-
种子是植物特有的繁殖器官,其质量高低影响农作物的产量和品质[1]。种子发芽是指种子从吸水膨胀开始产生的一系列有序的细胞分裂和增大、细胞壁和原生质发生水合以及各种酶的活化等过程,是种子植物生命周期中不可或缺的关键步骤[2]。种子发芽的速度和整齐度影响着作物的产量和质量。种子通过类朊病毒蛋白(FLOE1)感知外界水分状况,发生相分离,继而引发水合作用,启动萌发进程[3]。
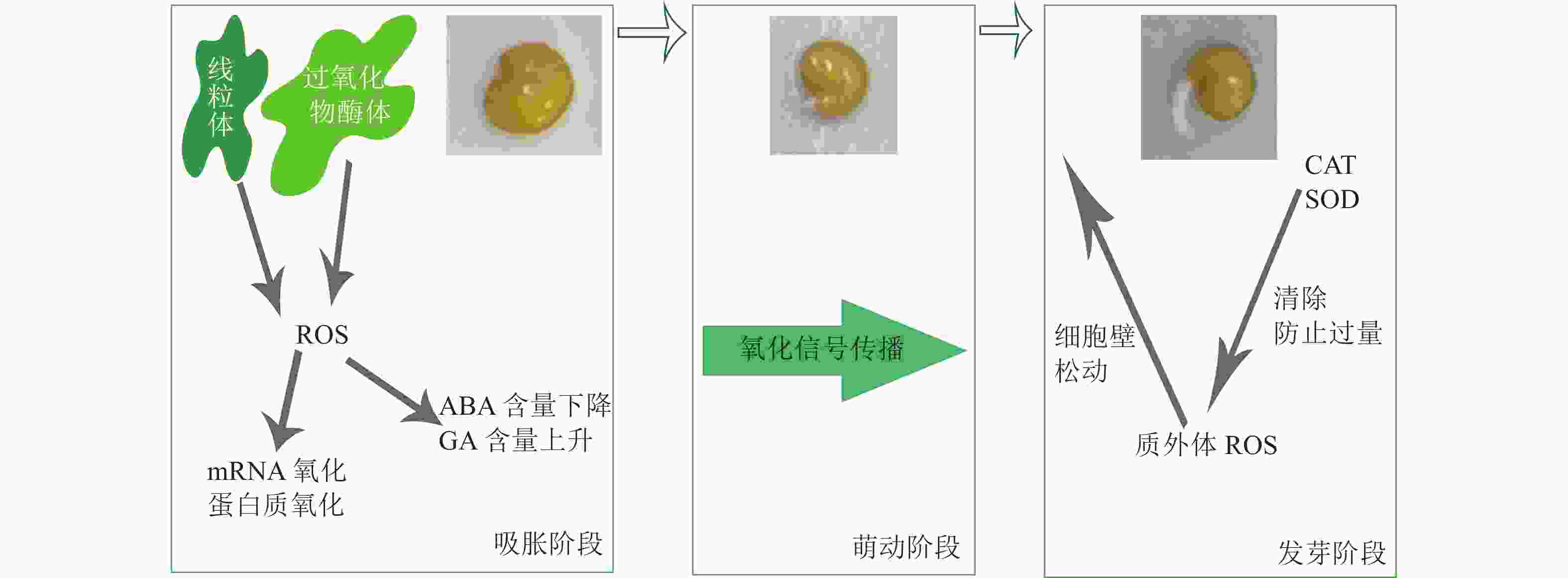
种子萌发通常分为吸胀、萌动和发芽3个阶段[4]。在第1阶段种子快速吸水,休眠解除。种子的生理活动被激活,如蛋白质、酶等大分子和细胞器等得到伸展和修复,DNA连接酶修复[2]。种子的呼吸作用、糖酵解、磷酸戊糖途径等开始启动[5]。在这一阶段种子的含水量从低于干质量的8%~10%增加到高于干质量的50%[6]。第2阶段水分吸收很少,种子内部的代谢开始加强,生物大分子和细胞器活化、修复,在此基础上,种胚细胞恢复生长致使种皮破裂[7]。同时,在这一阶段产生胚胎发育所需的酶与物质,种胚细胞开始合成与分泌赤霉素(GA),诱导α-淀粉酶表达,促进胚乳分解,为胚细胞的生化反应提供原料[8]。此外,该阶段新陈代谢变得活跃,使用新转录的mRNA合成蛋白质和新线粒体,以产生足够的能量来完成萌发[9]。第3阶段种子又重新开始快速吸收水分,胚乳破裂和胚根突出。该阶段吸收的水分是完成发芽和幼苗生长相关的水分[10]。水分的快速增长使细胞伸长以及DNA复制和细胞分裂[2]。
种子萌发受到GA和脱落酸(ABA)之间平衡的调控[11]。GA是种子萌发必不可少的激素,GA缺失突变的拟南芥Arabidopsis thaliana[12]、番茄Solanum lycopersicum[13]、黄瓜Cucumis sativus[14]种子无法萌发。对一些寄生植物而言,独角金内酯是种子萌发所必需的[15-16]。近几年研究结果表明,生长素(AUX)、细胞分裂素(CTK)、水杨酸(SA)等可以间接调控种子的休眠与萌发[17]。活性氧(reactive oxygen species, ROS)是在胁迫条件下调节植物生长发育及胁迫响应的多功能信号分子。ROS的主要形式有超氧阴离子(
${\rm{O}}_2^{\cdot-}$ )、过氧化氢(H2O2)、羟自由基(·OH)、单态氧(1O2)、羟基自由基(HO·)等[18]。近年来,越来越多的研究发现很多作物的种子发芽都与ROS的产生密切相关[19-21]。ROS在种子中的作用并不像之前认为的那样消极,而是对于种子萌发起到一定的积极作用[22-24]。 -
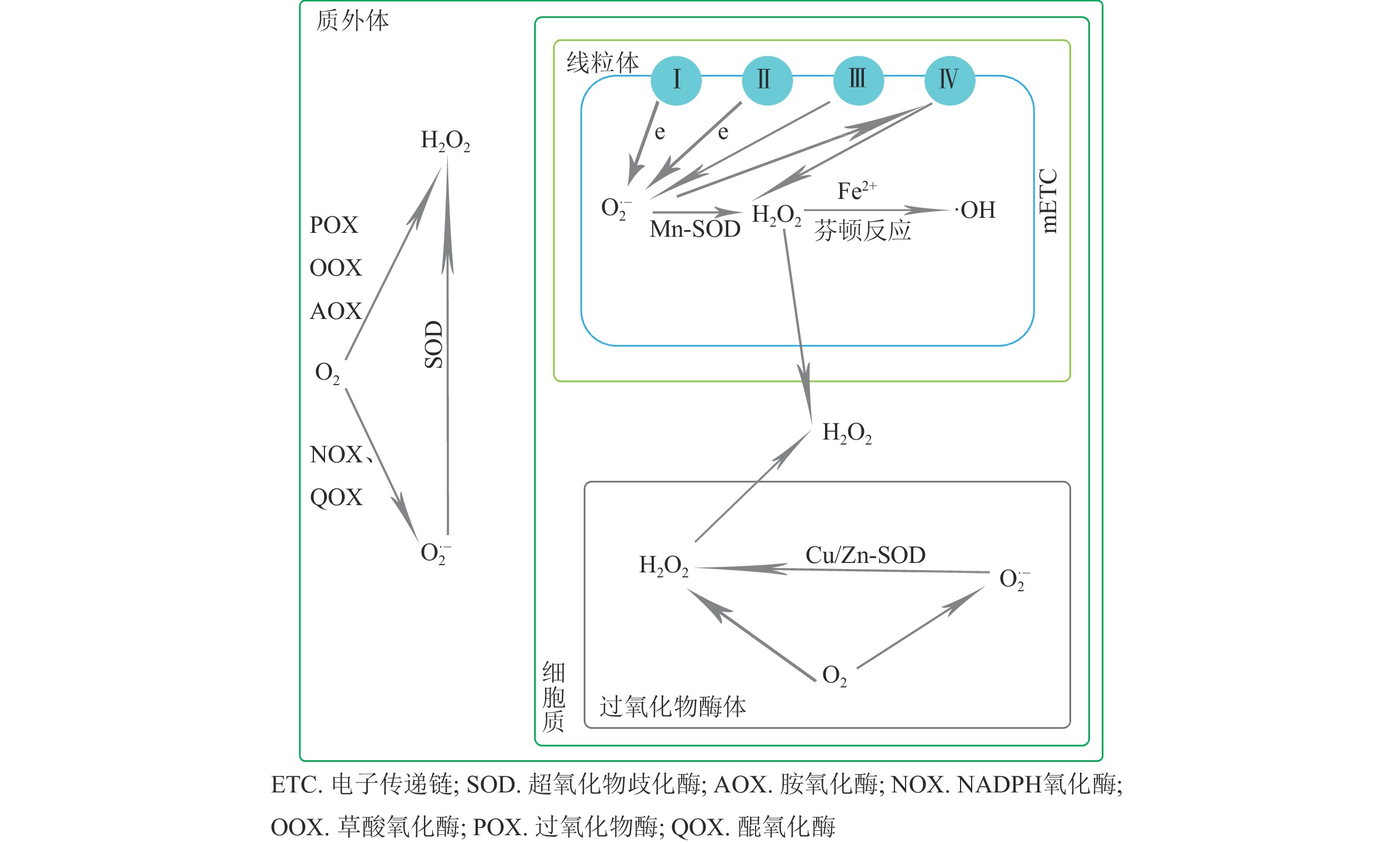
在植物体内,ROS主要产生于叶绿体、线粒体、过氧化物酶体、质膜等。种子中不含叶绿体,因此在种子萌发过程中,ROS产生的主要部位是线粒体、过氧化物酶体和质膜[25](图1)。氧气(O2)被激活产生ROS有2条途径:①吸收足够能量以改变1个不成对电子的旋转方向从而生成1O2;②以单电子形式逐步被还原成
${\rm{O}}_2^{\cdot-} $ 、H2O2和·OH[26]。在种子萌发完成第1阶段后,种子内线粒体呼吸作用恢复,部分电子泄漏传递给O2,将O2还原,形成ROS[27]。线粒体中ROS的产生与其中的电子传递链(mETC)关系密切,mETC需要约2%的O2在复合物Ⅰ和复合物Ⅱ处生成
${\rm{O}}_2^{\cdot-} $ [28],然后被Mn-超氧化物歧化酶(SOD)催化形成相对稳定、存活期较长且具有透膜性的H2O2[29],H2O2可以与还原的Fe2+通过芬顿(Fenton)反应进一步生成反应活性较高的·OH[30],·OH还可以由只有中度反应活性的${\rm{O}}_2^{\cdot-} $ 和H2O2通过哈勃·韦斯反应转化而来[31]。过氧化物酶体是微体的一种,其正常的代谢过程就会产生ROS[18],是胞内产生H2O2的最主要部位。过氧化物酶体还通过光呼吸乙醇酸氧化酶反应(GOX)、脂肪酸β-被酰基辅酶A氧化酶(ACO)氧化、黄素氧化酶的酶反应以及${\rm{O}}_2^{\cdot-} $ 自由基被SOD歧化来生成H2O2[32-33]。质膜普遍存在电子传递氧化还原酶而产生ROS。NADPH氧化酶(NOX)催化电子由胞质NADPH向O2转移从而生成${\rm{O}}_2^{\cdot-} $ ,${\rm{O}}_2^{\cdot-} $ 自发歧化或被SOD催化形成H2O2。 -
植物体内ROS的水平必须受到严格的调控,以免植物体内ROS积累过多而导致氧化爆发,导致大面积的细胞损伤和死亡[34]。植物体内ROS的清除主要通过抗氧化酶促系统和非酶促系统进行[35]。
-
抗氧化酶保护系统由SOD、过氧化氢酶(CAT)、过氧化物酶(POD)、抗坏血酸过氧化物酶(APX)及谷胱甘肽过氧化物酶(GPX)等组成的[36]。SOD是一种抗氧化金属酶,根据其活性部位的金属离子,可以大致分为Cu/Zn-SOD、Mn-SOD、Fe-SOD三大类[35]。它存在于线粒体、过氧化物酶体等细胞器以及细胞质基质中,能催化
${\rm{O}}_2^{\cdot-} $ 形成H2O2和O2,H2O2可以在POD、CAT等的催化下转化为水分子(H2O)而得以清除[37]。CAT是唯一不需要能量的ROS清除酶,它可促使H2O2分解为O2和H2O。CAT在种子和幼苗中含量非常丰富,它可以清除乙醛酸循环中脂肪降解过程产生的过量H2O2[38]。POD是以H2O2为电子受体直接氧化酚类或胺类化合物的抗氧化酶,具有消除H2O2和酚类胺类毒性的双重作用。APX利用抗坏血酸(AsA)作为特定的电子供体,在细胞质、线粒体和过氧化物酶体等细胞器中将H2O2还原为H2O,其对H2O2具有比CAT和POD更高的亲和力[35, 39]。 -
非酶清除机制由低分子量抗氧化剂介导,包括谷胱甘肽(GSH)、抗坏血酸(ASA)、类胡萝卜素(Car)、类黄酮等[40]。在植物中,GSH主要有2种清除ROS的方式:第一,直接与1O2、HO−和
${\rm{O}}_2^{\cdot-} $ 等发生化学反应,清除ROS;第二,GSH作为供氢体,通过与脱氢抗坏血酸还原酶(DHAR)反应使ASA从氧化型转变为还原型,增加细胞中还原型ASA的含量,还原型ASA可以在APX作用下清除H2O2。Car的抗氧化活性主要是由于共轭双键结构能够使未配对电子离域,是β-胡萝卜素在不降解的情况下猝灭1O2的原因[35]。ASA是水溶性抗氧化剂,可以清除多种类型的自由基[35],在APX的作用下,ASA直接与H2O2发生反应,防止或减少ROS对植物造成损害[41]。 -
0.3 μmol·L−1H2O2浸种处理后,小麦Triticum aestivum种子发芽势与发芽率分别提高25%和10% [42]。用体积分数为1%的H2O2浸种6 h,花生Arachis hypogaea的发芽率上升20%[43]。外源添加H2O2,白沙蒿Artemisia sphaerocephala种子发芽率显著高于对照[44]。对种子进行外源H2O2处理,可以上调ABA分解代谢基因和GA生物合成基因,促进大麦Hordeum vulgare [45-46]、小麦[47]、拟南芥[48]、豌豆Pisum sativum [49]等种子萌发。
-
盐[21]、干旱[50]、高温[50]、重金属[51-52]等胁迫,导致萌发种子中
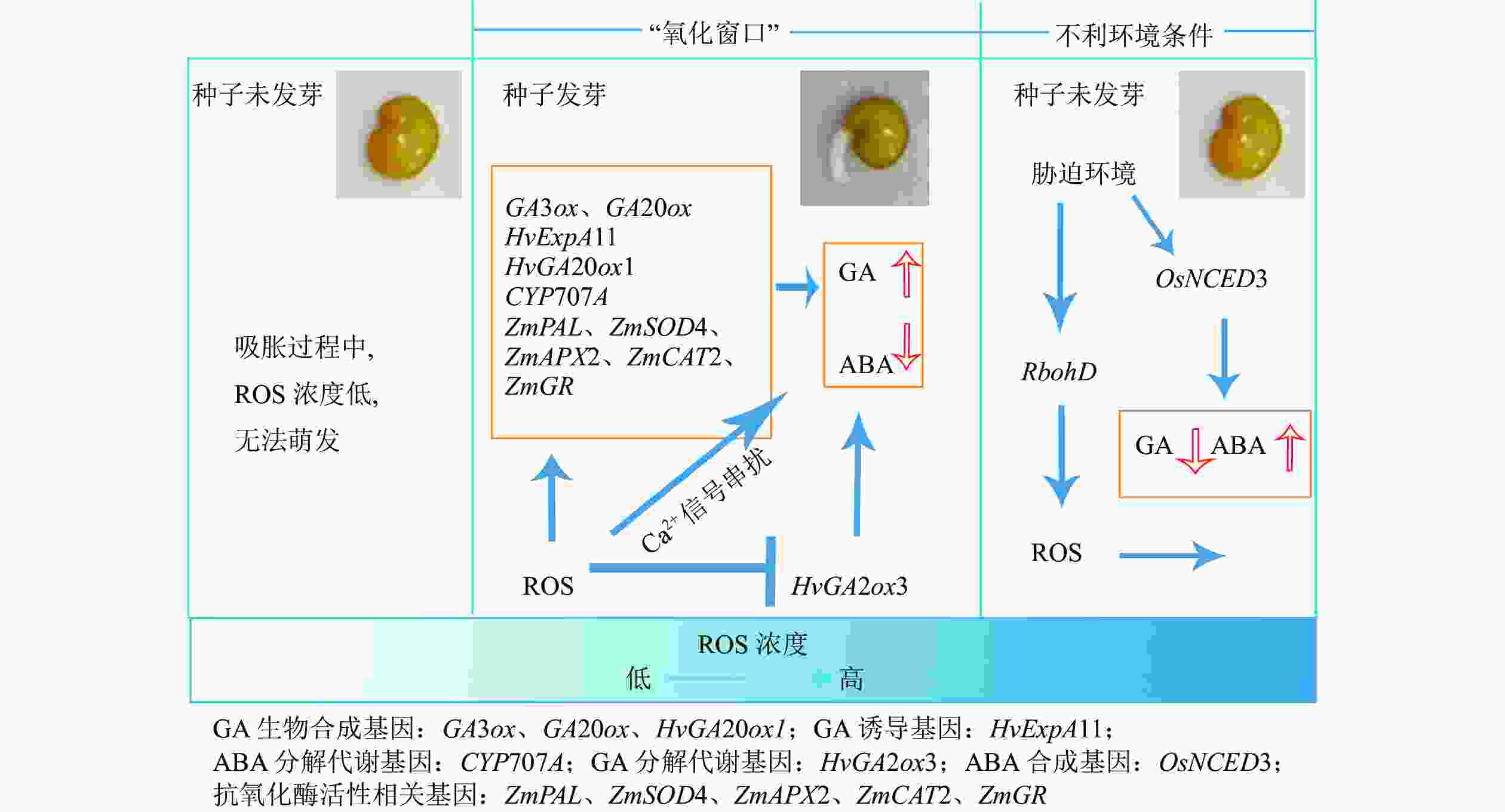
${\rm{O}}_2^{\cdot-} $ 、H2O2和丙二醛(MDA)浓度增加,ABA累积,从而抑制种子萌发。在盐胁迫下,ABI4 (abscisic acid-insensitive 4)与RbohD (NADPH氧化酶基因)和VTC2 (参与ROS产生和清除的关键基因)结合,增强RbohD表达,促进ROS积累,从而导致细胞膜损伤和种子活力下降[21]。用1.2 μmol·L−1H2O2浸种处理,小麦种子发芽势和发芽率分别比对照下降了10%和20%[42]。用1~10 mmol·L−1H2O2浸种处理,白沙蒿种子的发芽率比对照降低20%~40%;外源添加10~100 mmol·L−1的DPI (H2O2清除剂)处理白沙蒿种子,其发芽率比对照降低30%[44]。1 mmol·L−1 DPI处理大麦种子,显著延迟大麦种子发芽[53]。综上所述,ROS在一定的浓度范围内可以对种子萌发起到积极作用,即“氧化窗口”[23](图2)。若ROS的产生和清除之间不能达到平衡,即超出“氧化窗口”的范围,则种子萌发会延迟或抑制。如在白沙蒿种子中,其“氧化窗口”(H2O2浓度)为H2O2 30~70 mmol·L−1,高于或低于这个范围的H2O2浓度都会抑制种子萌发[44]。表1列出了ROS对部分种子萌发的影响。
表 1 ROS对种子萌发的影响
Table 1. Effects of ROS on seed germination
作物 内容 影响 文献 作物 内容 影响 文献 拟南芥 发芽、ROS、盐胁迫 抑制 [21] 绿豆 发芽、Ca2+ 促进 [61] 水稻 高温、干旱、ROS、ABA 抑制 [50] 水稻 NADPH氧化酶、萌发 促进 [62] 拟南芥 发芽、ABA 抑制 [54] 生菜 发芽、胚乳弱化 促进 [63] 大麦 发芽、NADPH氧化酶 促进 [55] 拟南芥 镉胁迫 抑制 [51] 向日葵 休眠缓解 促进 [56] 拟南芥 盐胁迫 促进 [64] 向日葵 休眠缓解、ABA、乙烯 促进 [57] 拟南芥 萌发、光 促进 [65] 大麦 ABA、ROS、发芽 促进 [45] 拟南芥 萌发、ABA、GA 促进 [48] 玉米 诱变剂、ROS 抑制 [58] 马蹄苋和乌伦杜娃 砷、锌胁迫 抑制 [52] 西瓜 GA3、种子活力 促进 [59] 大麦 发芽、GA、NADPH氧化酶 促进 [66] 烟草 ROS、GA信号 促进 [60] 豌豆 发芽、ABA 促进 [49] 说明:水稻Oryza sativa,向日葵Helianthus annuu,玉米Zea mays,西瓜Citrullus lanatus,烟草Nicotiana tabacum,绿豆Vigna radiate,生菜Lactuca sativa,马蹄苋Anadenanthera peregrina,乌伦杜娃Myracrodruon urundeuva -
ROS对种子中核酸、蛋白质、多糖等生物大分子氧化起着重要作用(图3)。在有胚乳种子萌发过程中,胚乳弱化是种子萌发的前提条件[67]。蛋白GhHSP 24.7调节mETC中细胞色素C/C1(CytC/C1)并诱导ROS产生,从而加速胚乳分解并促进种子萌发[68]。·OH和超氧自由基可直接裂解细胞壁多糖,使细胞壁松动,有利于种子萌发[69-70]。氰化氢和甲基紫精(产生ROS的化合物)导致胚胎蛋白氧化,会使种子休眠释放[71]。
-
GA是种子萌发必不可少的激素。H2O2能够增强GA诱导基因HvExpA11和GA合成基因HvGA20ox1的表达,抑制参与GA分解代谢基因HvGA2ox3的表达,促进GA的积累,使大麦种子休眠释放,进而萌发[46]。NOX产生的
$ {\rm{O}}_2^{\cdot-} $ 和H2O2通过促进HvKAO1和HvGA3ox1基因转录以促进大麦中GA的生物合成,从而加速发芽[66]。H2O2通过增强GA3ox和GA20ox的表达,促进GA3的生物合成,加速拟南芥种子萌发[48]。ABA能够维持种子休眠、抑制种子萌发。H2O2可以上调ABA分解代谢基因CYP707A,降低种子中ABA的浓度,从而促进种子萌发。ABI1和ABI2是ABA信号转导的重要调节因子[72],起负调控作用,H2O2能使ABI1和ABI2型2C蛋白磷酸酶失活[73−74],增强ABA信号传导。胁迫条件致使种子中ROS浓度增加,氧化应激诱导水稻种子中ABA合成关键基因OsNCED3的表达,抑制种子萌发[50]。
SA是一种小分子酚类物质,参与植物的种子发芽、开花和生理生化等生命进程[75]。胁迫条件下,氧代谢失调,积累大量的ROS,SA可以充当抗氧化剂来清除植物体内的ROS,减少氧化损伤,促进种子萌发[76]。在低温胁迫下,用50 mmol·L−1 H2O2处理,玉米种子发芽率为90%,种子内源SA浓度在0~72 h内迅速升高、可能与上调的ZmPAL(参与SA生物合成)表达密切相关;SA+H2O2显著增加了内源性H2O2和SA浓度、抗氧化酶活性及其相应基因ZmPAL、ZmSOD4、ZmAPX2、ZmCAT2和ZmGR的表达[77]。10和20 mmol·L−1H2O2对豌豆种子浸种24 h,促进豌豆种子萌发,但SA的浓度下降50%[78]。在正常条件下,SA会增加ROS介导的氧化损伤,并诱导H2O2的产生,对发芽产生不利影响;在胁迫条件下,SA可增强种子发芽[79]。
乙烯对打破种子休眠有重要作用[80]。100 mmol·L−1H2O2处理大豆Glycine max种子,可以刺激乙烯的生物合成,促进大豆种子的发芽[81]。
-
H2O2像Ca2+一样是一种普遍存在的第2信使,是一种多功能的生理信号剂[82]。 ROS和Ca2+之间相互调节,ROS可以调节细胞Ca2+信号传导,而Ca2+信号传导对于ROS的产生是必需的[83-84]。H2O2促进了ABA胁迫下甜瓜Cucumis melo种子中的Ca2+内流,从而增加了甜瓜种子内H2O2的积累[85]。而甜瓜和拟南芥种子中的H2O2缺乏阻止了ABA应激下的Ca2+,因此,在ABA胁迫下,Ca2+信号参与H2O2诱导的ABA/GA3平衡和随后的种子萌发。
-
抗氧化系统包括抗氧化酶系统和抗氧化剂。在非生物胁迫下,种子萌发会产生ROS,POD、CAT、SOD和APX的活性会增强,清除过量的ROS,促进种子萌发[86]。在莴苣Lactuca sativa种子中LsSOD1和LsSOD2的基因表达水平在种子萌发的时间点前都有所增加[87]。应用抗氧化剂褪黑素处理老化玉米种子,不仅提高了发芽率,还诱导种子中SOD和CAT活性[88]。0.5 g·L−1的GSH浸种8 h,低温胁迫下玉米种子的发芽势与发芽率分别提高了22.01%、11.84%;并且种子内的POD、SOD、APX分别增加了2.23%、23.40%和15.27%,MDA减少了35.19%[89]。20 mg·L−1 ASA处理铜胁迫下的蚕豆Vicia faba种子,其发芽势、发芽率、发芽指数和活力指数分别增加了6.75%、5.05%、14.10%和52.96%[90]。5 μmol·L−1的槲皮素(类黄酮)处理番茄Solanum lycopersicum种子降低H2O2和
$ {\rm{O}}_2^{\cdot-} $ ,提高了发芽率和种子活力[91]。1 mmol·L−1 DPI浸种处理,大麦种子发芽率降低40%[66]。用100、300和500 µg·L−1抗冻蛋白(AFPs)处理番茄种子,降低了抗氧化相关基因SOD和CAT1的表达水平,清除冷胁迫下ROS的过量产生,并降低氧化胁迫以促进种子萌发[92]。CaHsfA1d是A类热激蛋白,它在高温下转录上调,在热胁迫下维持H2O2动态平衡[93]。拟南芥Hsf3(热休克转录因子3)参与调节APX2的表达以清除H2O2[94]。
-
在种子萌发中,ROS起到了很重要的作用。ROS通过生物大分子氧化、种皮弱化促进种子休眠的释放,在种子萌发的早期阶段,ROS生成并参与环境条件的感知和转导,如激素信号,最后阶段,ROS会攻击细胞壁多糖并导致承重结构破坏,致使细胞壁松动,大量吸水促使胚根伸长。ROS的稳态参与种子萌发,其浓度影响种子萌发与休眠。当种子中ROS浓度过高时,会引起氧化损伤,影响种子萌发,只有适宜的ROS浓度,才能增强GA合成基因、ABA分解代谢基因和抗氧化酶活性相关基因的表达,达到促进种子萌发的作用,这被称为“氧化窗口”。在种子内,若ROS的浓度在这个“氧化窗口”以上或以下水平都不能对种子萌发起到积极作用。
国内外关于ROS在种子萌发方面的研究多集中于种子内ROS产生的种类、部位,对种子的毒害作用,ROS与GA、ABA之前的调控以及在胁迫条件下对种子萌发的影响。但是,对于ROS与其他激素途径的作用、调控种子内ROS的相关基因以及如何精细调控促进种子萌发的“氧化窗口”范围研究较少。关于ROS对其他激素途径的作用还需进一步研究,另外,可以利用转录组学和代谢组学的技术,及时观察种子吸胀后特定的表达特征,精准调控“氧化窗口”的范围,筛选出能调控ROS浓度的相关基因,进而为ROS在种子萌发中的作用提供新思路。
Research progress on the role of reactive oxygen species in seed germination
-
摘要: 种子是农业生产中最基本的生产资料。种子成功整齐发芽是作物生长发育、高产稳产的第1步。活性氧(ROS)是一类多功能化合物,在种子萌发过程中发挥着关键作用。本研究介绍了ROS的种类、产生部位以及对种子萌发的“氧化窗口”效应,总结了ROS调控种子萌发的机制并对未来研究提出展望。目前ROS调控种子萌发的研究集中于:①当条件合适时,ROS维持在一个适当的水平,启动赤霉素(GA)等信号转导途径、抑制脱落酸(ABA)途径,调控细胞膨大,促进种子萌发;②当面临不利环境时,ROS的过量积累会对生物大分子造成氧化损伤,诱导ABA途径,抑制种子萌发。ROS通过生物分子氧化、种皮弱化和胚乳衰退解除种子休眠。今后,需进一步探索对种子萌发起到积极作用的“氧化窗口”范围,同时结合转录组学和代谢组学技术筛选种子萌发中调控ROS含量相关基因,更好地了解ROS促进种子萌发的机制。图3表1参94Abstract: Seed is the most basic resource for agricultural production. The germination of seeds is essential for plant growth and development, and affects crop yield and quality. Reactive oxygen species (ROS) are multifunctional compounds that play a key role in seed germination. In this study, the species, production site and“oxidation window”effect of ROS on seed germination were introduced, the mechanism of ROS regulation on seed germination was summarized. Current studies on ROS regulation of seed germination mainly focus on: (1) When these conditions are permissive for germination, ROS levels are maintained at a level which triggers cellular events associated with germination, such as inducing of GA signaling and inhibition of ABA signaling. (2) However, when seeds are exposed to abiotic stresses, the over accumulation of ROS induces ABA signaling, promotes oxidative damage and thus inhibits seed germination. Moreover, the release of seed dormancy by ROS would be related to oxidation of biomacromolecule, the weakening of seed coat and recession of endosperm. The present review would shed a new light on the signalling roles of ROS in seed physiology. The scope of “oxidation window”which plays a positive role in seed germination should be further explored in future research, and transcriptomics and metabolomics techniques should be combined to screen genes related to the regulation of ROS content in seed germination, so as to better understand the mechanism of ROS promoting seed germination. [Ch, 3 fig. 1 tab. 94 ref.]
-
Key words:
- seed germination /
- reactive oxygen species /
- metabolic /
- hormone /
- review
-
表 1 ROS对种子萌发的影响
Table 1. Effects of ROS on seed germination
作物 内容 影响 文献 作物 内容 影响 文献 拟南芥 发芽、ROS、盐胁迫 抑制 [21] 绿豆 发芽、Ca2+ 促进 [61] 水稻 高温、干旱、ROS、ABA 抑制 [50] 水稻 NADPH氧化酶、萌发 促进 [62] 拟南芥 发芽、ABA 抑制 [54] 生菜 发芽、胚乳弱化 促进 [63] 大麦 发芽、NADPH氧化酶 促进 [55] 拟南芥 镉胁迫 抑制 [51] 向日葵 休眠缓解 促进 [56] 拟南芥 盐胁迫 促进 [64] 向日葵 休眠缓解、ABA、乙烯 促进 [57] 拟南芥 萌发、光 促进 [65] 大麦 ABA、ROS、发芽 促进 [45] 拟南芥 萌发、ABA、GA 促进 [48] 玉米 诱变剂、ROS 抑制 [58] 马蹄苋和乌伦杜娃 砷、锌胁迫 抑制 [52] 西瓜 GA3、种子活力 促进 [59] 大麦 发芽、GA、NADPH氧化酶 促进 [66] 烟草 ROS、GA信号 促进 [60] 豌豆 发芽、ABA 促进 [49] 说明:水稻Oryza sativa,向日葵Helianthus annuu,玉米Zea mays,西瓜Citrullus lanatus,烟草Nicotiana tabacum,绿豆Vigna radiate,生菜Lactuca sativa,马蹄苋Anadenanthera peregrina,乌伦杜娃Myracrodruon urundeuva -
[1] 胡宗英, 孙泽威. 植物种子在农业中的重要地位和作用[J]. 安徽农学通报, 2013, 19(24): 46 − 47. HU Zongying, SUN Zewei. Important position and role in agriculture for seed [J]. Anhui Agricultural Science Bulletin, 2013, 19(24): 46 − 47. [2] WEITBRECHT K, MÜLLER K, LEUBNER-METZGER G. First of the mark: early seed germination [J]. Journal of Experimental Botany, 2011, 62(10): 3289 − 3309. [3] DORONE Y, BOEYNAEMS S, FLORES E, et al. A prion-like protein regulator of seed germination undergoes hydration-dependent phase separation [J]. Cell, 2021, 184(16): 4284 − 4298. [4] PEREIRA A, OLIVERIRA H C, FRACETO L F, et al. Nanotechnology potential in seed priming for sustainable agriculture [J]. Nanomaterials, 2021, 11(2): 267 − 276. [5] BOTHA F C, POTGIETER G P, BOTHA A M. Respiratory metabolism and gene expression during seed germination [J]. Plant Growth Regulation, 1992, 11(3): 211 − 224. [6] BAILLY C. The signalling role of ROS in the regulation of seed germination and dormancy [J]. Biochemical Journal, 2019, 476(20): 3019 − 3032. [7] HAN Chao, YANG Pingfang. Studies on the molecular mechanisms of seed germination [J]. Proteomics, 2015, 15(10): 1671 − 1679. [8] 房艺. 拟南芥NFYA1在种子萌发阶段的功能[D]. 济南: 山东农业大学, 2012. FANG Yi. The Function of Arabidopsis NFYA1 Germination [D]. Ji’nan: Shandong Agricultural University, 2012. [9] ORACK K, STAWSKA M. Cellular recycling of proteins in seed dormancy alleviation and germination [J/OL]. Frontiers in Plant Science, 2016(7): 1128[2022-11-02]. doi:10.3389/fpls.2016.01128. [10] SRIVASTAVA A K, KUMAR J S, SUPRASANNA P. Seed“primeomics”: plants memorize their germination under stress [J]. Biological Reviews of the Cambridge Philosophical Society, 2021, 96(5): 1723 − 1743. [11] NÉE G, XIANG Yong, SOPPE W J. The release of dormancy, a wake-up call for seeds to germinate [J]. Current Opinion in Plant Biology, 2017, 35: 8 − 14. [12] CHEN Huhui, RUAN Jiuxiao, CHU Pu, et al. AtPER1 enhances primary seed dormancy and reduces seed germination by suppressing the ABA catabolism and GA biosynthesis in Arabidopsis seeds [J]. The Plant Journal, 2020, 101(2): 310 − 323. [13] GONG Xuemei, BEWLEY J D. A GAMYB-like gene in tomato and its expression during seed germination [J]. Planta, 2008, 228(4): 563 − 572. [14] LI Caixia, DONG Shaoyun, BECKLES D M, et al. The qLTG1.1 candidate gene CsGAI regulates low temperature seed germination in cucumber [J]. Theoretical and Applied Genetics, 2022, 135(8): 2593 − 2607. [15] BUNSICK M, TOH S, WONG C, et al. SMAX1-dependent seed germination bypasses GA signalling in Arabidopsis and Striga [J]. Nature Plants, 2020, 6(6): 646 − 652. [16] DVORAKOVA M, HYLOVA A, SOUDEK P, et al. Triazolide strigolactone mimics as potent selective germinators of parasitic plant Phelipanche ramose [J]. Pest Management Science, 2019, 75(7): 2049 − 2056. [17] 黎家, 李传友. 新中国成立70年来植物激素研究进展[J]. 中国科学(生命科学), 2019, 49(10): 1227 − 1281. LI Jia, LI Chuanyou, Seventy-year major research progress in plant hormones by Chinese scholars [J]. Scientia Sinica Vitae, 2019, 49(10): 1227 − 1281. [18] WASZCZAK C, CARMODY M, KANGACTIVE J. Reactive oxygen species in plant signaling [J]. Annual Review of Plant Biology, 2018, 69: 209 − 236. [19] GOMES M P, GARCIA Q S. Reactive oxygen species and seed germination [J]. Biologia, 2013, 68: 351 − 357. [20] GUO Ziting, ZHAO Jinjin, WANG Meiping, et al. Sulfur dioxide promotes seed germination by modulating reactive oxygen species production in maize [J/OL]. Plant Science, 2021, 312: 111027[2022-11-02]. doi: 10.1016/j.plantsci.2021.111027. [21] LUO Xiaofeng, DAI Yujia, ZHENG Chuan, et al. The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress [J]. New Phytologist, 2021, 229(2): 950 − 962. [22] BAILLY C. Active oxygen species and antioxidants in seed biology [J]. Seed Science Research, 2004, 14(2): 93 − 107. [23] BAILLY C, EL-MAAROUF-BOUTEAU H, CORBINEAU F. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology [J]. Comptes Rendus Biologies, 2008, 331(10): 806 − 814. [24] ORACZ K, KARPIÑSKI S. Phytohormones signaling pathways and ROS involvement in seed germination [J/OL]. Frontiers in Plant Science, 2016(7): 864[2022-11-02]. doi:10.3389/fpls.2016.00864. [25] FAROOQ M A, ZHANG Xiaomeng, ZAFAR M M, et al. Roles of reactive oxygen species and mitochondria in seed germination [J/OL]. Frontiers in Plant Science, 2021(12): 781734[2022-11-02]. doi: 10.3389/fpls.2021.781734. [26] 张梦如, 杨玉梅, 成蕴秀, 等. 植物活性氧的产生及其作用和危害[J]. 西北植物学报, 2014, 34(9): 1916 − 1926. ZHANG Mengru, YANG Yumei, CHENG Yunxiu, et al. Generation of reactive oxygen species and their functions and deleterious effects in plants [J]. Acta Botanica Boreali-Occidentalia Sinica, 2014, 34(9): 1916 − 1926. [27] KRANNER I, ROACH T, BECKETT R P, et al. Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum [J]. Journal of Plant Physiology, 2010, 167(10): 805 − 811. [28] MURPHY M P. How mitochondria produce reactive oxygen species [J]. Biochemical Journal, 2009, 417(1): 1 − 13. [29] WINTERBOURN C C. The biological chemistry of hydrogen peroxide [J]. Methods in Enzymology, 2013, 528: 3 − 25. [30] RICHARDS S L, WILKINS K A, SWARBRECK S M, et al. The hydroxyl radical in plants: from seed to seed [J]. Journal of Experimental Botany, 2015, 66(1): 37 − 46. [31] SINGH A. Chemical and biochemical aspects of superoxide radicals and related species of activated oxygen [J]. Canadian Journal of Physiology and Pharmacology, 1982, 60(11): 1330 − 1345. [32] ORTEGA-VILLASANTE C, BURÉN S, BARÓN-SOLA Á, et al. In vivo ROS and redox potential fluorescent detection in plants: present approaches and future perspectives [J]. Methods, 2016, 109: 92 − 104. [33] CORPAS F J, BARROSO J B, del RÍO L A. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells [J]. Trends in Plant Science, 2001, 6(4): 145 − 150. [34] 杨利, 王波, 李文姣, 等. 干旱胁迫下ROS的产生、清除及信号转导研究进展[J]. 生物技术通报, 2021, 37(4): 194 − 203. YANG Li, WANG Bo, LI Wenjiao, et al. Research progress on production, scavenging and signal transduction of ROS under drought stress [J]. Biotechnology Bulletin, 2021, 37(4): 194 − 203. [35] AHMAD P, JALEEL C A, SALEM M A. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress [J]. Critical Reviews in Biotechnology, 2010, 30(3): 161 − 175. [36] 皮明雪. BnTR1在甘蓝型油菜抗旱和耐盐中的功能鉴定[D]. 扬州: 扬州大学, 2017. PI Mingxue. Functional Identification of BnTR1 in Drought Resistance and Salt Tolerance of Brassica napus [D]. Yangzhou: Yangzhou University, 2017. [37] 丁福章, 李继新, 雷波, 等. 超氧化物歧化酶在烟草上的应用研究进展[J]. 安徽农业科学, 2008(5): 1897 − 1898, 1914. DING Fuzhang, LI Jixin, LEI Bo, et al. Research progress on application of superoxide dismutases in tobacco [J]. Journal of Anhui Agricultural Sciences, 2008(5): 1897 − 1898, 1914. [38] HUCHZERMEYER B, MENGHANI E, KHARDIA P, et al. Metabolic pathway of natural antioxidants, antioxidant enzymes and ROS providence[J/OL]. Antioxidants, 2022, 11(4): 761[2022-10-20]. doi: 10.3390/antiox11040761. [39] ANJUM N A, SHARMA P, GILL S S, et al. Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants [J]. Environmental Science and Pollution Research, 2016, 23(19): 19002 − 19029. [40] MANSOOR S, ALIWANI O, LONE J K, et al. Reactive oxygen species in plants: from source to sink [J/OL]. Antioxidants, 2022, 11(2): 225[2022-11-02]. doi: 10.3390/antiox11020225. [41] GALLIE D R. L-ascorbic acid: a multifunctional molecule supporting plant growth and development [J/OL]. Scientifica, 2013: 795964[2022-11-02]. doi: 10.1155/2013/795964. [42] 周亚洁, 陈朋, 周鑫惠, 等. 不同品种小麦种子萌发及幼苗发育对外源过氧化氢处理的响应[J]. 湖北农业科学, 2022, 61(16): 5 − 11, 17. ZHOU Yajie, CHEN Peng, ZHOU Xinhui, et al. Response of seed germination and seedling development of different wheat varieties to the treatment of exogenous H2O2 [J]. Hubei Agricultural Sciences, 2022, 61(16): 5 − 11, 17. [43] 张雅婷. 外源过氧化氢预处理对花生种子低温萌发的影响[D]. 合肥: 安徽农业大学, 2020. ZHANG Yating. Effect of Exogenous H2O2 Pre-treatment on Low Temperature Germination of Peanut Seeds [D]. Hefei: Anhui Agricultural University, 2020. [44] 冯潇. 过氧化氢对白沙蒿种子萌发的影响[D]. 兰州: 兰州大学, 2019. FENG Xiao. The Effect of H2O2 on Seed Germination of Artemisia sphaerocephala [D]. Lanzhou: Lanzhou University, 2019. [45] ISHIBASHI Y, AOKI N, KASA S, et al. The interrelationship between abscisic acid and reactive oxygen species plays a key role in barley seed dormancy and germination [J/OL]. Frontiers in Plant Science, 2017, 8: 275[2022-11-02]. doi: 10.3389/fpls.2017.00275. [46] BAHIN E, BAILLY C, SOTTA B, et al. Crosstalk between reactive oxygen species and hormonal signalling pathways regulates grain dormancy in barely [J]. Plant,Cell and Environment, 2011, 34(6): 980 − 993. [47] YU Yonglong, ZHEN Shoumin, WANG Shu, et al. Comparative transcriptome analysis of wheat embryo and endosperm responses to ABA and H2O2 stresses during seed germination [J/OL]. BMC Genomics, 2016, 17: 97[2022-11-02]. doi: 10.1186/s12864-016-2416-9. [48] LIU Yinggao, YE Nenghui, LIU Rui, et al. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination [J]. Journal of Experimental Botany, 2010, 61(11): 2979 − 2990. [49] BARBA-ESPIN G, NICOLAS E, ALMANSA M S, et al. Role of thioproline on seed germination: interaction ROS-ABA and effects on antioxidative metabolism [J]. Plant Physiology and Biochemistry, 2012, 59: 30 − 36. [50] LIU Juan, HASANUZZAMAN M, WEN Huili, et al. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice [J]. Protoplasma, 2019, 256(5): 1217 − 1227. [51] NOURIMAND M, TODD C D. There is a direct link between allantoin concentration and cadmium tolerance in Arabidopsis [J]. Plant Physiology and Biochemistry, 2019, 135: 441 − 449. [52] GOMES M P, CARNEIRO M M L C, NOGUEIRA C O G, et al. The system modulating ROS content in germinating seeds of two Brazilian savanna tree species exposed to As and Zn [J]. Acta Physiologiae Plantarum, 2013, 35: 1011 − 1022. [53] ISHIBASHI Y, TAWARATSUMIDA T, ZHENG Shaohui, et al. NADPH oxidases act as key enzyme on germination and seedling growth in barley (Hordeum vulgare L. ) [J]. Plant Production Science, 2010, 13(1): 45 − 52. [54] BAEK D, CHA J Y, KANG S, et al. The Arabidopsis a zinc finger domain protein ARS1 is essential for seed germination and ROS homeostasis in response to ABA and oxidative stress [J/OL]. Frontiers in Plant Science, 2015, 6: 963[2022-11-02]. doi: 10.3389/fpls.2015.00963. [55] ISHIBASHI Y, KASA S, SAKAMOTO M, et al. A role for reactive oxygen species produced by NADPH oxidases in the embryo and aleurone cells in barley seed germination [J/OL]. PLoS One, 2015, 10(11): e0143173[2022-11-02]. doi: 10.1371/journal.pone.0143173. [56] MORSCHER F, KRANNER I, ARC E, et al. Glutathione redox state, tocochromanols, fatty acids, antioxidant enzymes and protein carbonylation in sunflower seed embryos associated with after-ripening and ageing [J]. Annals of Botany, 2015, 116(4): 669 − 678. [57] El-MAAROUF-BOUTEAU H, SAJJAD Y, BAZIN J, et al. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination [J]. Plant,Cell and Environment, 2015, 38(2): 364 − 374. [58] ZHANG Yifei, SHI Haojie, DENG Benliang. Mutagen-induced phytotoxicity in maize seed germination is dependent on ROS scavenging capacity [J/OL]. Scientific Reports, 2018, 8(1): 14078[2022-11-02]. doi: 10.1038/s41598-018-32271-y. [59] HE Yong, YE Zhenxiao, YING Quansheng, et al. Glyoxylate cycle and reactive oxygen species metabolism are involved in the improvement of seed vigor in watermelon by exogenous GA3 [J]. Scientia Horticulturae, 2019, 247: 184 − 194. [60] LI Zhan, GAO Yue, ZHANG Yuchan, et al. Reactive oxygen species and gibberellin acid mutual induction to regulate tobacco seed germination [J/OL]. Frontiers in Plant Science, 2018, 9(9): 1279[2022-11-02]. doi: 10.3389/fpls.2018.01279. [61] SINGH K L, CHAUDHURI A, KAR R K. Role of peroxidase activity and Ca2+ in axis growth during seed germination [J]. Planta, 2015, 242(4): 997 − 1007. [62] LI Wenyan, CHEN Bingxian, CHEN Zhongjian, et al. Reactive oxygen species generated by NADPH oxidases promote radicle protrusion and root elongation during rice seed germination [J/OL]. International Journal Mololecular Science, 2017, 18(1): 110[2022-11-02]. doi: 10.3390/ijms18010110. [63] ZHANG Yu, CHEN Bingxian, XU Zhenjiang, et al. Involvement of reactive oxygen species in endosperm cap weakening and embryo elongation growth during lettuce seed germination [J]. Journal of Experimental Botany, 2014, 65(12): 3189 − 3200. [64] ORTIZ-ESPIN A, IGLESIAS-FERNÁNDEZ R, CALDERÓN A, et al. Mitochondrial AtTrxo1 is transcriptionally regulated by AtbZIP9 and AtAZF2 and affects seed germination under saline conditions [J]. Journal of Experimental Botany, 2017, 68(5): 1025 − 1038. [65] LARIGUET P, RANOCHA P, MEYER M, et al. Identification of a hydrogen peroxide signalling pathway in the control of light-dependent germination in Arabidopsis [J]. Planta, 2013, 238(2): 381 − 395. [66] KAI K, KASA S, SAKAMOTO M, et al. Role of reactive oxygen species produced by NADPH oxidase in gibberellin biosynthesis during barley seed germination [J/OL]. Plant Signaling & Behavior, 2016, 11(5): e1180492. doi: 10.1080/15592324.2016.1180492. [67] ZHANG Keliang, YAO Linjun, ZHANG Yin, et al. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination [J]. Planta, 2019, 249(2): 291 − 303. [68] MA Wei, GUAN Xueying, LI Jie, et al. Mitochondrial small heat shock protein mediates seed germination via thermal sensing [J]. Proceedings of the National Academy of Sciences, 2019, 116(10): 4716 − 4721. [69] SCHWEIKERT C, LISZKAY A, SCHOPFER P. Scission of polysaccharides by peroxidase-generated hydroxyl radicals [J]. Phytochemistry, 2000, 53(5): 565 − 570. [70] YANG Xueqin, ZHANG Fan, YANG Mei, et al. The NADPH-oxidase LsRbohC1 plays a role in lettuce (Lactuca sativa) seed germination [J]. Plant Physiology and Biochemistry, 2020, 154: 751 − 757. [71] ORACZ K, EL-MARROUF B H, FARRANT J M, et al. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation [J]. The Plant Journal, 2007, 50(3): 452 − 465. [72] RODRIGUEZ P L, BENNING G, GRILL E. ABI2, a second protein phosphatase 2C involved in abscisic acid signal transduction in Arabidopsis [J]. FEBS Letters, 1998, 421(3): 185 − 190. [73] MEINHARD M, GRILL E. Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis [J]. FEBS Letters, 2001, 508(3): 443 − 446. [74] MEINHARD M, RODEIGUEZ P L, GRILL E. The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling [J]. Planta, 2002, 214(5): 775 − 782. [75] 樊清清, 王英英, 冯晓东. 低温胁迫下水杨酸对茄子种子萌发及幼苗生长的影响[J]. 延安大学学报(自然科学版), 2020, 39(2): 71 − 75. FAN Qingqing, WANG Yingying, FENG Xingdong. Effects of salicylic acid on seed germination and seedling growth of eggplant under low temperature stress [J]. Journal of Yan’an University (Natural Science Edition), 2020, 39(2): 71 − 75. [76] WANI A B, CHADAR H, WANI A H, et al. Salicylic acid to decrease plant stress [J]. Environmental Chemistry Letters, 2017, 15(1): 101 − 123. [77] LI Zhan, XU Jungui, GAO Yue, et al. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L. ) seed germination [J/OL]. Frontiers in Plant Science, 2017, 8: 1153[2022-11-02]. doi:10.3389/fpls.2017.01153. [78] BARBA-ESPIN G, DIAZ-VIVANCOS P, CLEMENTE-MORENO M J, et al. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings [J]. Plant,Cell and Environment, 2010, 33(6): 981 − 994. [79] GAO Wenrui, LIU Yan, HUANG Juan, et al. MES7 modulates seed germination via regulating salicylic acid content in Arabidopsis [J/OL]. Plants, 2021, 10(5): 903[2022-11-22]. doi:10.3390/plants10050903. [80] MATILLA A J, MATILLA-VÁZQUEZ M A. Involvement of ethylene in seed physiology [J]. Plant Science, 2008, 175(1/2): 87 − 97. [81] ISHIBASHI Y, KODA Y, ZHENG Shaohui, et al. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species [J]. Annals of Botany, 2013, 111(1): 95 − 102. [82] GECHEV T S, BREUSEGEM F, V STONE J M, et al. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death [J]. Bioessays, 2006, 28(11): 1091 − 1101. [83] 赵欢欢. 活性氧对大白菜自交不亲和性影响的研究[D]. 济南: 山东农业大学, 2021. ZHAO Huanhuan. The Effect of Reactive Oxygen Species on Self-incompatibility in Chinese Cabbage [D]. Ji’nan: Shandong Agricultural University, 2021. [84] 侯晓媛, 李雪, 鲁严. 钙信号和活性氧相互作用的研究进展[J]. 中国细胞生物学学报, 2019, 41(9): 1837 − 1844. HOU Xiaoyuan, LI Xue, LU Yan. Interplay between calcium signaling and reactive oxygen species [J]. Chinese Journal of Cell Biology, 2019, 41(9): 1837 − 1844. [85] CHENG Mengjie, GUO Yanliang, LIU Qing, et al. H2O2 and Ca2+ signaling crosstalk counteracts ABA to induce seed germination [J/OL]. Antioxidants, 2022, 11(8): 1594[2022-11-02]. doi: 10.3390/antiox11081594. [86] 王志恒, 黄思麒, 邹芳, 等. 温度与NaCl处理对甜高粱种子萌发及幼苗抗氧化酶活性的影响[J]. 中国农业科技导报, 2020, 22(9): 42 − 51. WANG Zhiheng, HUANG Siqi, ZOU Fang, et al. Effects of temperature and NaCl on seed germination and seedling antioxidant enzyme activities of sweet sorghum [J]. Journal of Agricultural Science and Technology, 2020, 22(9): 42 − 51. [87] 肖健. 莴苣超氧化物歧化酶基因克隆及其在种子萌发中的功能研究[D]. 广州: 华南农业大学, 2018. XIAO Jian. Gene Cloning and Functional Analysis of Superoxide Dismutase during Lettuce Seed Germination [D]. Guangzhou: South China Agricultural University, 2018. [88] DENG Benliang, YANG Kejun, ZHANG Yifei, et al. Can antioxidant’s reactive oxygen species (ROS) scavenging capacity contribute to aged seed recovery? contrasting effect of melatonin, ascorbate and glutathione on germination ability of aged maize seeds [J/OL]. Free Radical Research, 2017, 51(9/10): 765-771[2022-11-02]. doi:10.1080/10715762.2017.1375099. [89] 李林. 外源谷胱甘肽对低温胁迫下玉米幼苗的缓解效应研究[D]. 哈尔滨: 黑龙江大学, 2022. LI Lin. Alleviating Effect of Exogenous Glutathione on Maize Seedlings under Low Temperature Stress [D]. Harbin: Heilongjiang University, 2022. [90] 秦永燕, 王雪婷, 班子茹, 等. 抗坏血酸对铜胁迫蚕豆种子萌发和根尖细胞损伤的缓解作用[J]. 农业研究与应用, 2022, 35(2): 12 − 17. QIN Yongyan, WANG Xueting, BAN Ziru, et al. Remission effect of ascorbic acid on seed germination and root tip cell damage of Vicia faba under copper stress [J]. Agricultural Research and Application, 2022, 35(2): 12 − 17. [91] YANG Jiale, ZHANG Lixiang, JIANG Li, et al. Quercetin alleviates seed germination and growth inhibition in Apocynum venetum and Apocynum pictum under mannitol-induced osmotic stress [J]. Plant Physiology and Biochemistry, 2021, 159: 268 − 276. [92] KYU S Y, NAING A H, WIN P P, et al. Tomato seeds pretreated with antifreeze protein type Ⅰ (AFP Ⅰ) promotes the germination under cold stress by regulating the genes involved in germination process [J]. Plant Signaling &Behavior, 2019, 14(12): 1682796[2022-11-02]. doi: 10.1080/15592324.2016.1682796. [93] GAI Wenxian, MA Xiao, LI Yang, et al. CaHsfA1d improves plant thermotolerance via regulating the expression of stress- and antioxidant-related genes [J/OL]. International Journal of Molecular Science, 2020, 21(21): 8374[2022-11-02]. doi: 10.3390/ijms21218374. [94] PANCHUK I I, VOLKOV R A, SCHÖFFL F. Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis [J]. Plant Physiology, 2002, 129(2): 838 − 853. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20220681







 下载:
下载: