分别在无菌水(ck)、50、100、200 mg·L−1的赤霉素(GA3)溶液中浸泡48 h,用于后续萌发试验。
冷层积处理:随机选取800粒种子,均分成8份,分别埋入含有湿润的混合基质(珍珠岩∶泥炭土=1∶1,体积比)的密封袋中,放入4 ℃的冰箱保存;分别在0、14、28、42、56、70、84和98 d取出1份种子,分别均匀撒播在垫有2层湿润滤纸的培养皿中,进行萌发试验。
黑暗处理:随机选取200粒种子,均分成2份。分别均匀撒播在垫有2层湿润滤纸的培养皿中,1份用锡纸包裹培养皿(确保无光透入),另1份不作避光处理,分别进行萌发试验。
经上述3种方式处理的种子,均置于变温(25 ℃ 16 h /15 ℃ 8 h)、光周期为光照16 h/黑暗8 h,光强为2 500 lx的培养箱中进行种子萌发试验,其中变温和光周期的时间同步。另随机选取200粒种子,均分成2份,分别均匀撒播在垫有2层湿润滤纸的培养皿中,其中1份置于上述的变温条件下,另1份则置于恒温25 ℃,两者的光周期(光照16 h/黑暗8 h)与光强(2 500 lx)设置相同。
所有萌发试验均进行3次重复。试验开始后每日观测,及时取出萌发种子和霉变种子,种子萌发以胚根突破种皮为标志,连续7天无种子萌发则发芽结束,统计发芽率和发芽势。发芽率=(发芽种子总数/供试种子总数)×100%,发芽势=(发芽高峰日发芽种子数/供试种子总数)×100%。
-
数据处理和作图采用Excel 2021、AutoCAD 2024和Origin 2024,数据分析采用SPSS 26.0。数据以平均值±标准差表示。
-
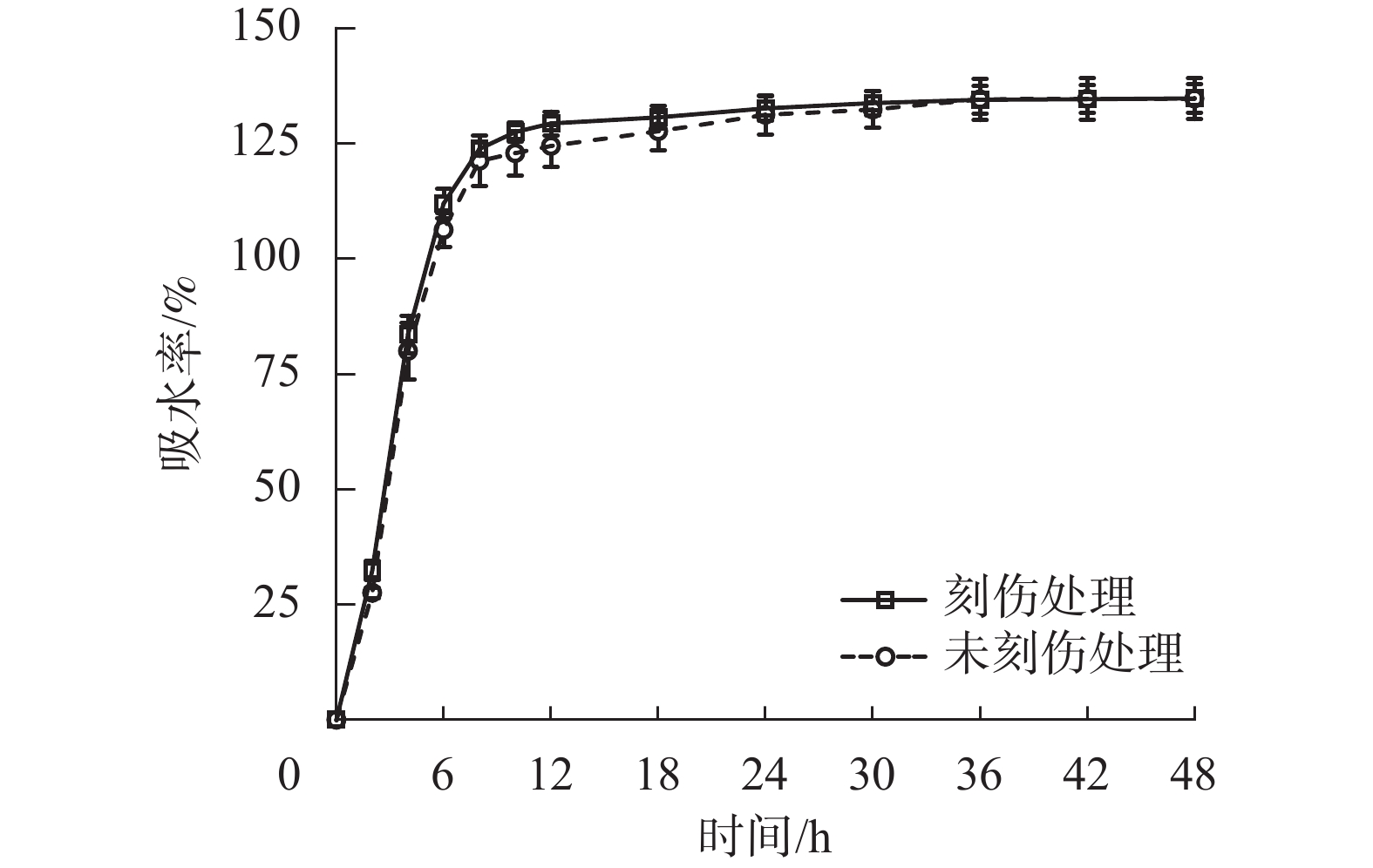
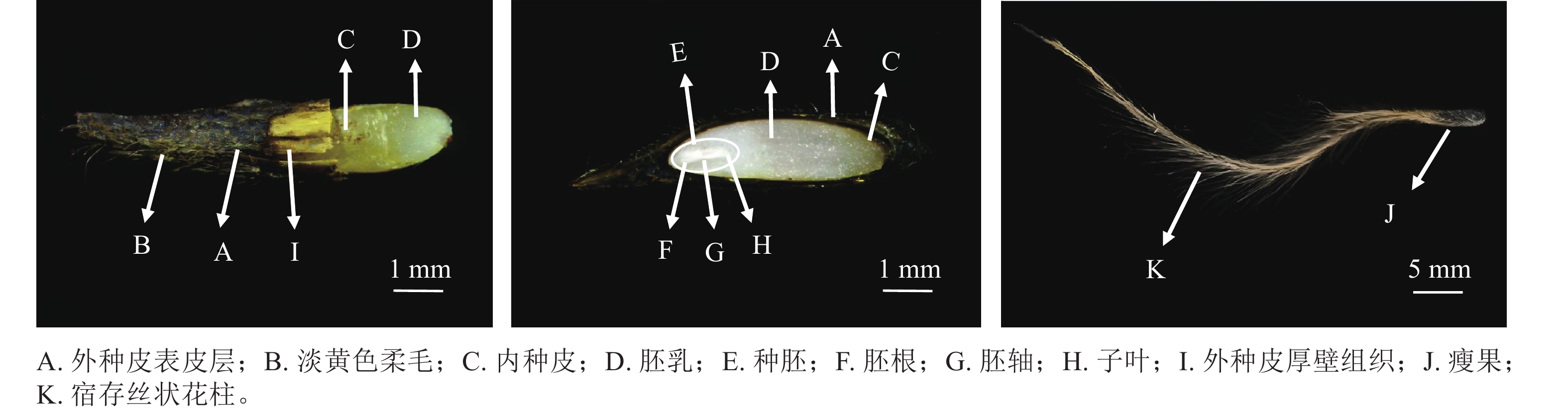
如图1所示:舟柄铁线莲种子分为瘦果和宿存丝状花柱2个部分,瘦果外形呈椭圆形或狭长椭圆形;外种皮呈黑褐色,粗糙且被淡黄色短柔毛,结构木质化;内种皮呈金黄色,光滑且薄;宿存花柱具有稀疏展开的淡黄色柔毛;种胚具乳白色胚乳层,胚乳紧包着种胚;种子成熟时种胚已分化但发育不完全,具明显的子叶、胚轴和胚根结构。种子长为(0.66±0.06) cm,宽为(0.19±0.07) cm,厚为(0.15±0.04) cm,宿存花柱长为(3.20±0.45) cm,千粒重为(7.48±0.12) g,相对含水量为(6.69±0.32)%。
-
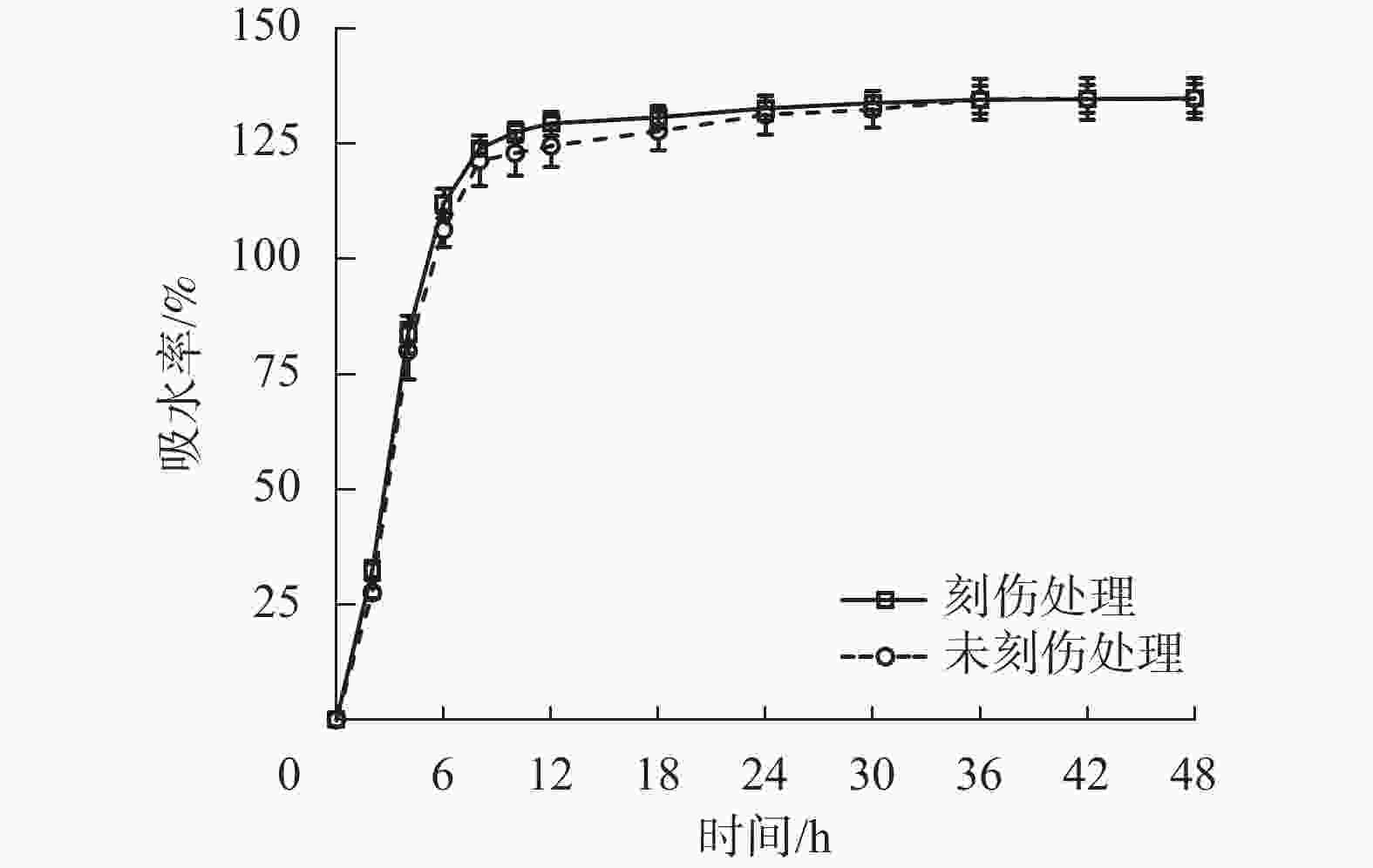
为探究舟柄铁线莲种皮是否存在吸水阻碍,对刻伤种皮和不刻伤种皮的种子进行吸水试验。2种处理的吸水率变化情况如图2所示。刻伤种子和不刻伤种子在0~8 h均为快速吸水阶段,在8~30 h均为缓慢吸水阶段,在30~48 h均为吸水饱和阶段。刻伤种皮的种子吸水率为(117.49±29.73)%,不刻伤种皮的种子吸水率为(114.82±30.97)%,两者无明显差异。以上结果说明舟柄铁线莲种皮不存在吸水阻碍,排除物理休眠现象。
-
如表1所示:各部位不同浸提液对白菜种子的发芽无显著抑制作用。相对于ck,种皮和胚乳的浸提液对白菜种子的胚根生长有显著促进作用(P<0.05),但对下胚轴生长无显著影响。说明舟柄铁线莲种皮和种胚中的内含物对种子萌发均无显著抑制作用。
浸提液 萌发率/% 胚根长/cm 下胚轴长/cm 无菌水(ck) 0.88±0.08 a 0.88±0.07 b 0.77±0.05 ab 0.05 g·mL−1种皮 0.85±0.03 a 1.22±0.15 a 0.72±0.12 ab 0.10 g·mL−1种皮 0.82±0.04 a 1.25±0.15 a 0.59±0.29 b 0.05 g·mL−1胚乳 0.80±0.04 a 1.13±0.29 a 0.85±0.13 a 0.10 g·mL−1胚乳 0.88±0.02 a 0.94±0.27 ab 0.68±0.13 ab 说明:同列不同小写字母表示不同处理间存在显著差异(P<0.05)。 Table 1. Effects of different extracts from different parts of C. dilatata seeds on the germination of B. rapa var. glabra seeds
-
由表2可知:与变温和光暗交替处理相比,恒温和光暗交替处理并未显著改变种子的发芽率和发芽势,但使平均胚根萌动时间延长了9 d;而变温和全黑暗处理则显著降低了发芽率(P<0.05),并使平均胚根萌动时间缩短了10 d,但发芽势无显著变化。由此可知,在变温和光暗交替的环境条件下,舟柄铁线莲种子的萌发效果最好。
处理类型 发芽率/% 发芽势/% 平均胚根萌动时间/d 变温+光暗交替 21.67±2.52 a 2.33±0.58 a 75 恒温+光暗交替 17.33±5.51 ab 2.00±1.00 a 84 变温+全黑暗 8.67±3.06 b 1.33±0.58 a 65 说明:变温为25 ℃ 16 h/15℃ 8 h,恒温为25 ℃,光暗交替为光照16 h/黑暗8 h,全黑暗为黑暗24 h。同列不同小写字母表示不同处理间存在显著差异(P<0.05)。 Table 2. Effects of different light and temperature treatments on seed germination of C. dilatata
-
如表3所示:种子经GA3处理后的发芽率显著高于无GA3处理的(P<0.05)。种子经50 mg·L−1 GA3处理后,发芽率高达(68.33±3.51)%,发芽势为(8.00±1.00)%,平均胚根萌动时间为23 d,但随GA3浓度升高,发芽率和发芽势均呈下降趋势,平均胚根萌动时间也随之延长。该结果说明GA3对克服舟柄铁线莲种子休眠至关重要,经50 mg·L−1 GA3处理后的种子萌发效果最好,高浓度的GA3反而抑制萌发。
GA3浓度/
(mg·L−1)发芽率/
%发芽势/
%平均胚根萌动
时间/d0 21.67±2.52 c 2.33±0.58 b 75 50 68.33±3.51 a 8.00±1.00 a 23 100 39.00±5.57 b 4.00±1.00 b 32 200 38.67±2.52 b 3.00±1.00 b 34 说明:同列不同小写字母表示不同处理间存在显著差异(P<0.05)。 Table 3. Effects of different concentrations of gibberellin treatment on seed germination of C. dilatata
-
由表4可知:与未处理的种子相比,种子经冷层积处理后能够有效缩短平均胚根萌动时间(21~45 d)。随着冷层积的时间增加,发芽率先升高后降低,发芽势先升高后趋于平稳,而平均胚根萌动时间则不断缩短直至稳定。其中,冷层积70 d时,发芽率达到最高,为(64.33±5.51) %,随后发芽率逐渐降低;冷层积56~98 d时,发芽势出现显著差异(P<0.05)且较为稳定;冷层积84 d时,平均胚根萌动时间最短(21 d)。该结果表明冷层积对舟柄铁线莲种子萌发有明显的促进作用,能够大幅度缩短胚根萌动时间,但长时间的冷层积会导致种子耗尽养分或失活,不利于种子萌发。
冷层积时间/d 发芽率/% 发芽势/% 平均胚根萌动时间/d 0 21.67±2.52 e 2.33±0.58 c 75 14 27.00±3.61 de 2.67±0.58 bc 45 28 31.67±2.52 cde 4.00±1.00 abc 37 42 35.00±3.61 cd 4.67±1.53 abc 32 56 42.67±4.73 bc 6.00±2.00 ab 27 70 64.33±5.51 a 7.33±1.15 a 25 84 53.67±4.16 b 6.33±1.53 a 21 98 28.33±5.13 de 6.67±1.15 a 23 说明:同列不同小写字母表示不同处理间存在显著差异(P<0.05)。 Table 4. Effect of different duration of cold stratification treatment on seed germination of C. dilatata
-
目前,BASKIN等[15]的二叉式检索表是最全面的种子休眠分类系统,划分不同休眠类型的判定依据主要包括种皮透水性、胚胎是否分化、胚是否发育完全、胚根和胚芽是否同时萌发等因素。物理休眠是由于种皮不透水而阻碍种胚萌发的一种休眠类型[16]。在本研究中,刻伤与不刻伤的种子均能正常吸水,说明舟柄铁线莲种子不存在物理休眠或物理生理组合休眠,这与周睿等[13]对全缘铁线莲C. integrifolia的研究结果一致。本研究中,以无菌蒸馏水为溶剂,舟柄铁线莲种胚与胚乳的浸提液均无法抑制白菜种子萌发,这可能是舟柄铁线莲种子的内源抑制物是脂溶性的,无菌蒸馏水无法有效提取[17]。
生理休眠类型的种子胚胎已分化且发育完全,而形态休眠和形态生理休眠类型的种子胚胎未分化或已分化但发育不全,在种胚已分化但发育不完全时,种子可以在适宜环境中1个月内生根发芽时,则归为形态休眠类型;若种子超过1个月没有出现根突破种皮的现象,则被认为是形态生理休眠类型[15]。本研究中,舟柄铁线莲种子在成熟时胚胎已分化但发育不全,但在适宜的培养条件下,种子在75 d后才出现根突破种皮的现象,表明舟柄铁线莲种子属于形态生理休眠类型,这与YANG等[9]的研究结果一致,他们发现C. taeguensis和C. terniflora的种子在温度为25 ℃/15 ℃的培养箱中培养100 d以上才会萌发,均属于形态生理休眠类型。
形态生理休眠类型具有形态休眠和生理休眠2种特点,即种子胚芽发育不完全,需要经历生理休眠才能萌发[18]。根据种胚破除休眠以及生长发育所需的温度和对GA3的响应,形态生理休眠类型可分为2个类别,1类是简单型,种胚在暖层积(≥15 ℃)中才能正常生长发育;另1类是复杂型,种胚的生长发育需要在冷层积(0~10 ℃)中进行[19]。本研究中,种子在冷层积过程中未出现种胚萌发的现象,但冷层积一定时间后,再转移到温度为25 ℃/15 ℃的培养箱中进行层积时,种胚能够正常生长发育,发芽率提高了,胚根萌动时间也大幅度缩短,该现象说明舟柄铁线莲种子经冷层积解除休眠后,种胚需要暖层积才能正常生长发育,属于简单形态生理休眠类型,这与JANG等[8]对圆锥铁线莲和C. trichotoma的休眠特性研究结果一致。本研究还发现舟柄铁线莲种子经50 mg·L−1 GA3和冷层积70 d分别处理后,2种处理方式对发芽率和发芽势的提升作用无明显差异,说明GA3可以代替冷层积打破舟柄铁线莲种子的休眠。因此,舟柄铁线莲种子的休眠类型可以进一步细分为非深度简单形态生理休眠类型。
-
植物激素能够调节种子体内的内源激素含量变化实现萌发的所需[20]。研究表明:在种子休眠和萌发时,植物内部的2种激素脱落酸(ABA)和GA3会相互影响。ABA浓度高会导致种子休眠并抑制萌发,而GA3则会降低ABA含量并促进萌发[21]。相关研究发现:适量浓度的GA3对毛蕊铁线莲C. lasiandra和女萎的种子萌发有显著的促进作用,低浓度的6-苄氨基嘌呤(6-BA)有利于提高山木通C. finetiana的种子发芽率[22]。本研究中使用50 mg·L−1 GA3浸泡舟柄铁线莲种子24 h后,平均胚根萌动时间提前了52 d,发芽率也提高了2倍,但随GA3浓度升高,发芽率和发芽势均呈下降趋势,平均胚根萌动时间也随之延长。但也有研究认为GA3对钝萼铁线莲C. peterae和粉绿铁线莲C. glauca种子的发芽率没有显著提升作用,但可以加速种子萌发的时间[23−24]。由此可知:50 mg·L−1 GA3对舟柄铁线莲种子萌发有显著促进作用,但随着浓度的提升,反而会产生一些抑制作用。
-
光照对种子萌发是否为必要条件,取决于种子的自身遗传特性和共生环境[25]。在光照条件下,钝萼铁线莲种子的发芽率比在黑暗条件下高24%[24]。灌木铁线莲和灰叶铁线莲C. tomentella种子在全光照和全黑暗条件下的萌发率相同,但在全黑暗条件下种子发芽能力更强[26],这与本研究结果部分相似,舟柄铁线莲种子在全黑暗环境下的平均胚根萌动时间比在光暗交替环境下短10 d,但与光暗交替环境相比,舟柄铁线莲种子在全黑暗环境下的发芽率显著降低,说明舟柄铁线莲种子适合在光暗交替环境下萌发。
-
温度也是影响种子萌发进度的直接因素,不同种子萌发速率对温度的敏感程度存在差异[27]。如大叶铁线莲和东方铁线莲C. orientalis种子的萌发温度适应范围较广,在20 ℃/10 ℃到30 ℃/20 ℃的变温范围内种子萌发率均高于70%[28],灌木铁线莲种子在30 ℃/15 ℃的变温环境中发芽率最高[29]。本研究中,在25 ℃/15 ℃的变温环境下,舟柄铁线莲种子的平均胚根萌动时间比在恒温(25 ℃)环境下短9 d,但其发芽率和发芽势与恒温条件相比差异不显著,说明舟柄铁线莲种子在变温环境下更能促进种胚发育。
低温层积会使种子体内发生一系列生理反应,加快大分子储藏物质分解为小分子物质,满足种子萌发时所需物质,进而解除休眠[30]。本研究中,舟柄铁线莲种子在冷层积70 d后,发芽率达到最高,为(64.33±5.51)%,随着冷层积时间的增加,发芽率显著降低,表明冷层积对舟柄铁线莲种子萌发有显著促进作用,但长时间的冷层积反而会抑制种子萌发。这与林春新等[31]对东北铁线莲C. mandshusica的研究结果不一致,他们发现东北铁线莲种子因自身特性和生存环境,需在低温层积140~160 d后,发芽率才能到达70%。
-
舟柄铁线莲种子休眠类型属于非深度简单形态生理休眠类型,适合在变温(25 ℃ 16 h/15 ℃ 8 h)和光暗交替(光照16 h/黑暗8 h)的培养条件中萌发。低温层积70 d和50 mg·L−1 GA3处理均可以有效的打破舟柄铁线莲种子的休眠并缩短胚根突破种皮的周期。本研究仅对舟柄铁线莲种子休眠类型及破眠方法进行了初步探讨,但是对于种子萌发抑制物的成分、存在部位、发生机理等有待进一步研究。
Seed germination characteristics of wild plant Clematis dilatata with extremely small populations
doi: 10.11833/j.issn.2095-0756.20250440
- Received Date: 2025-08-20
- Accepted Date: 2025-12-18
- Rev Recd Date: 2025-12-15
-
Key words:
- dormancy type /
- morphophysiological dormancy /
- gibberellin /
- low temperature stratification
Abstract:
| Citation: | HE Shunyun, ZHAO Changgao, WANG Jichen, et al. Seed germination characteristics of wild plant Clematis dilatata with extremely small populations[J]. Journal of Zhejiang A&F University, 2026, 43(X): 1−8 doi: 10.11833/j.issn.2095-0756.20250440 |









 DownLoad:
DownLoad: