-
花色是植物最直观的观赏特性之一[1]。花色可以吸引昆虫、鸟类、蜂类等各种传粉媒介,从而帮助植物进行有效传粉,有助于植物的繁殖和生存[2]。花色还具有防止光氧化损伤以及提供对生物和非生物胁迫抵抗力的功能[3]。除此之外,不同颜色的花卉使人感到愉悦放松,起到改善生活的作用。因此,花色改良和培育是观赏植物花色创新育种的重要目标。植物花色的形成是多种色素共同作用与积累的结果[4]。影响花色的色素类型主要分为类黄酮化合物、类胡萝卜素和生物碱[5]。其中,类黄酮中的花青苷是大多数观赏植物形成丰富花色的决定性色素群[6],在菊花Chrysanthemums × morifolium[7]、大丽花Dahlia variabilis[8]、月季Rosa chinensis[9]、紫罗兰Matthiola incana[10]等中都有过相关报道。
梅花Prunus mume是中国十大传统名花之一,具有树形多变、花香清新、花色多样等观赏特征,其中花色是最为明显的观赏性状,直接影响植株的景观表现与经济价值。目前梅花有紫红、粉红、白、绿白、淡黄、复色等颜色[11]。赵昶灵等[11]经化学显色反应及紫外-可见光谱分析,证实梅花花色色素属于黄酮类化合物。张芹[12]对41个品种的花色苷分析表明:梅花花色表型可为2类,分别为含花青苷的粉、紫红色系与不含花青苷的白、淡黄色系,花色苷合成存在品种特异性分支选择,矢车菊素和芍药花素衍生物主导红色花形成;天竺葵素和飞燕草素衍生物参与部分紫红色花的呈色,而槲皮素衍物质则影响黄绿色花表型[13−14]。此外,黄色花主要源于类胡萝卜素,尤其是叶黄素的积累,并受到类黄酮辅助色素的调控[15]。探讨梅花不同品种花色素成分的差异,可揭示梅花花色的形成机制,为梅花品种资源开发和利用提供借鉴。本研究选取15个不同花色梅花品种,使用比色卡和色差仪测定花色表型,结合聚类分析分类花色;通过超高效液相色谱-串联质谱(UPLC-MS/MS)测定梅花花瓣花青苷与其他类黄酮化合物的质量分数,通过不同梅花品种花色表型与色素成分的关联分析,探讨梅花花色与花色素之间的关系,以期为梅花高观赏性品种选育及花青苷代谢分子机制解析提供理论依据。
-
参考陈俊愉等[16]的方案,观察浙江农林大学种质资源库梅花的表型,在资源库中选择尽可能不同的品种群,对生长健壮、长势一致的15个不同花色的梅花品种(表1)进行调查研究,测定不同品种盛花期花瓣颜色表型并采样。取样时每个品种随机选取5株,每株采集至少10朵盛开花朵,均取自树冠外围中上部花枝,并仅取最外层花瓣。将同一品种的花瓣样品分3份混合均匀,作为生物学重复。混合样品标记后用锡箔纸包裹并立即用液氮速冻,放置于−80 ℃冰箱保存备用。
品种群 编号 品种 品种群 编号 品种 朱砂品种群 M1 ‘晨晖朱砂’‘Chenhui Zhusha’ 宫粉品种群 M10 ‘春意早宫粉’‘Chunyizao Gongfen’ M2 ‘骨红朱砂’‘Guhong Zhusha’ M3 ‘粉红朱砂’‘Fenhong Zhusha’ 玉蝶品种群 M11 ‘六萼玉蝶’‘Liue Yudie’ M4 ‘先春朱砂’‘Xianchun Zhusha’ M12 ‘月光玉蝶’‘Yueguang Yudie’ M5 ‘银红朱砂’‘Yinhong Zhusha’ 跳枝品种群 M13 ‘单粉跳枝’‘Danfen Tiaozhi’ 宫粉品种群 M6 ‘桃红宫粉’‘Taohong Gongfen’ M7 ‘晓红宫粉’‘Xiaohong Gongfen’ 江梅品种群 M14 ‘江梅’‘Jiangmei’ M8 ‘童颜宫粉’‘Tongyan Gongfen’ M9 ‘早花宫粉’‘Zaohua Gongfen’ 垂枝品种群 M15 ‘单粉垂枝’‘Danfen Chuizhi’ Table 1. List of P. mume cultivars analyzed in this study
-
采用英国皇家园艺学会标准比色卡对15个不同花色梅花品种盛开期的花瓣进行颜色比对。使用色差仪测定花瓣的明度(L*)、红绿色度(a*)、黄蓝色度(b*)。L*代表明暗程度,数值越高颜色越亮;a*的正值代表红色,a*越大颜色越红;b*的正值表示黄色。每个品种测5朵花作为生物学重复,离体测量,以白纸为背景,测最外层花瓣上表皮中央位置。
-
将梅花花瓣取出后加入液氮充分研磨,精确称取0.3 g,加入V(甲醇)∶V(水)∶V(甲酸)∶V(四氢呋喃)=70∶27∶2∶1的提取液3 mL,在4 ℃冰箱内避光静置24 h后,超声30 min,在4 ℃、12 000 r·min−1条件下离心10 min,吸取上清液。用0.22 µm过滤头过滤上清液后,避光保存在−20 ℃冰箱供上机分析。进行UPLC-MS/MS检测梅花花瓣类黄酮化合物组分,每个样品3个生物学重复。
色谱柱:ACQUITY UPLC HSS T3 (1.8 µm,2.1 mm×100.0 mm),柱温为40 ℃,进样为2 μL,流速为0.4 mL·min−1,洗脱方式为梯度洗脱,流动相A为体积分数1%的甲酸,B为纯乙腈(洗脱程序百分值为体积分数)。梯度洗脱程序为:0 min,5%B;2.0 min,11%B;6.0 min,13%B;9.4 min,38%B;10.0 min,50%B;12.0 min,5%B;16.0 min,5%B。检测波长为350 nm、520 nm。
质谱条件:电喷雾离子源(ESI),正/负离子模式扫描;扫描范围为m/z 80~1 500;离子源温度:正模式为600 ℃,负模式为550 ℃;离子化电压为5 500 V;一级扫描参数:去簇电压(DP)为100 V;聚焦电压(CE)为10 V;二级扫描采用TOF MS-Product Ion-IDA模式,碰撞诱导解离(CID)能量为4 020 eV;进样前以校准液输送系统(CDS)泵校准质量轴,误差<2×10−6。结合各色素组分的保留时间、离子大小、二级碎片大小和文献,推测梅花花色素成分。花青苷结构参考文献[13, 17−21],类黄酮结构则参考文献[22−24]。
-
将标准品芦丁和矢车菊素3-O-葡萄糖苷(纯度质量分数>98%)配制成1 000 mg·L−1的母液,将标准品稀释成不同的梯度,暂放−20 ℃冰箱保存待测。以标准品浓度(x)为横坐标,色谱峰面积(y)为纵坐标,绘制标准曲线。其中:矢车菊素-3-O-葡萄糖苷标准曲线为y=18 871x−23 280,R2=0.999 5;芦丁标准曲线为y=9 174.6x+6 227.2,R2=0.999 8。将待测样品(鲜质量)与标准品同步处理,按标准曲线计算总花青苷(total anthocyanins,TA)、总黄酮(total flavonoids,TF)及各组分质量分数(μg·g−1)。
-
使用Excel 2021进行数据整理,以平均值表示结果。使用SPSS 27对15个梅花品种花色表型参数L*、a*、b*进行最远邻元素法系统聚类分析,对总花青苷、总类黄酮进行差异化分析,对类黄酮化合物进行主成分分析,筛选主要有效因子,采用步进法对关键色素组分进行线性逐步回归分析。使用GraphPad Prism 9和Origin 2025绘图,并对梅花花色表型与色素组分进行Pearson相关性分析。
-
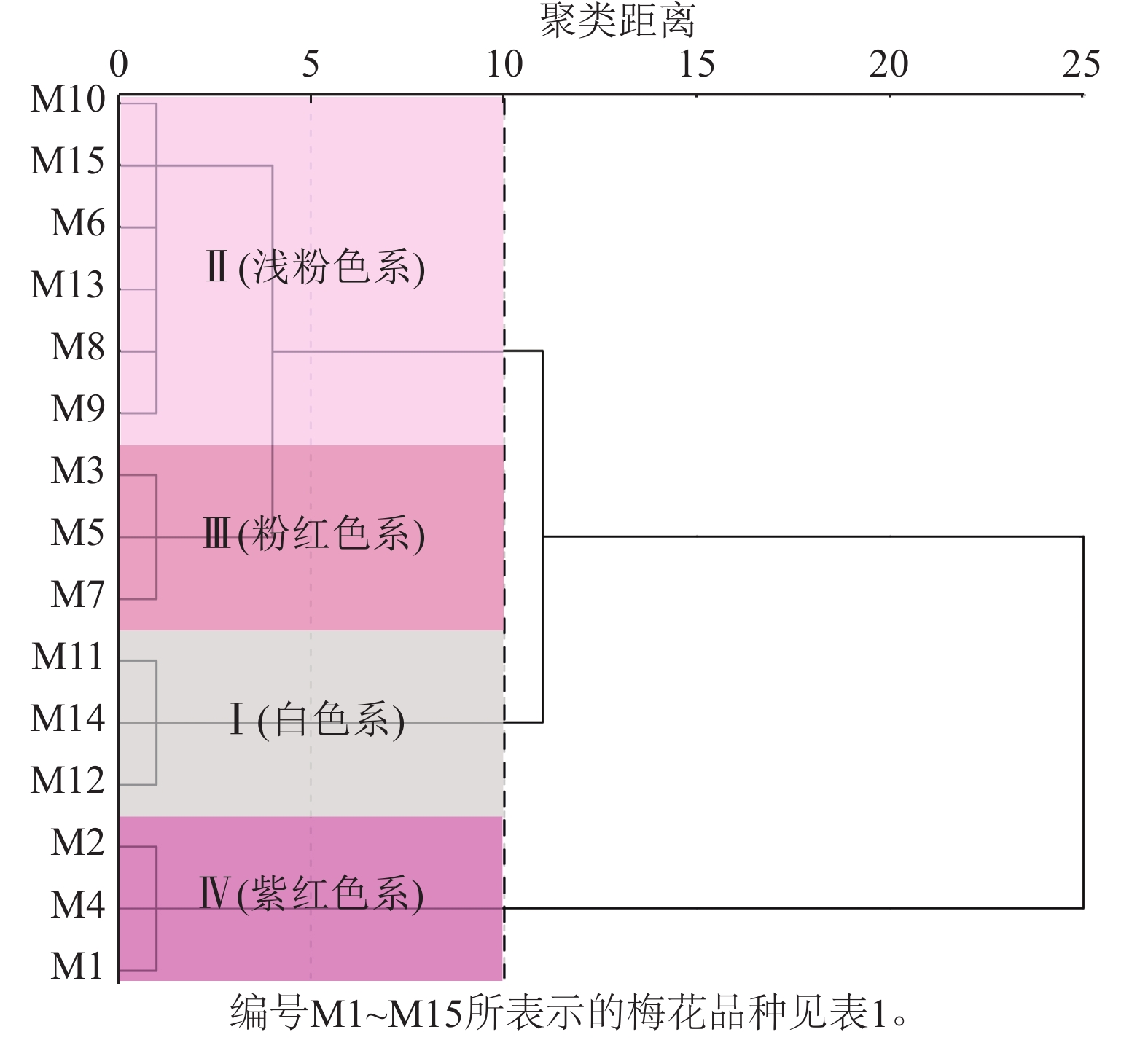
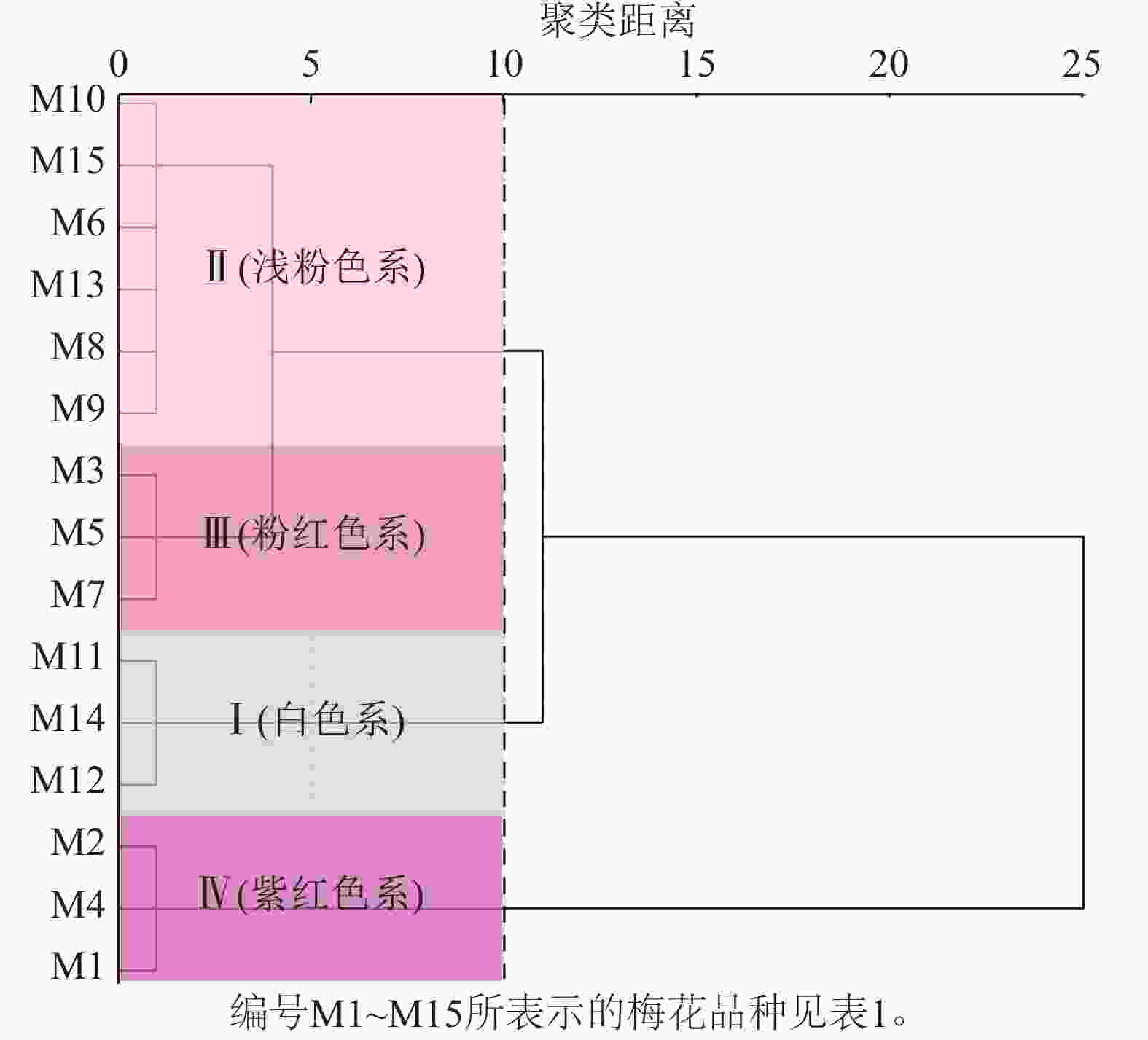
通过比色卡和色差仪测定15个梅花品种(图1)的花色表型。根据花色表型L*、a*、b*,对梅花品种进行聚类分析(图2)。聚类分析将花色分为4类,结合比色卡将这4类花色定义为:白色系(M11、M12、M14);浅粉色系(M6、M8、M9、M10、M13、M15);粉红色系(M3、M5、M7);紫红色系(M1、M2、M4)。
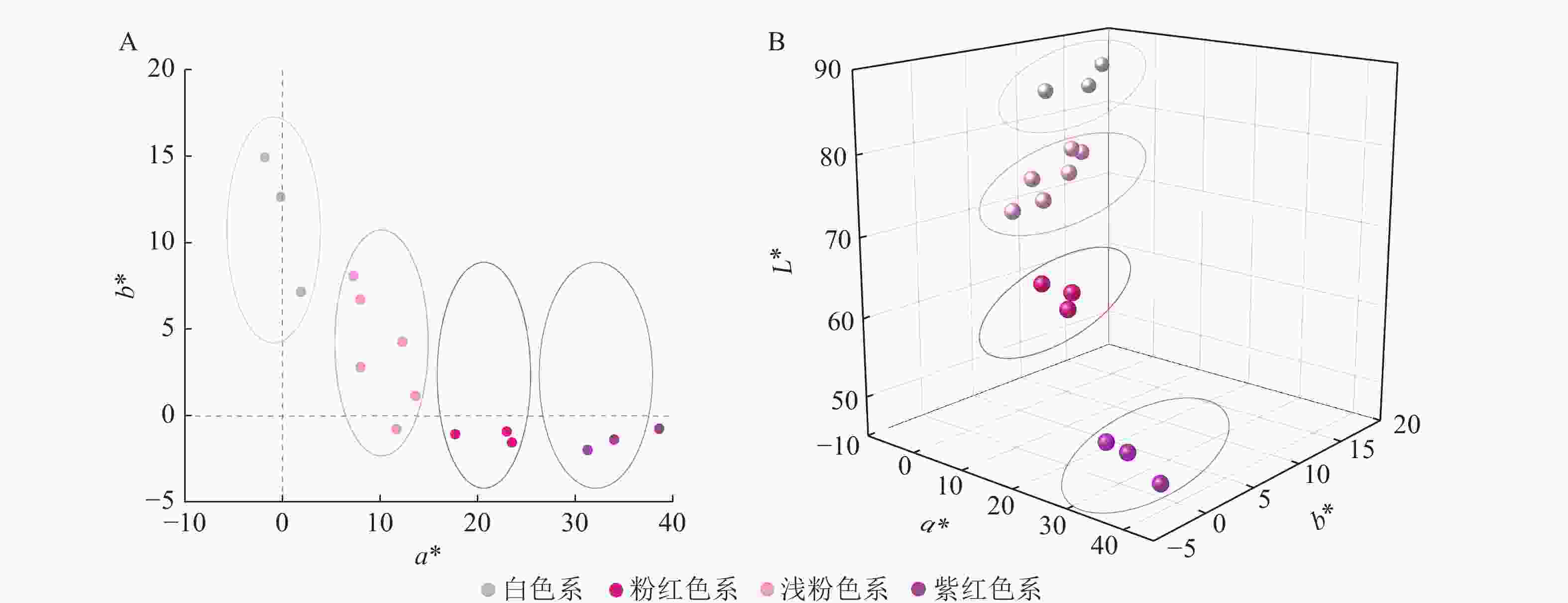
梅花品种紫红色系的a*为31.28~38.60,包括‘先春朱砂’‘骨红朱砂’‘晨晖朱砂’,a*最高;其次是粉红色系,a*为17.72~23.52,包括‘粉红朱砂’‘银红朱砂’‘晓红宫粉’;然后是浅粉色系,包括‘春意早宫粉’‘童颜宫粉’‘早花宫粉’‘桃红宫粉’‘单粉跳枝’‘单粉垂枝’;白色系的a*基本为负值。白色系的L*为84.88~86.92,包括‘六萼玉蝶’‘月光玉蝶’‘江梅’;紫红色系的L*为47.44~51.46,说明白色系L*最大,紫红色系L*最小。各色系的梅花b*均不高,其中白色系的b*最高,为7.18~14.94。可见,花瓣颜色越深,L*越低,a*越高。
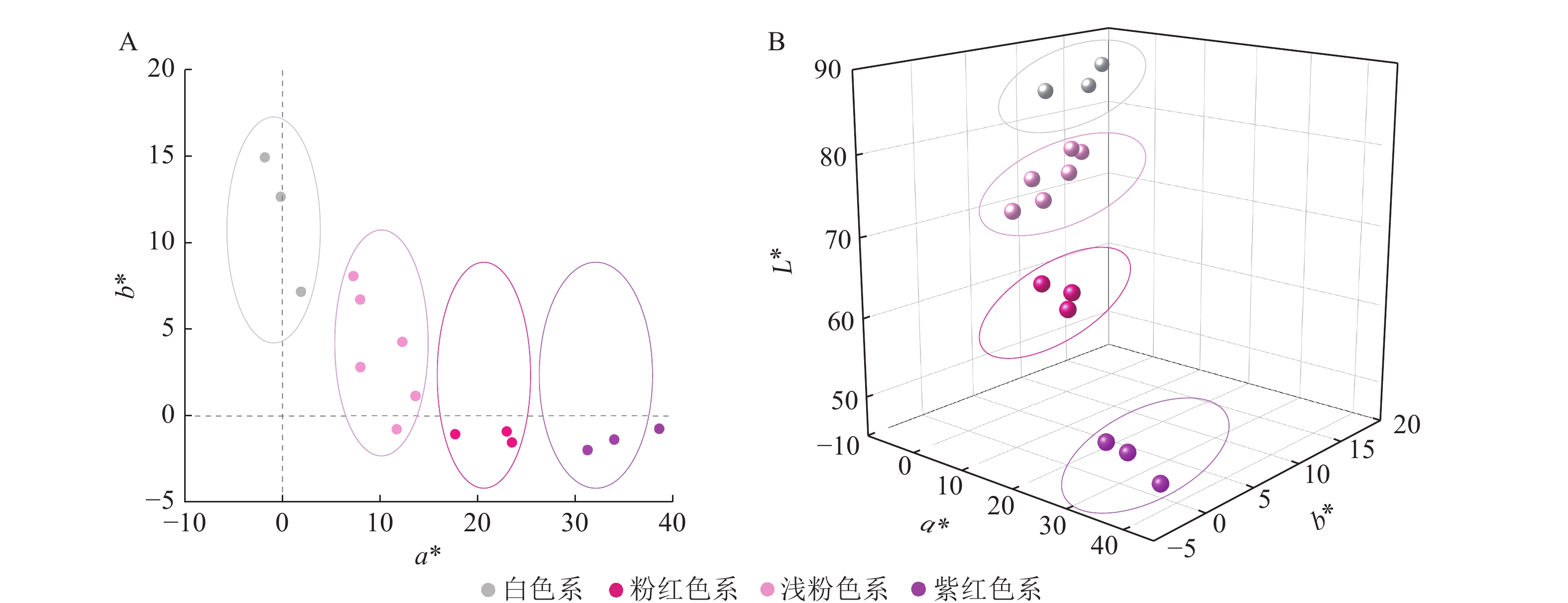
在a*和b*二维分布图(图3A)中,白色系位于第Ⅱ象限,浅粉色系集中于第Ⅰ象限,紫红色系与粉红色系位于第Ⅳ象限。在L*、a*、b*的三维分布图(图3B)中,各色系的参数分布集中、界限明显。
-
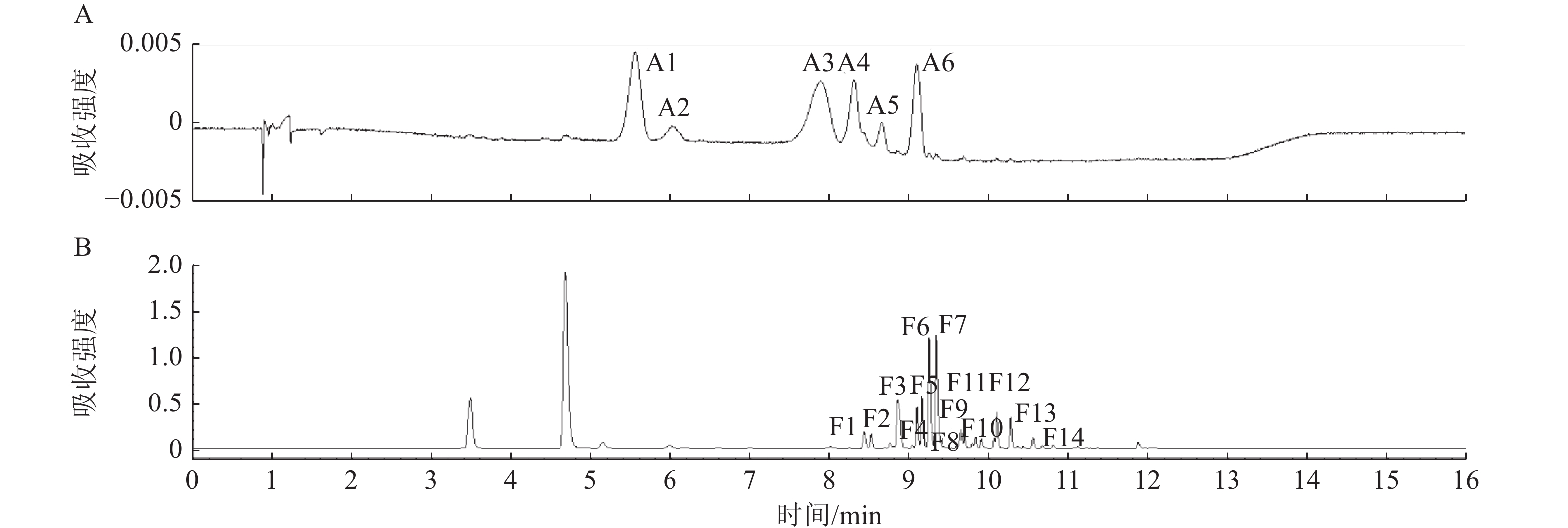
利用超高效液相色谱法(UPLC)在不同梅花品种花瓣中共中检测出6种花青苷成分和14种黄酮类物质。图4以‘桃红宫粉’为例展示UPLC色谱图。根据UPLC-MS/MS的结果(表2),推定4类花青素,分别为矢车菊素、芍药色素、飞燕草素、矮牵牛素;以及其他黄酮类物质,分别为2类黄酮醇苷元(槲皮素和异鼠李)和1类黄烷醇苷元(儿茶素)。
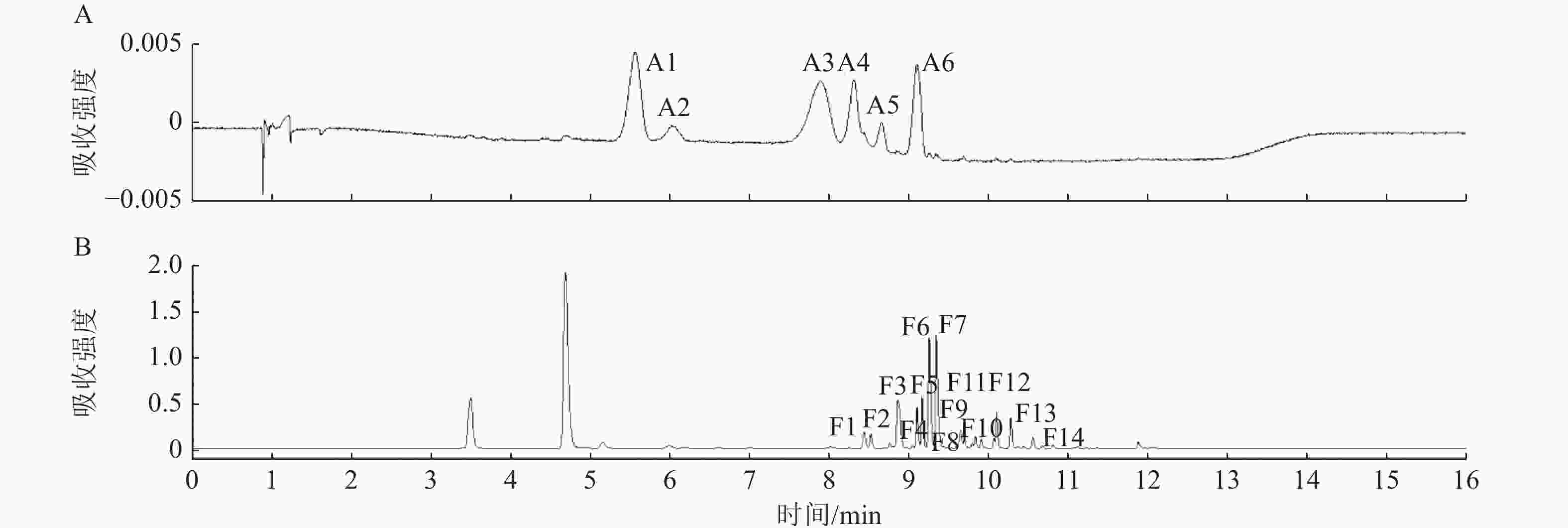
Figure 4. UPLC chromatograms of flavonoid extract from P. mume ‘Taohong Gongfen’ at detection wavelengths of 520 nm (A) and 350 nm (B)
峰 保留时
间/min分子m/z 碎片 分子式 推定物质 A1 5.64 449.10 287.10 C21H21O11 矢车菊素-3-O-葡萄糖苷 cyanidin-3-O-glucoside A2 6.13 595.17 287.10 C27H31O15 矢车菊素-3-O-芸香糖苷 cyanidin-3-O-rutinoside A3 8.01 463.12 301.10 C22H23O11 芍药花素-3-O-葡萄糖苷 peonidin 3-O-glucoside A4 8.35 609.18 301.10 C28H33O15 芍药花素-3-O-芸香糖苷 peonidin 3-O-rutinoside A5 8.68 757.22 611.20, 303.10 C33H41O20 飞燕草素-3-O-芸香糖鼠李糖苷 delphinidin-3-O-rutinoside-rhamnoside A6 9.11 771.24 625.20, 317.10 C34H43O20 矮牵牛素-3-O-芸香糖-5-O-鼠李糖苷 petunidin-3-O-rutinoside-5-O-rhamnoside F1 8.42 755.21 301.00 C33H40O20 槲皮素3-O-(2,6''-α-L-吡喃鼠李糖)-β-D-吡喃半乳糖苷
quercetin 3-O-(2'',6''-α-L-dirhamnopyranosyl)-β-D-galactopyranosideF2 8.51 755.21 301.00 C33H40O20 槲皮素-3-O-α-L-鼠李糖-β-D-吡喃葡萄糖苷
quercetin 3-O-α-L-rhamnopyranosyl-β-D-glucopyranosideF3 8.87 609.15 301.00 C27H30O16 槲皮素3-O-(6''-O-β-L-鼠李糖)-β-D-吡喃葡萄糖苷
quercetin 3-O-(6''-O-β-L-rhamnopyranosyl)-β-D-galactopyranosideF4 9.08 769.22 315.00 C34H42O20 香浦新苷 typhaneoside F5 9.15 609.15 301.00 C27H30O16 槲皮素-3-O-(2''-O-α-L-鼠李糖)-β-D-吡喃葡萄糖苷
quercetin-3-O-(2''-O-α-L-rhamnopyranosyl)-β-D-glucuronopyranosideF6 9.24 609.15 301.00 C27H30O16 槲皮素-3-O-芸香糖苷(芦丁) quercetin-3-O-rutinose(rutin) F7 9.33 463.08 301.00 C21H20O12 异槲皮素 isoquercetin F8 9.64 623.16 315.00 C28H32O16 异鼠李素-3-O-α-L-鼠李糖-β-D-吡喃半乳糖苷
isorhamnetin-3-O-α-L-rhamnopyranosyl-β-D-galactpyranosideF9 9.82 477.11 315.00 C22H22O12 异鼠李素-3-O-β-D-半乳糖苷 isorhamnetin 3-β-D-galactopyranoside F10 9.89 477.11 315.00 C22H22O12 异鼠李素-3-O-葡萄糖苷 isorhamnetin-3-O-glucoside F11 9.97 613.18 145.00 C27H34O16 儿茶素二己糖苷 catechin dihexoside F12 10.10 651.16 301.00 C29H32O17 槲皮素-3-O-α-鼠李糖-(1'''-6'')-(4''-O-乙酰)-β-D-半乳糖苷
quercetin-3-O-α-L-rhamnosyl-(1'''-6'')-(4''-O-acetyl)-β-D-galactosideF13 10.27 505.10 301.00 C23H22O13 槲皮素-3-O-6''-O-乙酰-β-D-吡喃葡萄糖苷
quercetin 3-O-(6''-O-acetyl)-β-D-glucopyranosideF14 10.56 665.18 315.00 C30H34O17 2''-O-乙酰-3'-O-甲基芦丁2''-O-acetyl-3'-O-methylrutin Table 2. LC-MS/MS parameters and identification of flavonoid compounds (anthocyanins and flavones) in P. mume
在520 nm区域的6个峰(A1~A6)中,具体结构的判定主要依据分子离子峰[M]+、苷元碎片离子[Y0]+和糖基丢失规律。A1和A2均产生m/z 287 [Y0]+碎片离子(矢车菊素特征),并分别因丢失162 Da葡萄糖基或308 Da芸香糖基被推定为矢车菊素-3-O-葡萄糖苷和矢车菊素-3-O-芸香糖苷;A3和A4则均产生m/z 301 [Y0]+碎片离子(芍药花素特征),推定为芍药花素-3-O-葡萄糖苷和芍药花素-3-O-芸香糖苷。A5和A6基于m/z 303 [Y0]+碎片离子(飞燕草素特征)和m/z 317 [Y0]+碎片离子(矮牵牛素特征)及连续的糖基丢失模式推定为飞燕草素-3-O-芸香糖鼠李糖苷和矮牵牛素-3-O-芸香糖-5-O-鼠李糖苷。
在350 nm区域检测到14种类黄酮成分(F1~F14),其具体结构的判定主要依据去质子化分子离子峰[M-H]-、苷元碎片离子[Y0]-和糖基修饰规律。F1、F2、F3、F5、F6、F7、F12和F13均产生m/z 301 [Y0]-碎片离子(槲皮素特征),通过不同的糖基组合,如葡萄糖、鼠李糖、半乳糖及乙酰化修饰,推定为槲皮素-3-O-半乳糖苷、槲皮素-3-O-芸香糖苷(芦丁)等多种糖苷形式;F4、F8、F9、F10和F14则均产生m/z 315 [Y0]-碎片离子(异鼠李素特征),并据此推定为异鼠李素-3-O-葡萄糖苷等衍生物;F11基于其分子离子m/z 613 [M-H]-及分子式被推定为儿茶素二己糖苷。具体鉴定结果见表2。
-
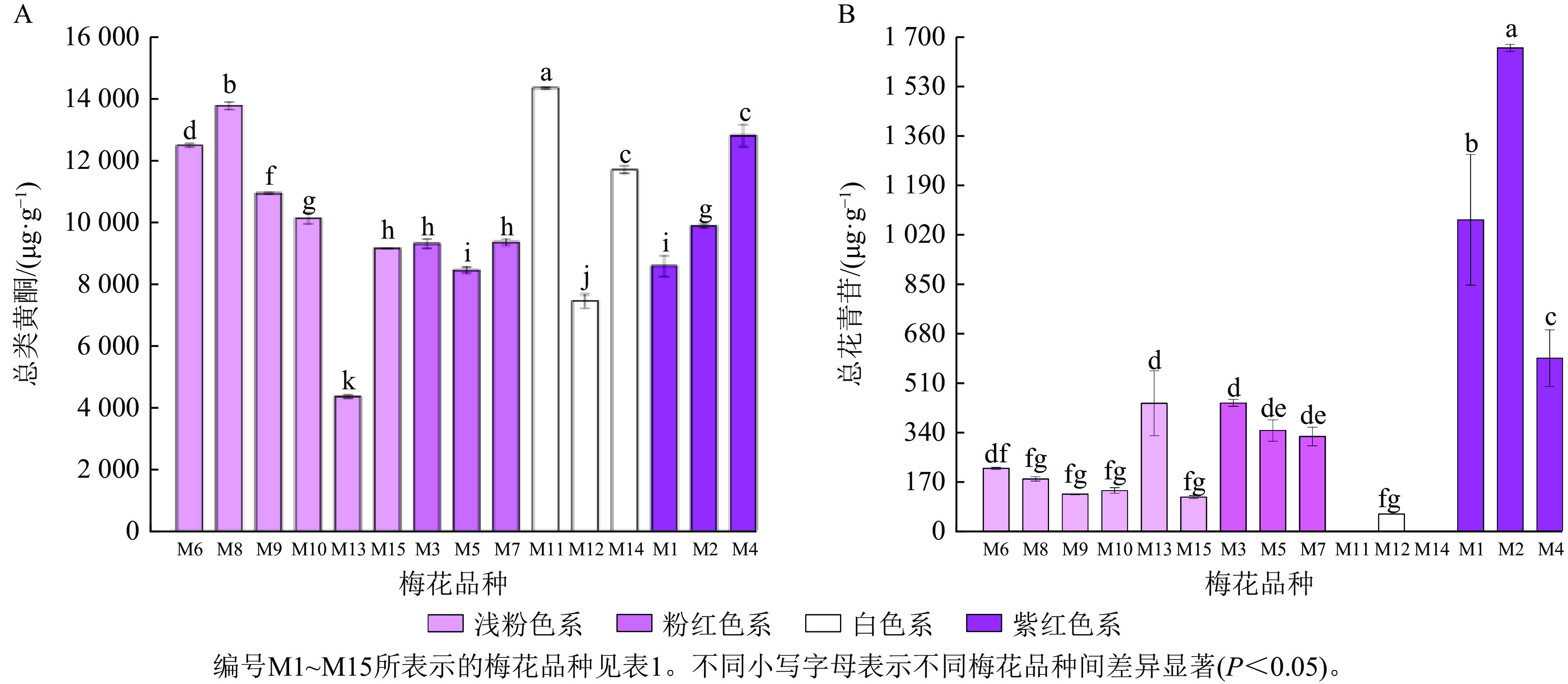
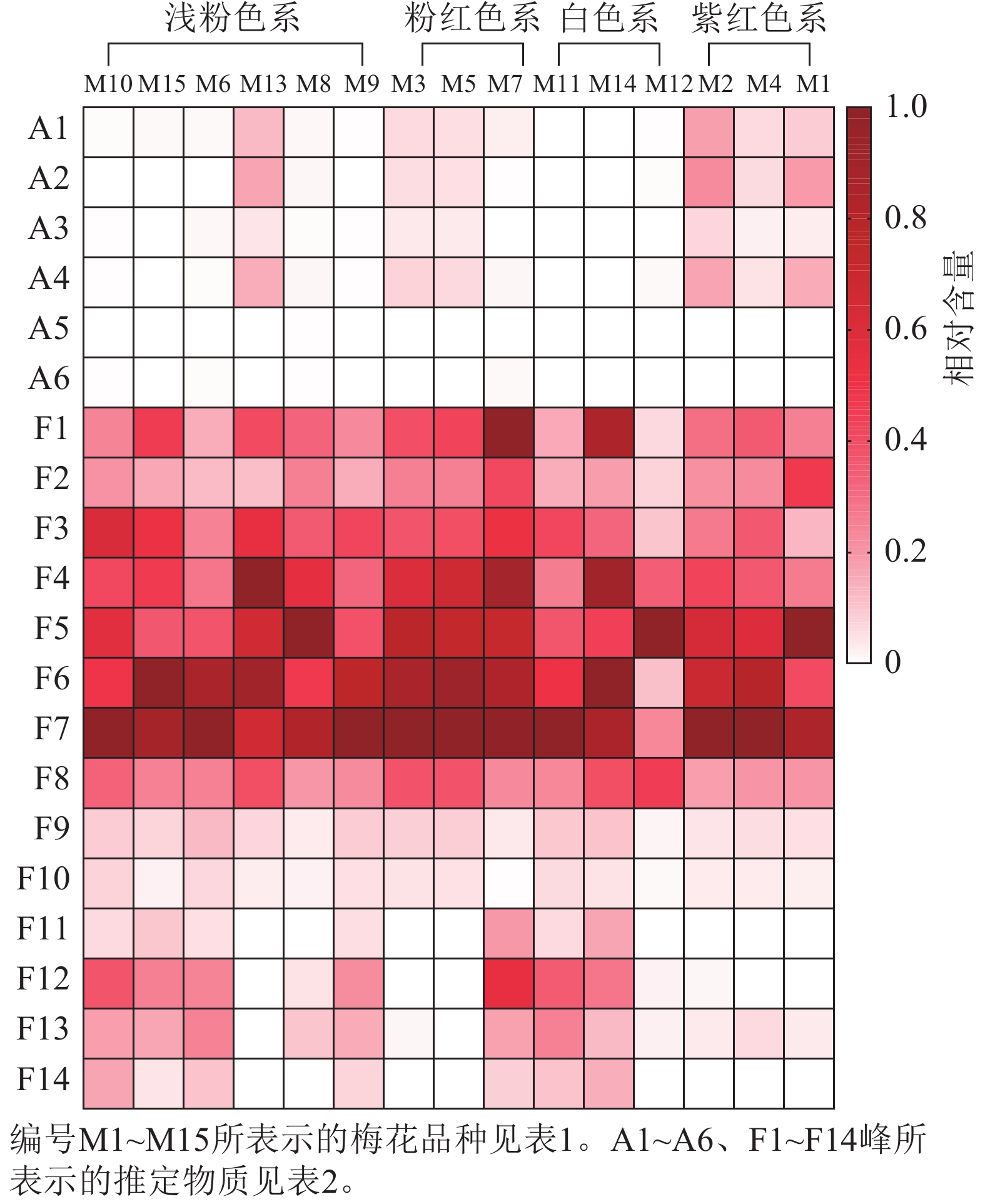
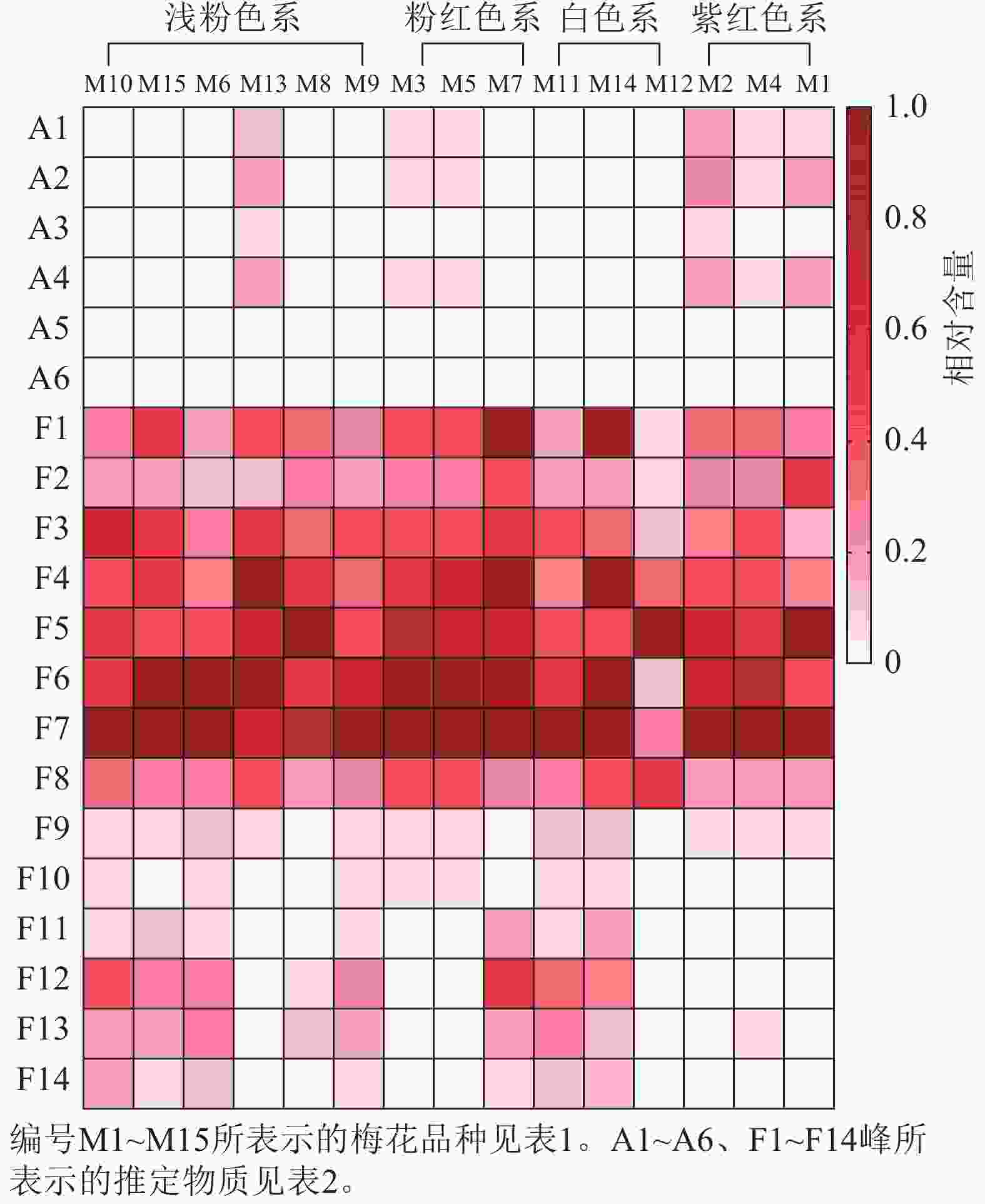
不同梅花品种的总花青苷和总类黄酮积累模式有一定差异。紫红色系品种花瓣的总花青苷质量分数最高,白色系品种不含或仅含有少量总花青苷;总类黄酮在不同色系品种中的分布没有明显的规律(图5)。为更好比较不同品种梅花各单体类黄酮化合物的积累差异,将鉴定出的6种花青苷化合物、14种类黄酮化合物进行热图分析,对总花青苷和总类黄酮进行差异分析。如图6所示:紫红、粉红、浅粉色系品种中均含有花青苷,其中,紫红色系‘骨红朱砂’‘晨晖朱砂’和浅粉色系‘单粉跳枝’的花青苷矢车菊素-3-O-葡萄糖苷、矢车菊素-3-O-芸香糖苷、芍药花素-3-O-葡萄糖苷、芍药花素-3-O-芸香糖苷相对含量较高;粉红色系‘粉红朱砂’‘银红朱砂’和紫红色系‘先春朱砂’的矢车菊素-3-O-葡萄糖苷、矢车菊素-3-O-芸香糖苷、芍药花素-3-O-葡萄糖苷、芍药花素-3-O-芸香糖苷相对含量也较其他品种高。而4个色系的花青苷飞燕草素-3-O-芸香糖鼠李糖苷和矮牵牛素-3-O-芸香糖-5-O-鼠李糖苷相对含量均较低。
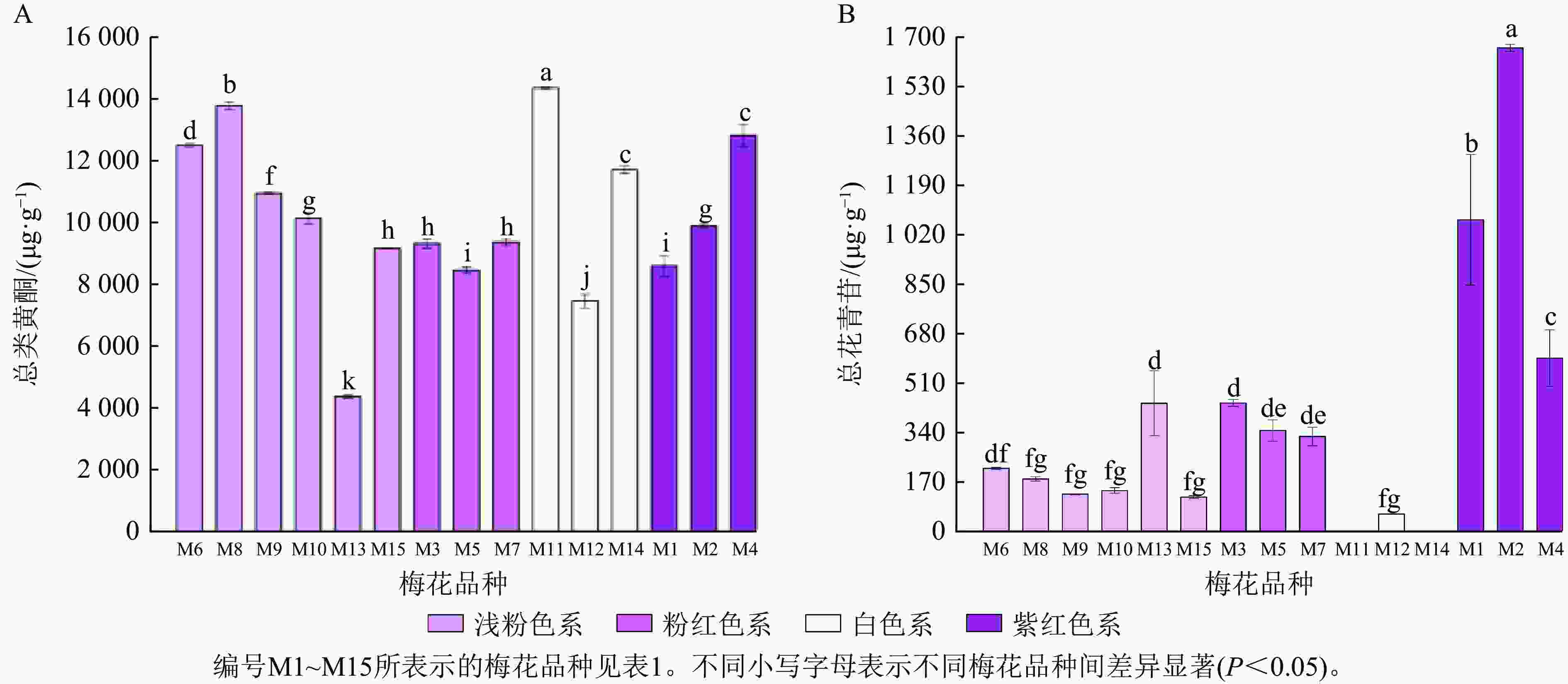
Figure 5. Bar charts of total anthocyanins (A) and total flavonoids (B) of different P. mume cultivars
紫红、粉红、浅粉色、白色系品种中均含有类黄酮,并且在前8个类黄酮成分中富集。类黄酮F1~F14中前10个成分是所有品种共有的成分,后4个成分只在部分品种中出现。其中,异槲皮素相对含量最高,其次是槲皮素-3-O-(2''-O-α-L-鼠李糖)-β-D-吡喃葡萄糖苷和槲皮素-3-O-芸香糖苷。相对含量较高的有香浦新苷、槲皮素-3-O-α-L-鼠李糖-β-D-吡喃葡萄糖苷、槲皮素3-O-(2,6''-α-L-吡喃鼠李糖)-β-D-吡喃半乳糖苷、异鼠李素-3-O-α-L-鼠李糖-β-D-吡喃半乳糖苷和F2槲皮素-3-O-α-L-鼠李糖-β-D-吡喃葡萄糖苷。
-
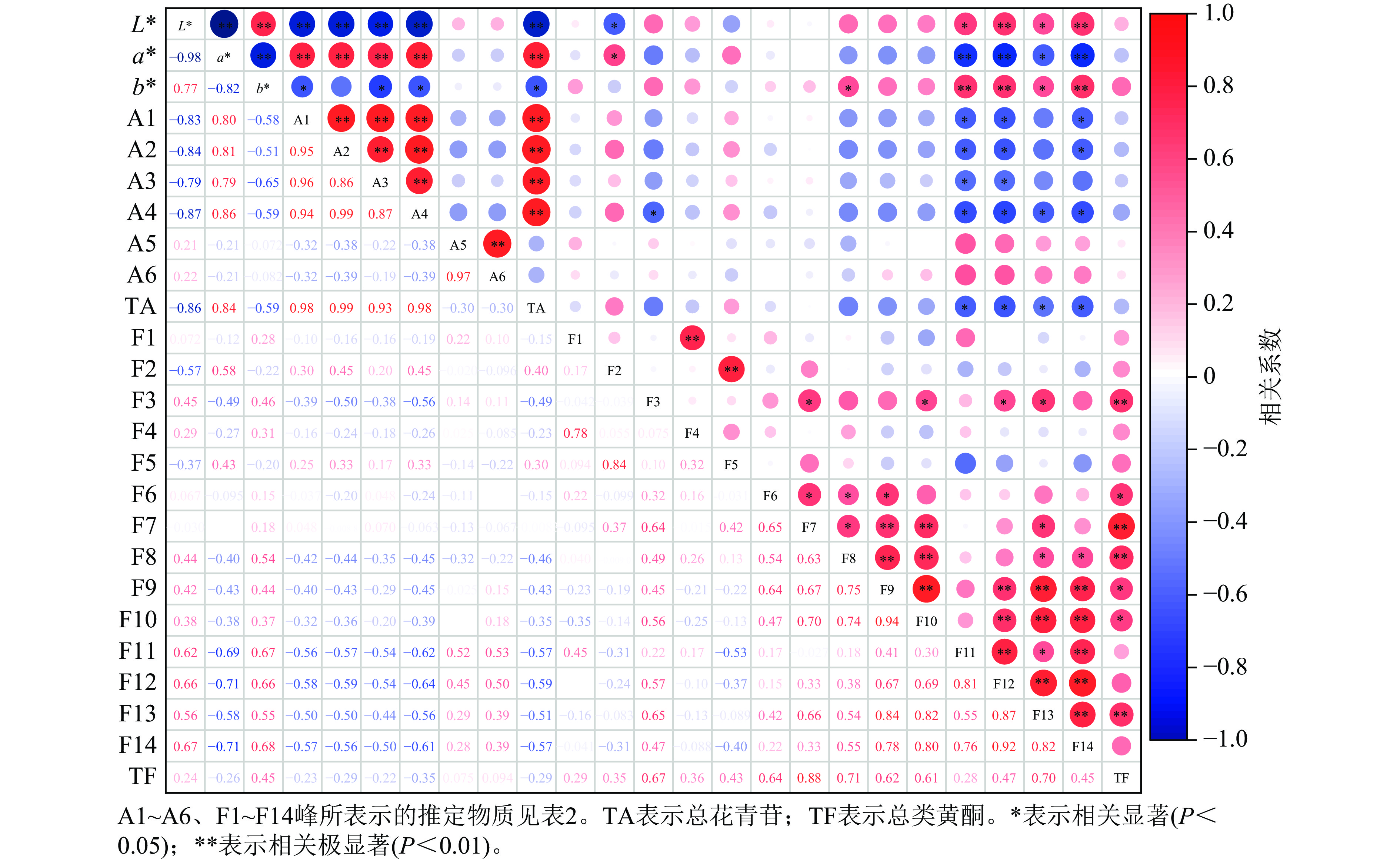
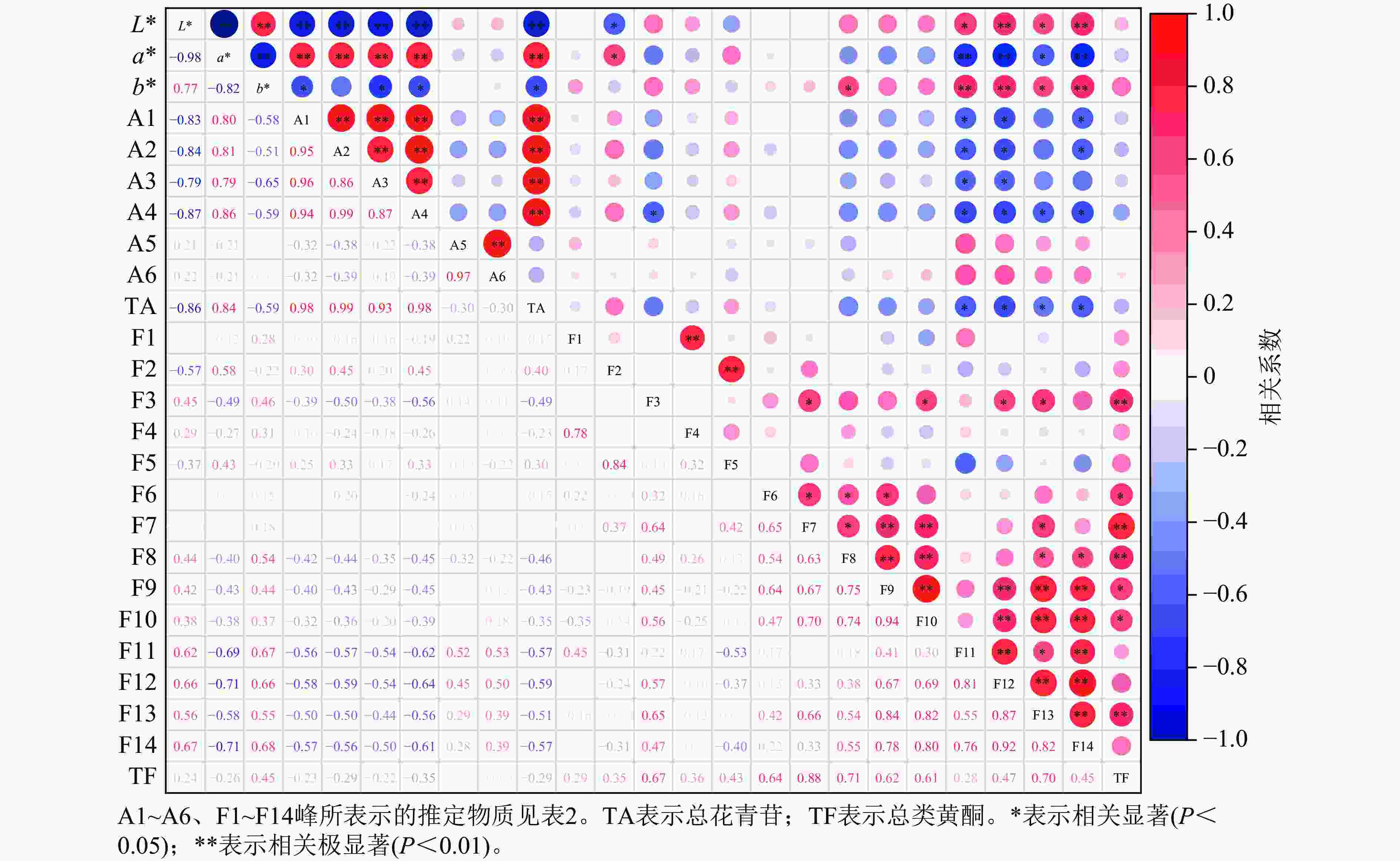
相关性分析(图7)显示:L*与矢车菊素-3-O-葡萄糖苷、矢车菊素-3-O-芸香糖苷、芍药花素-3-O-葡萄糖苷、芍药花素-3-O-芸香糖苷和总花青苷呈极显著负相关(P<0.01)。a*与矢车菊素-3-O-葡萄糖苷、矢车菊素-3-O-芸香糖苷、芍药花素-3-O-葡萄糖苷、芍药花素-3-O-芸香糖苷和总花青苷呈极显著正相关(P<0.01)。a*越高,花瓣颜色越红,说明这4种花青苷的积累对花瓣红色形成起作用。b*与矢车菊素-3-O-葡萄糖苷、芍药花素-3-O-葡萄糖苷、芍药花素-3-O-芸香糖苷和总花青苷呈显著负相关(P<0.05)。
L*与槲皮素-3-O-α-鼠李糖-(1'''-6'')-(4''-O-乙酰)-β-D-半乳糖苷、2''-O-乙酰-3'-O-甲基芦丁呈极显著正相关(P<0.01);与儿茶素二己糖苷、槲皮素-3-O-6''-O-乙酰-β-D-吡喃葡萄糖苷呈显著正相关(P<0.05),表明这4种类黄酮组分积累会提高梅花花瓣亮度;但L*与槲皮素-3-O-α-L-鼠李糖-β-D-吡喃葡萄糖苷呈显著负相关(P<0.05)。a*与儿茶素二己糖苷、槲皮素-3-O-α-鼠李糖-(1'''-6'')-(4''-O-乙酰)-β-D-半乳糖苷和2''-O-乙酰-3'-O-甲基芦丁呈极显著负相关(P<0.01);与槲皮素-3-O-6''-O-乙酰-β-D-吡喃葡萄糖苷呈显著负相关(P<0.05);但与槲皮素-3-O-α-L-鼠李糖-β-D-吡喃葡萄糖苷呈显著正相关(P<0.05)。b*与儿茶素二己糖苷、2''-O-乙酰-3'-O-甲基芦丁呈极显著正相关(P<0.01);与槲皮素-3-O-α-鼠李糖-(1'''-6'')-(4''-O-乙酰)-β-D-半乳糖苷、槲皮素-3-O-6''-O-乙酰-β-D-吡喃葡萄糖苷呈显著正相关(P<0.05),表明随着这4种类黄酮组分的增加,花瓣黄色程度相应增强。
-
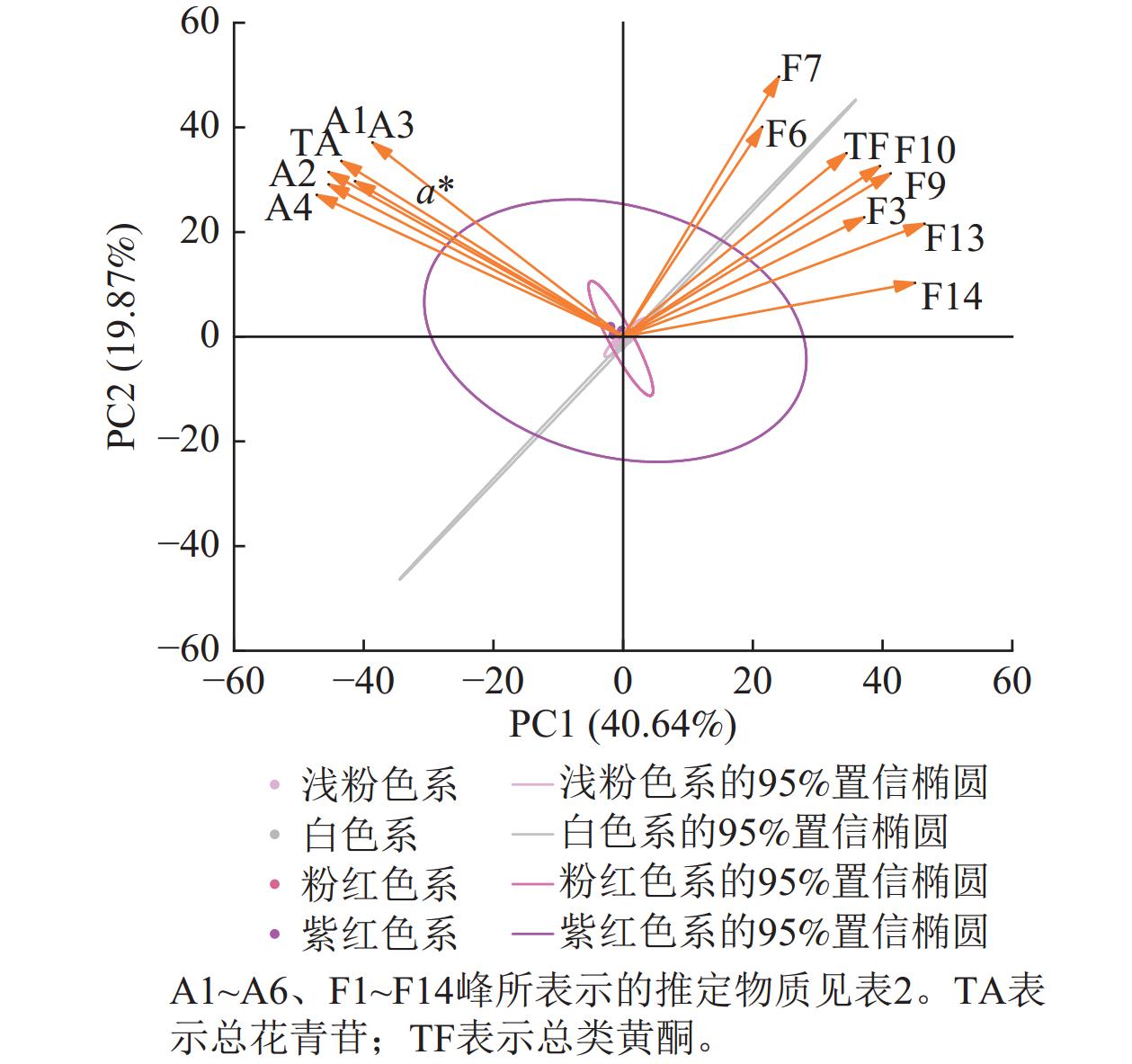
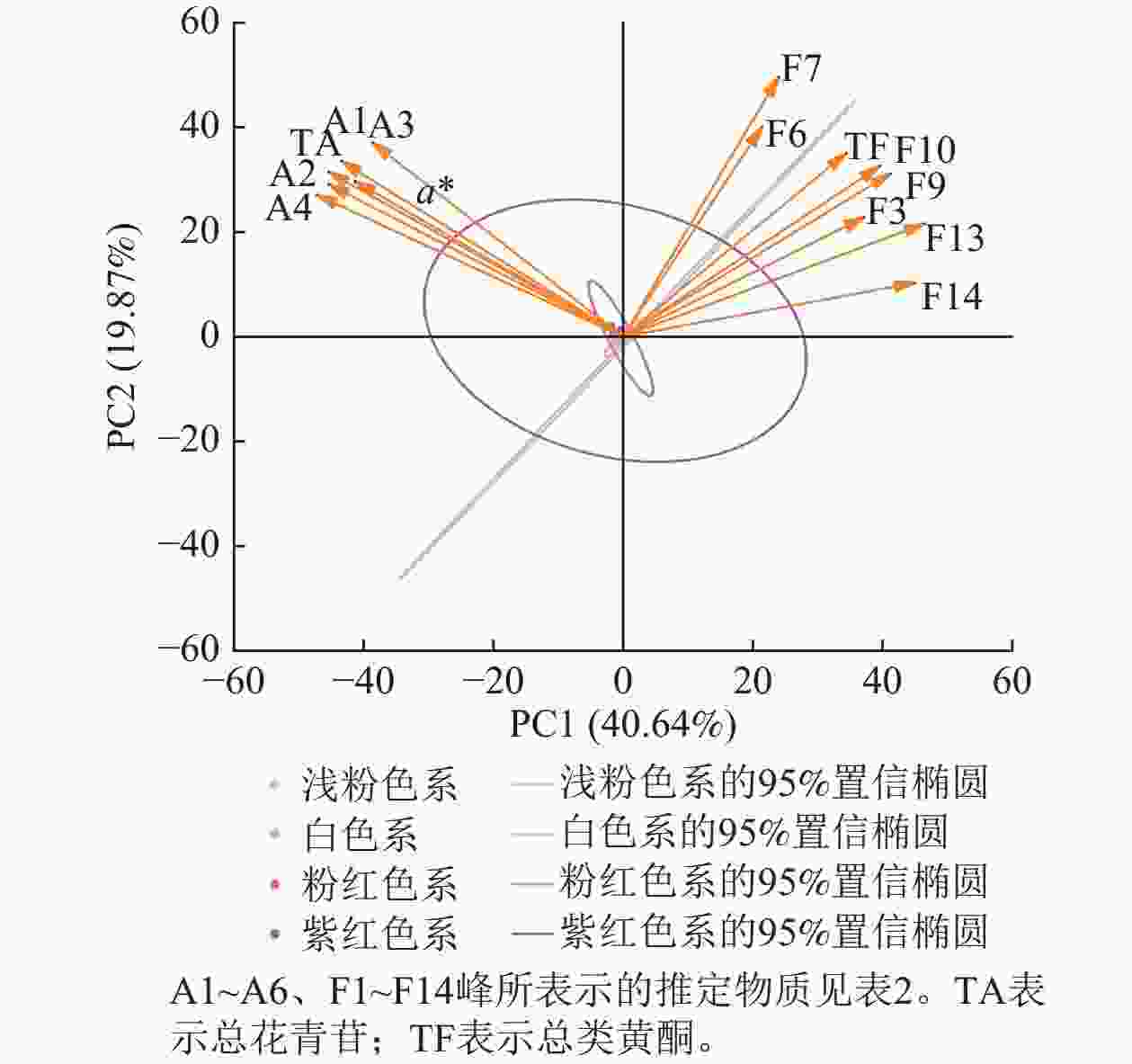
将梅花花色表型和色素组分进行主成分分析发现:前6个主成分贡献率为92.37%,其中,第1主成分(PC1)贡献率为40.64%,第2主成分(PC2)贡献率为19.87%。筛选出20种主要色素、a*和b*。主成分因子载荷和得分图(图8)显示:后4个主成分贡献率较低,可能仅在特定品种中发挥作用。第1主成分包括矢车菊素-3-O-葡萄糖苷、矢车菊素-3-O-芸香糖苷、芍药花素-3-O-葡萄糖苷、芍药花素-3-O-芸香糖苷、总花青苷和a*,说明花青苷是梅花红色调的主要贡献者,a*与上述花青苷相对含量高度相关,说明矢车菊素和芍药花素衍生物主导红色花色。第2主成分包括是槲皮素3-O-(6''-O-β-L-鼠李糖)-β-D-吡喃葡萄糖苷、槲皮素-3-O-芸香糖苷、异槲皮素、异鼠李素-3-O-β-D-半乳糖苷、异鼠李素-3-O-葡萄糖苷、槲皮素-3-O-6''-O-乙酰-β-D-吡喃葡萄糖苷、2''-O-乙酰-3'-O-甲基芦丁和总类黄酮。
-
多元回归分析发现:(1) L*的回归方程为y=87.151−0.012x1−0.016x2 (R2=0.819,P<0.05),其中,y代表L*,x1代表总花青苷质量分数,x2代表槲皮素-3-O-α-L-鼠李糖-β-D-吡喃葡萄糖苷质量分数,以上2种物质的积累可显著降低花瓣的亮度。(2) a*的回归方程为y=7.065+0.081x (R2=0.746,P<0.01),其中,y代表a*,x代表芍药花素-3-O-芸香糖苷质量分数,其积累量可显著提高花瓣红色程度。(3) b*的回归方程为y=7.104−0.061x (R2=0.363,P<0.05),其中,y代表b*,x代表芍药花素-3-O-葡萄糖苷质量分数,其积累量可降低花瓣的黄色程度。可见,梅花花色与色素组分之间显著相关(P<0.05)。
-
早前研究表明:类黄酮化合物是影响梅花花色的主要色素,红色梅花含花青苷和类黄酮,白色梅花仅含类黄酮[11]。张芹[12]研究发现:除白色系以外,其他色系中也含有矢车菊素-3-O-葡萄糖苷、矢车菊素-3-O-鼠李糖葡萄糖苷、芍药花素-3-O-葡萄糖苷、芍药花素-3-O-鼠李糖葡萄糖苷4种核心花青苷,并推断其他色系的代谢途径是矢车菊素苷分支。吴思惠等[13]利用HPLC-MS等方法,不仅验证白色梅花中芦丁、槲皮素等黄酮的特异性积累,且揭示矢车菊素和芍药花素衍生物主导红色花色形成,槲皮素衍生物影响黄绿花色。WANG等[14]研究发现:在部分紫红色梅花品种中,花青苷代谢途径是天竺葵素和飞燕草素分支。本研究通过UPLC-MS/MS技术验证了前人研究中矢车菊素与芍药花素糖苷的核心地位,揭示特定花青苷组分和其他类黄酮组分对梅花花色差异的影响,提出“花青苷主导-类黄酮协同”的代谢网络。
本研究表明:在15个梅花品种花瓣中共检测出6种花青苷成分,14种类黄酮成分,除已报道的矢车菊素和芍药色素外[11],还存在少量的飞燕草素和矮牵牛素。矢车菊素和天竺葵素多使花与果实呈现红色,芍药素对植物呈现紫红色具有重要作用,飞燕草素和矮牵牛素负责蓝色和蓝紫色[25−27]。矢车菊素是大多植物中最常见的花青苷成分,例如梅花[12]、玫瑰Rusa rugosa[28]和山茶Camellia japonica[29]等。芍药色素在观赏植物花色形成中起关键作用,如在牡丹Paeonia suffruticosa[30]、长春花Catharanthus roseus[31]、芍药Paeonia lactiflora[32]等植物中,该色素是红色系花色的主要呈色物质之一,而在夏蜡梅Sinicalycanthus chinensis和光叶红蜡梅Calycanthus floridus var. glaucus中,紫红色花的形成则主要由矢车菊素主导[33]。类似地,换锦花Lycoris sprengeri的花色苷主要成分包括矢车菊素、天竺葵素和飞燕草素[34]。本研究发现:在梅花紫红、粉红、浅粉色系品种中均含有花青苷,以矢车菊素和芍药花素为主,其中矢车菊素-3-O-芸香糖苷和芍药花素-3-O-芸香糖苷相对含量较高。白色系品种中几乎不含有花青素苷,但类黄酮含量较高。
相关分析发现:L*与儿茶素二己糖苷、槲皮素-3-O-α-鼠李糖-(1'''-6'')-(4''-O-乙酰)-β-D-半乳糖苷、槲皮素-3-O-6''-O-乙酰-β-D-吡喃葡萄糖苷、2''-O-乙酰-3'-O-甲基芦丁呈显著正相关,表明这4种类黄酮成分积累会提高梅花花瓣亮度。a* 与矢车菊素-3-O-葡萄糖苷、矢车菊素-3-O-芸香糖苷、芍药花素-3-O-葡萄糖苷、芍药花素-3-O-芸香糖苷、总花青苷和槲皮素-3-O-α-L-鼠李糖-β-D-吡喃葡萄糖苷呈显著正相关,说明这4种花青苷成分和类黄酮积累对梅花花瓣红色形成起一定作用。b*与儿茶素二己糖苷、槲皮素-3-O-α-鼠李糖-(1'''-6'')-(4''-O-乙酰)-β-D-半乳糖苷、槲皮素-3-O-6''-O-乙酰-β-D-吡喃葡萄糖苷、2''-O-乙酰-3'-O-甲基芦丁呈显著正相关,说明随着这4种类黄酮成分的增加,花瓣黄色程度相应增强。
主成分分析表明:决定梅花红色的色素主要为花青苷。多元线性回归分析表明:总花青苷积累显著降低了L*。a*的核心贡献因子为芍药花素-3-O-芸香糖苷,这与第1主成分中以芍药花素-3-O-芸香糖苷为重要载荷的结论一致。芍药花素-3-O-葡萄糖苷降低了b*,证实花青苷主导核心呈色。
-
本研究通过多维度分析揭示了梅花花色形成的代谢调控规律,明确了花青苷是花色分化的核心色素。矢车菊素和芍药花素衍生物主导梅花红色花色形成,其中芍药花素-3-O-芸香糖苷通过显著正向调控红度成为红色表型的关键贡献者。主成分分析与多元线性回归分析共同证实:花青苷与a*呈极显著正相关,其积累通过降低L*加深花色。
Analysis of flower color phenotypes and pigment components in different Prunus mume cultivars
doi: 10.11833/j.issn.2095-0756.20250486
- Received Date: 2025-08-31
- Accepted Date: 2025-09-29
- Rev Recd Date: 2025-09-26
- Available Online: 2025-10-29
- Publish Date: 2025-10-20
-
Key words:
- Prunus mume /
- cultivars /
- flower color phenotypes /
- anthocyanin /
- flavonoid
Abstract:
| Citation: | XIA Mengxue, YANG Yu, NING Aoxiang, et al. Analysis of flower color phenotypes and pigment components in different Prunus mume cultivars[J]. Journal of Zhejiang A&F University, 2025, 42(5): 956−966 doi: 10.11833/j.issn.2095-0756.20250486 |











 DownLoad:
DownLoad: