-
当前,树体高大、通风透光性差和管理粗放是导致针叶树种种子园采种难和雌球花花量不足的重要原因,制约着林木种子园的精细化经营和管理[1]。截顶影响营养物质流向和分配,促进结实母枝更新,是植株达到矮化效应的重要方法,对实现“果园式”林木种子园具有重要指导意义[2−3]。国外学者对花旗松Pseudotsuga menziesii进行夏季截顶和修枝后发现:虽然树冠体积减少了,但是枝条上雌雄球花密度显著增加,通过调控栽植间距,提高了单位面积球果产量[4];国内学者研究表明:截冠可提高樟子松Pinus sylvestris var. mongholica壮龄母树的产量和种子质量[3];对马尾Pinus massoniana截顶处理后发现:中产和高产无性系的雌球花量能提高20%以上[1]。
赤霉素(GAs)被认为是一种重要的双萜类植物生长调节剂,参与松树球花分化与茎伸长等许多重要的生长发育过程[5−6]。FERNÁNDEZ等[7]对辐射松Pinus radiata不同组织中内源GAs测定后发现:GA3在营养芽和雄球花中质量分数较高但比较稳定,而GA4则差异明显,推测GA4可能与它们的发育调节有关。赤霉素等激素比值的变化可表征树体内激素的不同吸收或运转规律。较高水平的Z型细胞分裂素和较低水平的脱落酸(ABA)及其代谢产物可能与雌球花的形成有关,在顶芽发育过程中GA和ABA往往表现出拮抗作用,外源注射GA4/7使顶梢ABA合成减少或通过其他途径加速了ABA代谢产物的分解,进而降低顶芽内源ABA及ABA分解代谢的主导产物ABA葡萄糖酯(ABA-ge)的质量分数,改变了它们的比值,进而提高雌球花的分化能力,增加雌球花的数量,但其机制尚不清楚[8−9]。
研究表明:外界因素首先通过植株内源激素质量分数及其比值的变化对生长和成花起作用[10]。营养芽转变为生殖芽也是植株体内各种激素在时间和空间上相互作用产生的综合结果,取决于促进和抑制开花这2类激素的平衡[11]。植物顶芽会抑制侧芽的发生,去除顶芽或抑制顶端优势则会促进侧芽的产生。截顶可通过抑制植株顶端优势形成,影响植株体内生长素和细胞分裂素的合成与再分配。在生产上,许多针叶树正是利用截顶与修枝、植物生长调节剂诱导等措施控制树体的生殖与营养生长平衡[12−13]。马尾松是中国南方主要的速生丰产优质用材树种和最重要的脂用树种[14],其雌球花多分布于主枝或侧枝顶端,生殖芽和营养芽都由新生枝梢产生,其生理状态与花芽分化密切相关[15−20]。因此,本研究采用盆栽控制试验,以高产的马尾松无性系为试材,在花原基形成前期设置生产上常用的截顶和赤霉素诱导试验,研究树体内源激素质量分数及其比值的变化和平衡,以期为马尾松结实母树的树体管理提供理论指导和技术支持。
-
试验地点位于浙江省淳安县林业总场有限公司姥山分场国家马尾松良种基地(29°32′34″N, 119°04′04″E)。供试材料选用马尾松第2代无性系种子园中结实能力强的209号无性系,于2017年选取无性系同一个母株上生长基本一致的穗条进行嫁接。2018年3月,将生长势相对一致、侧枝数量相近的无性系嫁接苗移栽至无纺布容器内。容器规格为直径70 cm,高度60 cm,每个容器内放入50 kg马尾松第2代无性系种子园内0~40 cm土层的酸性红壤,土壤pH 4.71、全氮3.59 g·kg−1、全磷0.34 g·kg−1、全钾16.90 g·kg−1、碱解氮248.00 mg·kg−1、速效磷5.48 mg·kg−1、速效钾228.00 mg·kg−1、有机质67.60 g·kg−1、交换性钙3.10 cmol·kg−1、交换性镁0.34 cmol·kg−1。每盆栽植1株,栽植后立即浇水,无性系植株按照完全随机配置,放置在铺设有地布的苗圃地内,株行距为1.5 m×1.5 m。苗木栽培常规管理。苗木培育期间,为保证生长条件和栽培管理一致,不施肥,干旱时滴灌,及时除草。
在处理前,对供试幼树的树高、地径、枝长、枝粗和轮枝数进行本底调查,树高为189.3~204.2 cm,地径为35.63~40.12 mm,枝长为45.5~96.5 cm,枝粗为17.89~21.68 mm,每株树均为3层轮枝。
-
设置未截顶、截顶后保留1层轮枝、截顶后保留2层轮枝、未截顶+100 mg·L−1赤霉素(GA4/7)、未截顶+200 mg·L−1 GA4/7和未截顶+400 mg·L−1 GA4/7共6个处理,分别记为NT、T1、T2、NT+G100、NT+G200和NT+G400。每个处理设置3个重复,每个重复3个分株。在2021年6月15日,T1和T2截顶处理采用修枝剪自下而上分别一次性截除第1层和第2层轮枝处以上的顶梢,NT+G100、NT+G200和NT+G400处理在植株叶面分别均匀喷施100、200和400 mg·L−1的GA4/7混合液,喷施至针叶湿润且叶尖有液体下滴时为止,NT处理以喷施去离子水作为对照。
-
各处理在每株自下而上的第1层轮枝处(H1)和第2层轮枝处(H2)选择东、西、南、北4个方位的一级侧枝及顶梢(H3)作为固定观测对象。分别于2021年花原基形成前期(S1,6月20日)、花原基形成期(S2,7月20日)和花原基形成后期(S3,8月20日)测定枝长和枝粗。同时,于S1、S2和S3时期,分别在H1处4个方位的一级侧枝顶端选取1.0 g以上针叶,置于液氮中速冻,带回实验室,保存于−80 ℃超低温冰箱中,用于内源激素质量分数测定,研究截顶和GA4/7诱导对花原基形成期前后的针叶主要激素质量分数及其比值影响。2022年4月25日调查每株观测枝的雌球花数量。
此外,还针对T1、T2和NT处理,分别在截顶处理(2021年6月15日)后1、4、7、10、13、16和19 d取样。每次取样选取第1层轮枝处(H1)、第2层轮枝处(H2)一级分枝顶端和顶梢处(H3)的针叶,每个部位取样1.0 g以上,带回实验室,保存于−80 ℃超低温冰箱中,研究截除顶梢后短期内内源激素质量分数在时间和空间上的变化特征。每个处理设置3个重复,每个重复3个分株。
-
委托南京瑞源生物技术有限公司采用安捷伦1290高效液相色谱仪串联Qtrap 6500质谱仪(AB公司)测定内源激素。测定的内源激素包括:吲哚乙酸(IAA)、脱落酸(ABA)、赤霉素(GA4和GA7)、水杨酸(SA)和玉米素核苷(ZR)。。
-
采用SPSS 20.0软件对不同截顶强度和赤霉素诱导处理的结果进行差异性分析。试验数据经Levene检验满足方差齐性后,采用单因素和Duncan差异性检验(P<0.05)进行方差分析和多重比较。图和表中数据均为平均值±标准误。
-
与S1时期相比,到S3时期时,T1和T2处理后H1处的枝长增长量分别比NT高181.55%和119.31% (P<0.05),枝粗增长量分别高出NT的35.78%和9.17% (P<0.05);H2处的枝长和枝粗增长量分别比NT高的150.45%和111.49% (P<0.05)。T1处理下的枝长和枝粗增长量略高于T2处理,但差异不显著(表1)。截顶后第2年,T1与T2处理的标准枝雌球花密度均显著高于NT,其中,T1处理H1处的雌球花密度较NT增加126.00%,T2处理H1和H2处的雌球花密度较NT分别增加82.67%和 66.52%。说明截顶削弱了顶端优势,促进下层结实母枝的生长和结实层下移,雌球花密度增加。
表 1 截顶和赤霉素诱导处理对雌球花密度和枝生长的影响
Table 1. Effect of top pruning and gibberellin induction on female cones density and branch growth
处理 H1 H2 H3 枝长净增
长量/cm枝粗净增
长量/cm雌球花密度/
(个·枝−1)枝长净增
长量/cm枝粗净增
长量/cm雌球花密度/
(个·枝−1)枝长净增
长量/cm枝粗净增
长量/cm雌球花密度/
(个·枝−1)NT 2.33±0.11 d 1.09±0.08 b 1.50±0.06 c 2.22±0.10 c 0.87±0.03 c 2.33±0.09 c 9.33±0.26 ab 1.66±0.09 a 2.94±0.13 b NT+G100 5.26±0.16 bc 1.03±0.06 b 2.93±0.12 ab 3.89±0.13 b 1.76±0.09 ab 3.17±0.15 ab 11.80±0.31 a 1.69±0.09 a 4.50±0.19 a NT+G200 8.42±0.25 a 1.18±0.09 ab 3.27±0.16 a 6.22±0.18 a 1.76±0.10 ab 3.39±0.16 ab 9.67±0.28 ab 1.06±0.04 ab 4.67±0.21 a NT+G400 6.14±0.17 b 1.56±0.11 a 2.67±0.10 ab 5.22±0.14 ab 2.01±0.12 a 3.33±0.16 ab 12.33±0.33 a 1.04±0.03 ab 3.33±0.15 b T1 6.56±0.18 b 1.48±0.09 a 3.39±0.16 a - - - - - - T2 5.11±0.14 bc 1.19±0.07 ab 2.74±0.11 ab 5.56±0.15 ab 1.84±0.11 ab 3.88±0.18 a - - - 说明:同列不同小写字母表示处理间差异显著(P<0.05);-表示无此项。 在S3时期,截顶与赤霉素处理相比,除T1和T2处理H1处的枝长增长量显著(P<0.05)低于NT+G200处理外,T1与T2处理的雌球花密度与NT+G100、NT+G200、NT+G400处理结果差异均不显著;T1和T2处理其他轮枝处的枝长和枝粗增长量与GA4/7各处理间差异不显著。说明截顶和赤霉素处理均促进了马尾松结实母枝生长和雌球花形成。
-
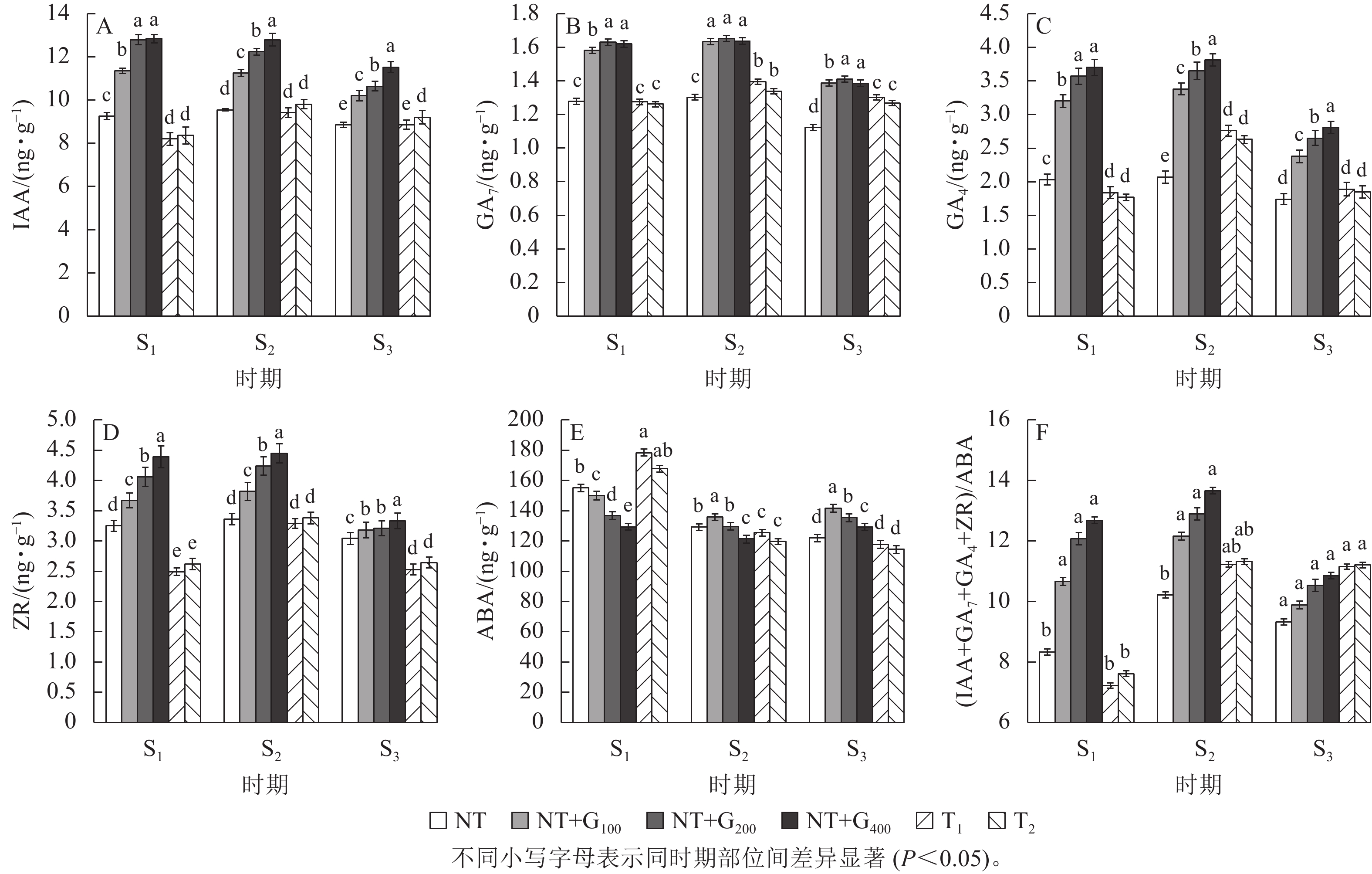
在S1时期,与NT相比,T1和T2处理的针叶IAA质量分数分别下降11.24%和9.62%,T2处理的IAA质量分数高于T1处理,但随着截顶程度的加重而降低(图1A);T1和T2处理的GA7质量分数分别下降0.27%和1.26% (图1B),GA4分别下降9.36%和12.62% (图1C),ZR分别下降23.38%和18.77% (图1D),而ABA分别增加15.09%和8.15%,T1处理的ABA质量分数高于T2处理(图1E),但T1和T2处理两者间差异不显著。

图 1 截顶及GA4/7诱导对不同时期主要激素质量分数及其比值变化的影响
Figure 1. Effect of top pruning and GA4/7 induction on changes in the content of major hormones and their ratios at different periods
在S2时期,无论截顶与否,针叶IAA、GA7、GA4和ZR质量分数均较S1时期增加,其中,T1处理的IAA、GA7、GA4和ZR质量分数分别增加了1.21、1.21、0.92和0.80 ng·g−1,T2处理分别增加了1.45、0.77、0.86和0.76 ng·g−1,显著高于NT处理的增加量(0.30、24.67、0.04和0.11 ng·g−1,P<0.05),截顶处理后ABA质量分数较S1时期降低,其中,T1和T2处理分别降低52.97和48.06 ng·g−1。说明受截顶影响,在之后1个月时间,IAA、GA7、GA4和ZR质量分数呈恢复增长变化,其质量分数在S2时期并未受截顶强度加重显著降低。
在S3时期,与S2时期相比,T1和T2处理下针叶IAA、GA7、GA4和ZR质量分数下降,其中,T1处理分别下降6.32%、7.21%、46.03%和30.04%,T2处理分别下降6.52%、5.52%、42.16%和28.03%;与S1时期相比,T1处理的针叶IAA、GA7、GA4和ZR质量分数分别增加7.34%、2.08%、2.65%和1.58%,T2处理分别增加9.30%、0.55%、4.32%和0.76%;而T1和T2处理的针叶ABA质量分数较S1和S2时期持续降低。
由图1F看出:在S1时期,T1和T2处理的(IAA+GA7+GA4+ZR)/ABA比值较低,分别为7.22和7.61,均低于NT (8.33),而在S2时期,T1和T2处理的(IAA+GA7+GA4+ZR)/ABA比值迅速增加,分别为11.32和11.23,均高于NT (10.21),进一步印证了在花原基形成前期实施截顶,内源激素的比值显著下降,在花原基形成期间,生长促进型激素恢复增长,抑制型激素下降,内源激素的比值显著增加。
-
由图1A~D可知:在S1时期,T1和T2处理的针叶IAA、GA7、GA4和ZR质量分数显著低于NT+G100、NT+G200和NT+G400处理,而ABA质量分数显著增加 (P<0.05),其中,T1处理下的IAA、GA7、GA4和ZR质量分数分别比GA4/7处理低38.25%~56.39%、24.05%~27.06%、73.91%~101.09%和47.39%~76.31%,ABA质量分数比GA4/7处理高15.92%~27.52%;T2处理下的IAA、GA7、GA4和ZR质量分数分别比GA4/7处理低35.77%~53.59%、25.30%~29.17%、80.79%~109.04%和40.08%~67.56%,ABA质量分数则比GA4/7处理高10.53%~22.88%。
在S1~S3期间,NT+G100、NT+G200和NT+G400处理的IAA质量分数逐渐降低,马尾松针叶GA7、GA4和ZR质量分数均先增加后降低,S3时期的激素质量分数低于S1时期,ABA则先降低后增加;截顶与GA4/7诱导后主要激素质量分数的变化趋势不同,T1和T2处理的马尾松针叶IAA、GA7、GA4和ZR质量分数均为先增加后降低,但是,S3时期的激素高于S1时期,ABA则为持续降低。从图1F可看出:在S2时期,NT+G400处理的(IAA+GA7+GA4+ZR)/ABA比值最高,比NT+G200、NT+G100、T2和T1处理依次高5.97%、12.34%、20.67%和21.64%。
-
由图2A可知:在花原基形成前期截除顶梢,T1和T2处理的马尾松针叶IAA质量分数呈先降低后增加的趋势,到第16天时比NT高33.33%~45.45%。截顶后针叶的GA7质量分数趋势变化不显著,T1处理和T2处理的针叶GA7质量分数也均无显著性差异(图2B)。截顶后马尾松针叶的GA4质量分数呈显著下降 (P<0.05),在第10天达到最低,并比NT处理显著低24.26%~35.32% (P<0.05),之后逐渐增加(图2C)。T1和T2处理的马尾松针叶ZR质量分数均比NT处理显著低4.39%~57.65% (P<0.05),而T1和T2处理间差异不显著(图2D)。说明截顶处理打破了马尾松原有的激素平衡,使针叶中的IAA和GA4生长促进型激素出现短期内先下降后升高的现象。T1和T2处理的ABA质量分数呈降低趋势,但始终高于NT处理(图2E)。在第1~4天时,T1和T2处理马尾松针叶的SA质量分数急剧上升,之后逐渐下降,至第16天时,与NT处理差异不显著(图2F)。
-
在不同高度处,未截顶的马尾松针叶IAA质量分数从高到低依次为H3、H2、H1,T2处理IAA质量分数为H2大于H1;在第10天之后,T1处理H1处的针叶IAA质量分数显著高于T2处理(图2A,P<0.05)。在第13天之后,未截顶H3处的马尾松针叶GA7质量分数与H2和H1处相比差异不显著(图2B),T2处理H1处针叶GA7质量分数和H1处差异不显著,未截顶的针叶GA4质量分数从高到低依次为 H3、H2、H1,T2处理GA4质量分数为H2大于H1,T1处理H1处针叶的GA4质量分数在截顶后第10天低于T2处理,之后逐渐高于T2处理(图2C)。未截顶的ZR质量分数从高到低依次为 H3、H2、H1,T2处理针叶的ZR质量分数在H2处最高,T1处理H1处的ZR质量分数始终最低(图2D)。说明截顶强度影响着不同轮枝处针叶的IAA、GA4和ZR激素质量分数。未截顶的ABA质量分数从高到低依次为H1、H2、H3,T2处理ABA质量分数为H1大于H2,T1处理H1处的针叶ABA质量分数比T2处理高2.85%~7.84% (图2E)。未截顶时,SA质量分数在H1、H2和H3处差异不显著,随截顶程度的加重,在第1~13天,T1处理H1处的SA质量分数比T2处理H1处显著高1.88%~90.88% (P<0.05,图2F)。
-
已有研究表明:通过截顶可以增加母树对光能的有效利用,影响叶面积与营养储藏水平,也对侧枝的生长具有重要影响[3, 21]。本研究发现:马尾松树体截顶后不仅促进了枝梢的生长,而且显著增加了雌球花密度。这与KOLPAK等[4]在夏季对花旗松进行截顶和修枝的研究结果相类似。截顶和修枝后花旗松枝条上的雌雄球花量增加,提高了单位面积球果产量。王福森等[2]研究也表明:樟子松截顶后促使母树结实层下移,平均单株球果数增加。截顶处理可以控制树体顶端优势,促使结实母枝更新。通过与赤霉素处理对比发现:截顶处理的雌球花密度与其差异不显著,说明截顶不仅可作为种子园树体管理的有效措施,而且也可促进雌球花形成。郑一等[1]研究马尾松不同结实能力无性系同样发现:截顶可提高中产和高产无性系的雌球花量20%以上。陈虎等[22]对16年生马尾松无性系截顶处理发现:随着截顶强度的增加,母树生长和结实能力增强,在保留1轮枝的情况下结实最多;HAN等[23]对红松P. koraiensis截干后同样得出结果枝数量增加,结实量提高的结果。因此,截顶处理可以控制马尾松树体顶端优势,促使结实母枝更新。植物激素,尤其赤霉素通过其自身前馈和反馈调节参与松树花器官发育并在调节植株生长、控制树势或适应逆境过程中发挥重要作用[7, 24]。马尾松顶端优势强,雌球花多分布在主枝或侧枝的顶端,植株顶芽会抑制侧芽的发生,去除顶芽或抑制顶端优势则会促进侧芽的产生。已有研究表明:树木木质部汁液中细胞分裂素和ABA质量分数与比值在胁迫信号传递中起着重要作用,较高质量分数的Z型细胞分裂素和较低水平的ABA及其代谢产物可能与雌球花的形成有关[25−26]。通过分析花原基形成期间3个阶段的主要激素质量分数与标准枝雌球花量相关性,均发现在花原基形成期,截顶处理后GA4、IAA、GA7和ZR质量分数与雌球花量相关性最高,与ABA质量分数呈显著负相关。研究表明:未截顶时,不同高度处的IAA、GAs和ZR质量分数从高到低依次为H3、H2、H1。在花原基形成前期截顶,受胁迫的影响,促进生长型激素IAA和GA4质量分数呈先降低后增加的动态变化,T2处理依然表现为H2大于H1,截顶后20 d左右,主要激素水平可恢复稳定;在花原基形成期,下部枝条的IAA、GAs和ZR质量分数较花原基形成前期显著增加,(IAA+GA+ZR)/ABA比值大幅提升,说明截顶削弱了顶端优势,促使下部侧枝的激素质量分数在空间上随高度的降低和截顶程度的加重而变化,打破了树体原有的养分平衡,可能通过内源激素的合成、极性运输和信号转导等途径,调节着营养生长与生殖生长之间的平衡。这可能是促进侧芽更快地生长和雌球花形成的主要原因之一[9, 20, 27]。
在顶芽发育过程中,GA和ABA往往表现出拮抗作用,外源注射GA4/7使顶梢ABA合成减少或通过其他途径加速了ABA代谢产物的分解,进而降低顶芽内源ABA及ABA分解代谢的主导产物ABA葡萄糖酯(ABA-ge)的质量分数。GA4/7的使用也可增加反式玉米素核苷(t-ZR)水平和降低异戊烯基腺嘌呤(IP型)细胞分裂素,改变了它们的比值,进而提高雌球花的分化能力,增加雌球花的数量[28−30]。本研究通过截顶处理和GA4/7诱导的对比试验同样得出:在花原基形成期,截顶和GA4/7诱导处理可显著降低ABA质量分数,增加ZR质量分数。国内外育种工作者已经利用GA4、GA7等极性较小的赤霉素可促进多种松树开花和诱导雌雄球花分化的能力,在生产上积累了丰富的经验[31−32]。虽然截顶处理与赤霉素诱导马尾松针叶的内源激素质量分数变化趋势有所不同,但均表明通过树体内主要激素质量分数及其比值平衡的变化,可以调控树体的生长发育,进而指导生产。此外,持续截顶或修枝措施,也是控制顶端优势的方式,对激素变化也有影响。但NEILSEN等[32]对辐射松修枝研究发现:持续修剪对枝条生长有长期影响,造成枝粗增长缓慢。
-
在花原基形成前期实施截顶和赤霉素处理均可促进马尾松结实母枝更新和雌球花形成,与针叶内源激素质量分数的变化密切相关。截除顶梢后,马尾松针叶的IAA、GA7、GA4和ZR质量分数显著下降,而ABA质量分数显著增加。截顶影响着内源激素重新分配,(IAA+GA7+GA4+ZR)/ABA比值在花原基形成期显著升高。截顶与GA4/7诱导后主要激素质量分数的变化趋势不同,在S1至S3时期,赤霉素诱导后的IAA质量分数逐渐降低,GA7、GA4和ZR质量分数先增加后降低,ABA质量分数则先降低后增加。研究结果表明:生产上通过持续截顶,优化截干技术和控制树势,配合激素诱导,可促进结实母树更新和调控雌球花形成。
Effects of top pruning and exogenous hormone application on endogenous hormone content and female bulb formation in Pinus massoniana
-
摘要:
目的 探究截除顶梢及植物生长调节剂诱导后马尾松Pinus massoniana内源激素质量分数变化对新枝生长与雌球花形成的影响。 方法 采用盆栽控制试验,以3年生209号马尾松无性系为试材,设置保留1层轮枝(T1)、保留2层轮枝(T2)、未截顶(NT)、未截顶+100 mg·L−1赤霉素 (GA4/7) (NT+G100)、未截顶+200 mg·L−1GA4/7(NT+G200)和未截顶+400 mg·L−1GA4/7 (NT+G400) 等6个处理,测定花原基形成前期(S1)、花原基形成期(S2)和花原基形成后期(S3)的针叶内源激素质量分数及其比值的变化,研究各处理对雌球花密度和枝生长的影响。 结果 与NT相比,T1处理的雌球花密度、枝长和枝粗分别增加126.00%、181.55%和35.78%,T2处分别理增加66.52%~82.67%、119.31%~150.45%和9.17%~111.49%;与GA4/7各处理相比,截顶处理后,除第1层轮枝处的枝长增长量显著(P<0.05)低于NT+G200处理外,T1和T2处理的雌球花密度、枝长与枝粗增长量与GA4/7其他处理间差异不显著。在S1时期,与NT相比, T1和T2处理的针叶吲哚乙酸(IAA)质量分数分别显著(P<0.05)下降11.24%和9.62%,脱落酸(ABA)质量分数显著(P<0.05)增加15.09%和8.15%,GA7、GA4、玉米素核苷(ZR)质量分数下降,但差异不显著,(IAA+GA7+GA4+ZR)/ABA比值分别为7.22和7.61;在S2时期,T1和T2处理下的针叶IAA、GA7、GA4和ZR质量分数均较S1时期增加,ABA质量分数降低,(IAA+GA7+GA4+ZR)/ABA比值升高;在S3时期,所测激素质量分数均较S2时期降低。截顶与GA4/7诱导后主要激素质量分数的变化趋势不同。在S1至S3时期,GA4/7诱导后的IAA质量分数逐渐降低,GA7、GA4和ZR质量分数先增加后降低,ABA质量分数则先降低后增加。在截顶后20 d内,IAA、GA7、GA4和ZR质量分数呈先降低后增加的恢复特征,ABA质量分数呈持续下降的动态变化,截顶强度影响着不同轮枝处针叶IAA、GA4、ABA和ZR激素质量分数的变化。 结论 在花原基形成前期实施截顶和GA4/7处理均可促进马尾松结实母枝更新和雌球花形成,与针叶内源激素质量分数的变化密切相关。图2表1参32 Abstract:Objective This study is to investigate the effects of the change in endogenous hormone content on the growth of new branches and formation of female cones in Pinus massoniana after top pruning and hormone induction. Method A pot control experiment was conducted using a 3-year-old clone 209 of P. massoniana as the test material. Six treatments were set up, including retaining one layer of branches (T1), retaining two layers of branches (T2), no top pruning (NT), no top pruning + 100 mg·L−1 GA4/7 (NT+G100), no top pruning + 200 mg·L−1 GA4/7 (NT+G200) and no top pruning + 400 mg·L−1 GA4/7 (NT+G400) to measure the changes in the content and ratio of endogenous hormones in conifers at the early stage of flower primordium formation (S1), the stage of flower primordium formation (S2) and the late stage of flower primordium formation (S3). The effects on female cone density and branch growth were studied. Result Compared with NT, the female cone density, branch length and branch diameter of T1 treatment increased by 126.00%, 181.55% and 35.78%, respectively, while those of T2 treatment increased by 66.52%−82.67%, 119.31%−150.45% and 9.17%−111.49%, respectively. Compared with GA4/7 treatments, there was no significant difference in the growth of female cone density, branch length and branch diameter between T1 and T2 treatments and other treatments with GA4/7 after top pruning, except that the growth of branch length at the first layer was significantly lower than that of NT+G200 treatment. In S1 period, compared with NT, the content of indoleacetic acid (IAA) in the needles of T1 and T2 treatments decreased significantly by 11.24% and 9.62% (P<0.05), the content of abscisic acid (ABA) increased significantly by 15.09% and 8.15% (P<0.05), and the content of GA7, GA4 and zeatin nucleoside (ZR) in the needles decreased significantly, but the difference was not significant, with (IAA+GA7+GA4+ZR)/ABA ratios of 7.22 and 7.61 respectively. At S2 stage, the contents of IAA, GA7, GA4 and ZR in needles treated with T1 and T2 increased compared with that in S1 stage, while the content of ABA decreased, and the ratio of (IAA+GA7+GA4+ZR)/ABA increased. At S3 stage, the measured hormone content was lower than that in S2 stage. The change trend of the main hormone content after top pruning and GA4/7 induction was different. From S1 to S3, IAA content gradually decreased after GA4/7 induction, GA7, GA4 and ZR content first increased and then decreased, and ABA content first decreased and then increased. Within 20 days after top pruning, IAA, GA7, GA4 and ZR contents decreased first and then increased, while the ABA content decreased continuously. The intensity of top pruning affected the changes of IAA, GA4, ABA and ZR hormone contents in needles at different whorls. Conclusion Both top pruning and GA4/7 treatment at the early stage of flower primordium formation can promote the regeneration of fruiting mother branches and the formation of female cones in P. massoniana, which is closely related to the change of endogenous hormone content in needles. [Ch, 2 fig. 1 tab. 32 ref.] -
Key words:
- top pruning /
- Pinus massoniana /
- female cones /
- endogenous hormones /
- floral primordium formation
-
表 1 截顶和赤霉素诱导处理对雌球花密度和枝生长的影响
Table 1. Effect of top pruning and gibberellin induction on female cones density and branch growth
处理 H1 H2 H3 枝长净增
长量/cm枝粗净增
长量/cm雌球花密度/
(个·枝−1)枝长净增
长量/cm枝粗净增
长量/cm雌球花密度/
(个·枝−1)枝长净增
长量/cm枝粗净增
长量/cm雌球花密度/
(个·枝−1)NT 2.33±0.11 d 1.09±0.08 b 1.50±0.06 c 2.22±0.10 c 0.87±0.03 c 2.33±0.09 c 9.33±0.26 ab 1.66±0.09 a 2.94±0.13 b NT+G100 5.26±0.16 bc 1.03±0.06 b 2.93±0.12 ab 3.89±0.13 b 1.76±0.09 ab 3.17±0.15 ab 11.80±0.31 a 1.69±0.09 a 4.50±0.19 a NT+G200 8.42±0.25 a 1.18±0.09 ab 3.27±0.16 a 6.22±0.18 a 1.76±0.10 ab 3.39±0.16 ab 9.67±0.28 ab 1.06±0.04 ab 4.67±0.21 a NT+G400 6.14±0.17 b 1.56±0.11 a 2.67±0.10 ab 5.22±0.14 ab 2.01±0.12 a 3.33±0.16 ab 12.33±0.33 a 1.04±0.03 ab 3.33±0.15 b T1 6.56±0.18 b 1.48±0.09 a 3.39±0.16 a - - - - - - T2 5.11±0.14 bc 1.19±0.07 ab 2.74±0.11 ab 5.56±0.15 ab 1.84±0.11 ab 3.88±0.18 a - - - 说明:同列不同小写字母表示处理间差异显著(P<0.05);-表示无此项。 -
[1] 郑一, 张含国, 张振, 等. 矮化对马尾松雌球花发生及枝梢生长的影响[J]. 东北林业大学学报, 2021, 49(10): 1 − 5. ZHENG Yi, ZHANG Hanguo, ZHANG Zhen, et al. Effect of dwarfing on occurrence of female strobilus of Pinus massoniana and its effect on branch growth [J]. Journal of Northeast Forestry University, 2021, 49(10): 1 − 5. [2] 王福森, 李树森, 李晶, 等. 樟子松无性系种子园矮化处理对结实及种子品质的影响[J]. 东北林业大学学报, 2017, 45(5): 26 − 28. WANG Fusen, LI Shusen, LI Jing, et al. Effects of dwarfing treatment of clonal seed orchard on seed and seed quality of Pinus sylvestris var. mongolica [J]. Journal of Northeast Forestry University, 2017, 45(5): 26 − 28. [3] 阎雄飞, 曹存宏, 袁小琴, 等. 截冠处理对种子园樟子松壮龄母树结实的影响[J]. 北京林业大学学报, 2019, 41(8): 48 − 56. YAN Xiongfei, CAO Cunhong, YUAN Xiaoqin, et al. Effects of top pruning on seed setting of aged mother trees in orchard of Pinus sylvestris var. mongolica [J]. Journal of Beijing Forestry University, 2019, 41(8): 48 − 56. [4] KOLPAK S E, SMITH J, ALBRECHT M J, et al. High-density miniaturized seed orchards of Douglas-fir [J]. New Forests, 2015, 46: 121 − 140. [5] 杨凯, 谷会岩. 红松果林从幼龄到开花阶段林木体内激素动态变化[J]. 林业科学, 2005, 41(5): 33 − 37. YANG Kai, GU Huiyan. Dynamic changes of hormone in the plants from teneral stage to hossomphase Pinus koraiensis fruit forests [J]. Scientia Silvae Sinicae, 2005, 41(5): 33 − 37. [6] 杨钧贺, 刘畅, 钮世辉, 等. 茎部形成层赤霉素在植物生长发育中的调控作用[J]. 北京林业大学学报, 2019, 41(7): 68 − 74. YANG Junhe, LIU Chang, NIU Shihui, et al. Regulatory effect of stem cambium gibberellin on plant growth and development [J]. Journal of Beijing Forestry University, 2019, 41(7): 68 − 74. [7] FERNÁNDEZ H, FRAGA M F, BERNARD P, et al. Quantification of GA1, GA3, GA4, GA7, GA9, and GA20 in vegetative and male cone buds from juvenile and mature trees of Pinus radiata [J]. Plant Growth Regulation, 2004, 40: 185 − 188. [8] NAMBARA E, MARION-POLL A. Abscisic acid biosynthesis and catabolism [J]. Annual Review of Plant Biology, 2005, 56: 165 − 185. [9] KONG Lisheng, PATRICK V D, ZAHARIA I. Effects of stem-injected gibberellins and 6-benzylaminopurine on phytohormone profiles and cone yield in two lodgepole pine genotypes [J]. Trees, 2018, 32(3): 765 − 775. [10] 李洪娜, 许海港, 任饴华, 等. 不同施氮水平对矮化富士苹果幼树生长、氮素利用及内源激素含量的影响[J]. 植物营养与肥料学报, 2015, 21(5): 1304 − 1311. LI Hongna, XU Haigang, REN Yihua, et al. Effect of different N application rates on plant growth, 15N-urea utilization and hormone content of dwarf apple trees [J]. Journal of Plant Nutrition and Fertilizers, 2015, 21(5): 1304 − 1311. [11] THOMAS L D, DAVID W P, STEWART B R. Correlation of endogenous gibberellin acid with initiation of mango shoot growth [J]. Journal of Plant Growth Regulation, 2001, 20: 308 − 315. [12] WEI Chunhua, ZHU Chunyu, YANG Liping, et al. A point mutation resulting in a 13 bp deletion in the coding sequence of Cldf leads to a GA-deficient dwarf phenotype in watermelon [J/OL]. Horticulture Research, 2019, 6: 132[2022-11-14]. doi: 10.1038/s41438-019-0213-8. [13] 殷东生, 吴海波, 张建瑛, 等. 环剥和去叶对红松雌球果和枝生长及不同组织和器官中养分含量的影响[J]. 应用生态学报, 2019, 30(11): 3671 − 3680. YIN Dongsheng, WU Haibo, ZHANG Jianying, et al. Effects of girdling and defoliation on the growth of female cones and branches and nutrient content in different tissues and organs of Pinus koraiensis [J]. Chinese Journal of Applied Ecology, 2019, 30(11): 3671 − 3680. [14] 季孔庶, 徐立安, 王登宝, 等. 中国马尾松遗传改良研究历程与成就[J]. 南京林业大学学报(自然科学版), 2022, 46(6): 10 − 22. JI Kongshu, XU Li’an, WANG Dengbao, et al. Progresses and achievements of genetic improvement on masson pine (Pinus massoniana) in China [J]. Journal of Nanjing Forestry University (Natural Sciences Edition), 2022, 46(6): 10 − 22. [15] 杨章旗. 马尾松不同年龄产脂量及松香组分分析[J]. 林业科学, 2014, 50(6): 147 − 151. YANG Zhangqi. Comparative study on the resin yield and rosin components of Pinus massoniana superior provenances among different ages [J]. Scientia Silvae Sinicae, 2014, 50(6): 147 − 151. [16] 谭小梅, 金国庆, 张一, 等. 截干矮化马尾松二代无性系种子园开花结实的遗传变异[J]. 东北林业大学学报, 2011, 39(4): 39 − 42. TAN Xiaomei, JIN Guoqing, ZHANG Yi, et al. Genetic variation of flowering and fruiting in dwarfed second-generation clonal seed orchard of Pinus massoniana [J]. Journal of Northeast Forestry University, 2011, 39(4): 39 − 42. [17] CASTILLO-LLANQUE F, RAPOPORT H F. Relationship between reproductive behavior and new shoot development in 5-year-old branches of olive trees (Olea europaea L. ) [J]. Trees, 2011, 25: 823 − 832. [18] 闫师杰, 郭李维, 吴彩娥, 等. 高效液相色谱法同时测定鸭梨种子中3种内源激素[J]. 分析化学, 2010, 38(6): 843 − 847. YAN Shijie, GUO Liwei, WU Cai’e, et al. Simultaneous determination of three kinds of endogenous hormones content in seeds of post-harvest yali pear by high performance liquid chromatography [J]. Chinesese Journal of Analytical Chemistry, 2010, 38(6): 843 − 847. [19] 王辉, 顾文亮, 庄辉发, 等. 不同外源激素对香草兰花芽分化的影响[J]. 热带农业科学, 2016, 36(11): 72 − 76. WANG Hui, GU Wenliang, ZHUANG Huifa, et al. Effects of plant external hormones on flower bud differentiation of vanilla [J]. Chinese Journal of Tropical Agriculture, 2016, 36(11): 72 − 76. [20] 邵苗苗, 朱亚艳, 秦雪, 等. 不同激素处理对马尾松花芽分化过程中营养物质含量的影响[J]. 陕西林业科技, 2014(1): 10 − 13, 17. SHAO Miaomiao, ZHU Yayan, QIN Xue, et al. Effect of different hormone treatments on nutrients concentration during flower budding period of Pinus massoniana [J]. Shaanxi Forest Science and Technology, 2014(1): 10 − 13, 17. [21] LANGE P W, RONDE C E, BREDENKAMP B V. The effects of different intensities of pruning on the growth of Pinus radiata in south Africa [J]. South African Forestry Journal, 1987, 143: 30 − 36. [22] 陈虎, 张明慧, 卢开成, 等. 修剪强度对马尾松成年种子园母树的影响[J]. 福建林业科技, 2017, 44(1): 38 − 42. CHEN Hu, ZHANG Minghui, LU Kaicheng, et al. Effect of different pruning intensity in the seed orchard of Pinus massoniana L. trees [J]. Journal of Fujian Forestry Science and Technology, 2017, 44(1): 38 − 42. [23] HAN S U, KANG K S, KIM C S, et al. Effect of top-pruning in a clonal seed orchard of Pinus koraiensis [J]. Annals of Forest Research, 2008, 51: 155 − 156. [24] 曲波, 张微, 陈旭辉, 等. 植物花芽分化研究进展[J]. 中国农学通报, 2010, 26(24): 109 − 114. QU Bo, ZHANG Wei, CHEN Xuhui, et al. Research progress of flower bud differentiation mechanism of plant [J]. Chinese Agricultural Science Bulletin, 2010, 26(24): 109 − 114. [25] MEIJÓN M, CAÑAL M J, FERNÁNDEZ H, et al. Hormonal profile in vegetative and floral buds of azalea: levels of polyamines, gibberellins, and cytokinins [J]. Journal of Plant Growth Regulation, 2010, 30: 74 − 82. [26] ALVAREZ S, MARSH E L, SCHROEDER S G, et al. Metabolomic and proteomic changes in the xylem sap of maize under drought [J]. Plant Cell and Environment, 2008, 31(3): 325 − 340. [27] SCHACHTMAN D P, GOODGER J Q. Chemical root to shoot signaling under drought [J]. Trends in Plant Science, 2008, 13(6): 281 − 287. [28] KONG Lisheng, von ADERKAS P, OWEN S J, et al. Comparison of endogenous cytokinins, ABA and metabolites during female cone differentiation in low and high cone-producing genotypes of lodgepole pine [J]. Trees, 2011, 25: 1103 − 1110. [29] KONG Lisheng, von ADERKAS P, ZAHARIA I, et al. Analysis of phytohormone profiles during male and female cone initiation and early differentiation in long-shoot buds of lodgepole pine [J]. Journal of Plant Growth Regulation, 2012, 31: 478 − 489. [30] SHEARER R C, STOEHR M U, WEBBE J E, et al. Seed cone production enhanced by injecting 38-year-old Larix occidentalis Nutt with GA4/7 [J]. New Forests, 1999, 18(3): 289 − 300. [31] ALMQVIST C. Timing of GA4/7 application and the flowering of Pinus sylvestris grafts in the greenhouse [J]. Tree Physiology, 2003, 23(6): 413 − 418. [32] NEILSEN W A, PINKARD E A. Effects of green pruning on growth of Pinus radiata [J]. Canadian Journal of Forest Research, 2003, 33(11): 2067 − 2073. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20220768






 下载:
下载: