-
土地盐碱化增大了土壤渗透压,导致植物吸水困难,对植物造成了生理干旱[1]。过多钠离子(Na+)、氯离子(Cl−)的积累,导致膜结构破坏,对植物造成渗透胁迫[2−3]。盐胁迫还导致植物内源活性氧(ROS)增加,引起细胞膜损伤甚至细胞死亡,抑制植株生长发育[4]。ROS作为响应盐胁迫的关键因子,在低水平下,诱导增强抗氧化酶活性,抵御盐胁迫;在高水平下,过量积累造成氧化胁迫,导致生物大分子产生不可逆的损伤,改变细胞形态结构,抑制植株生长发育[5]。为缓解ROS积累引起的氧化胁迫,植物通过增强抗氧化酶系统相关酶活性来降低体内的ROS,从而提高抗逆性[6]。植物的抗氧化酶系统主要包括超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、抗坏血酸过氧化物酶(APX)、过氧化物酶(PRX)等[7]。
在小麦Triticum aestivum中过表达TaPRX-2A,植株抗氧化能力增强,ROS下降,耐盐性增加[8]。拟南芥Arabidopsis thaliana AtPRX19参与胁迫(盐害、干旱、病虫害等)后的氧化应激反应,使得ROS增加,对植物造成氧化胁迫。盐胁迫下,拟南芥AtPRX19表达量上调,ROS减少,抵御胁迫能力增强[9]。在胡萝卜Daucus carota中异源表达OsPRX114同样降低过氧化氢(H2O2)水平,提高植株的耐盐性[10]。玉米Zea mays的PRX家族成员ZmPRX26、ZmPRX42、ZmPRX71、ZmPRX75和ZmPRX78参与了对包括盐胁迫在内多种非生物胁迫的响应[11]。部分PRX家族成员通过协调水杨酸(SA)、茉莉酸(JA)和乙烯(ET)等激素水平发挥作用[12]。
研究林木对盐胁迫的响应,揭示耐盐性相关机理,对培育耐盐性更强林木品种具有重要意义。为研究杨树Populus PRX家族对林木耐盐性的影响,本研究以银腺杨‘84K’ Populus alba × P. glandulosa ‘84K’ (84K杨)为材料,通过构建PagPRX19的过表达转基因株系,改变杨树H2O2水平,并分析了杨树耐盐相关生理指标,以期揭示PagPRX19参与调控杨树盐胁迫响应的机制,为杨树的分子育种提供理论依据。
-
研究材料为84K杨,过表达PagPRX19株系为本研究获得。
-
Phytozome v13数据库中获取毛果杨Populus trichocarpa和拟南芥的PRX家族的蛋白序列、蛋白编码区(CDS)序列、启动子序列。利用P1ant CARE 在线软件对PRX家族启动子顺式作用元件预测。使用MEGA v7软件,构建拟南芥和杨树的PRX家族的系统发育树,分析杨树和拟南芥PRX基因家族成员在进化关系上的同源性。利用MEGE v5.4.1 在线软件分析PRX家族基因保守结构域。
-
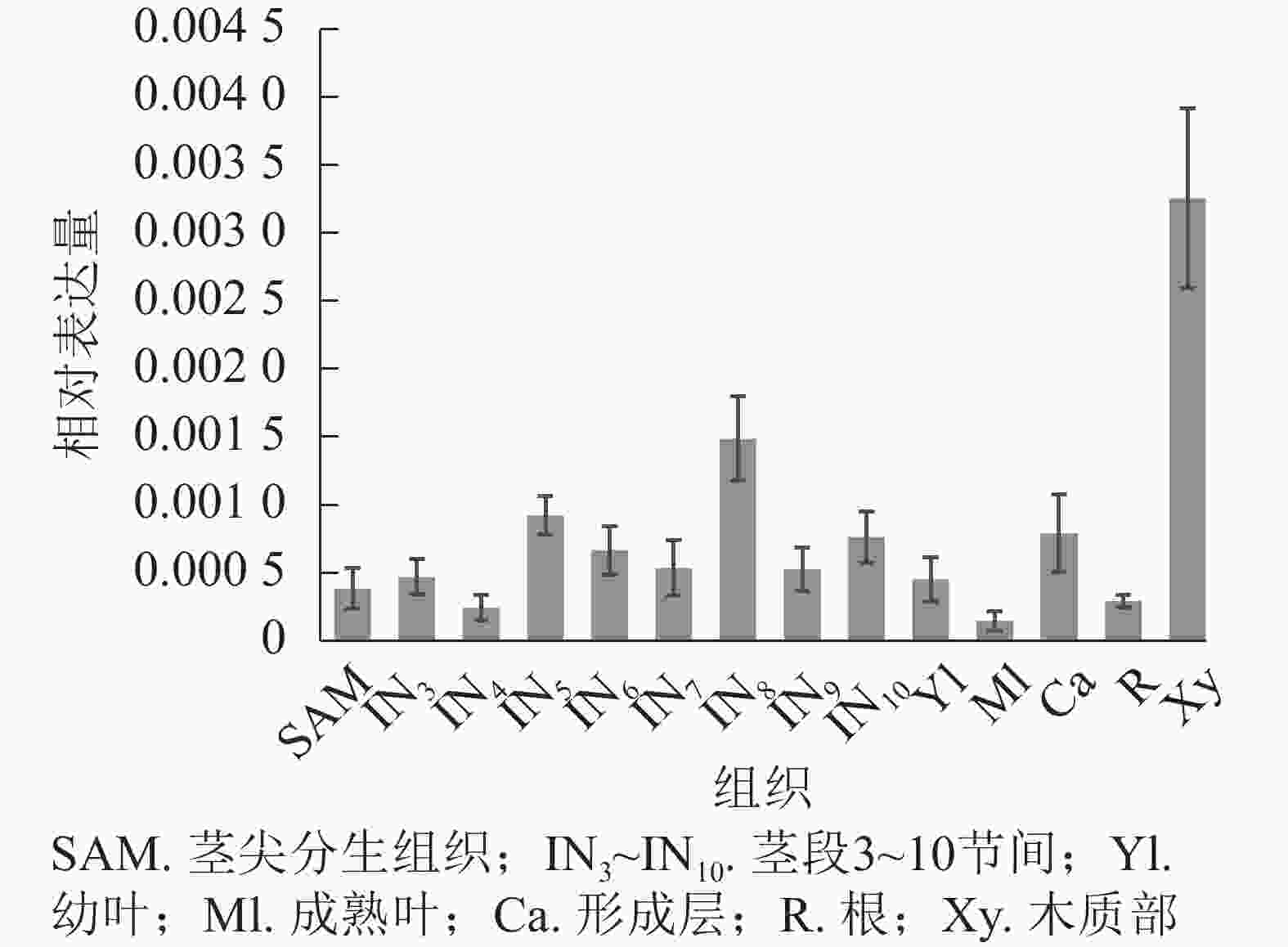
选用生长一致的84K杨树苗,分别从茎尖(SAM)、茎段3~10节间(IN3~IN10)、形成层(Ca)、幼叶(YL)、成熟叶(ML)、根(R)、木质部(Xy)取样,所有材料取3个生物学重复。采用实时荧光定量PCR (RT-qPCR)检测该基因在不同组织内的表达模式。
-
在84K杨基因组数据库中,通过Blast获取毛果杨PRX19同源基因PagPRX19,设计引物扩增获得目的基因(F端引物:ATGTATACAACAATCATGCCT,R端引物:TTATGTATGCATACTGCAAAC),使用Gateway技术构建过表达载体35S::PagPRX19,利用叶盘转化法转化84K杨获得过表达PagPRX19转基因苗[13]。
-
①组培盐胁迫处理。以组培培养45 d的过表达株系为材料,以非转基因植株为对照。盐胁迫组接顶芽于100 mmol·L−1氯化钠(NaCl)生根培养基,对照组接顶芽于0 mmol·L−1NaCl生根培养基。每组处理12瓶,每瓶接2株顶芽。培养温度为23~25 ℃,光周期为8 h/16 h(黑暗/光照)。处理25 d,观察植株在长期盐胁迫下耐盐能力。②土培盐胁迫处理。以土培生长2个月的过表达株系为实验材料,以非转基因植株为对照,每组处理5株。盐胁迫组隔2 d浇灌1次100 mmol·L−1的NaCl溶液,对照组隔2 d浇灌1次0 mmol·L−1的NaCl溶液。培养温度为23~25 ℃,光周期为8 h/16 h(黑暗/光照)。处理7 d,取样测定各项生理指标,处理12 d后拍照[14]。
-
①脯氨酸质量分数测定。采用脯氨酸测试盒(南京建成,A107-1-1)测定脯氨酸质量分数。②相对含水量测定。取新鲜叶片记录鲜质量(WF),超纯水(ddH2O)浸泡24 h记录质量(WT),65 ℃烘箱干燥3 d,记录干质量(WD)。叶片相对含水量=(WF−WD)/(WT−WD)×100%[15]。③丙二醛(MDA)质量摩尔浓度测定。称取植物叶片0.2 g,加入4 mL质量浓度为10%的三氯乙酸(TCA)溶液和石英砂研磨至匀浆,4 000 r·min−1离心10 min,取上清液2 mL,加入2 mL硫代巴比妥酸(TAB)溶液,对照为2 mL ddH2O。摇匀后沸水浴15 min,−20 ℃迅速冷却后4 000 r·min−1离心2 min,取上清液,在532和600 nm波长下测量吸光度。MDA浓度CMDA(μmol·L−1)=[D(532)−D(600)]/155×1 000,MDA质量摩尔浓度(nmol·g−1)=(CMDA×V提取液体积)/F样品质量[16]。④电解质渗透率测定。采用五点取样法取样,加入6 mL ddH2O,28 ℃摇床震荡1 h。取出测第1次电导值(G1)。沸水浴30 min,冷却至室温,摇匀测量第2次电导值(G2)。电解质渗透率=第1次电导值/第2次电导值×100%[17]。⑤ROS水平定性测量。DAB染色剂试剂盒(索莱宝,DA1010)染色检测H2O2;氯化硝基四氮唑蓝(NBT)粉末(索莱宝,N8140)染色检测超氧阴离子(O2 .−)。
-
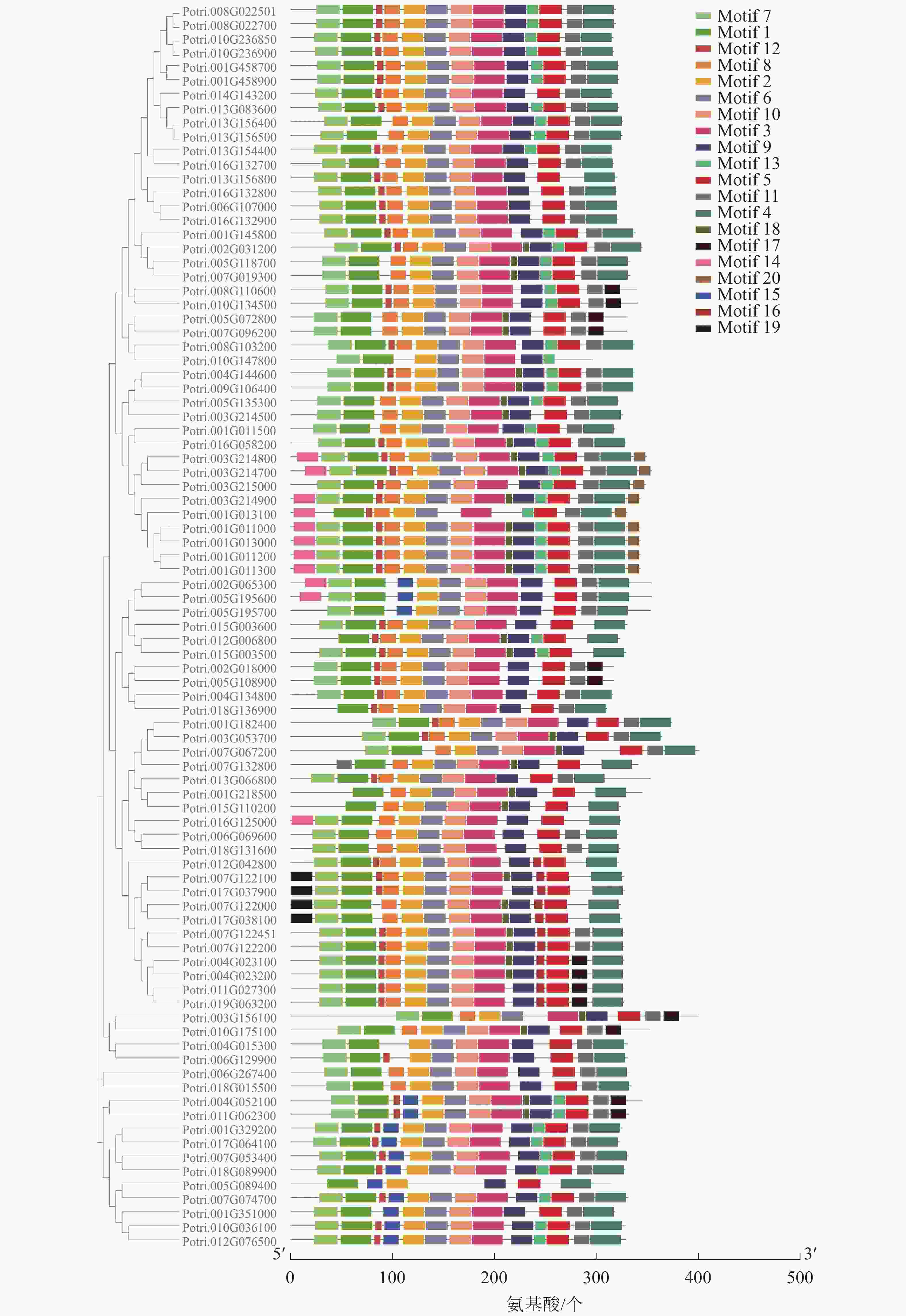
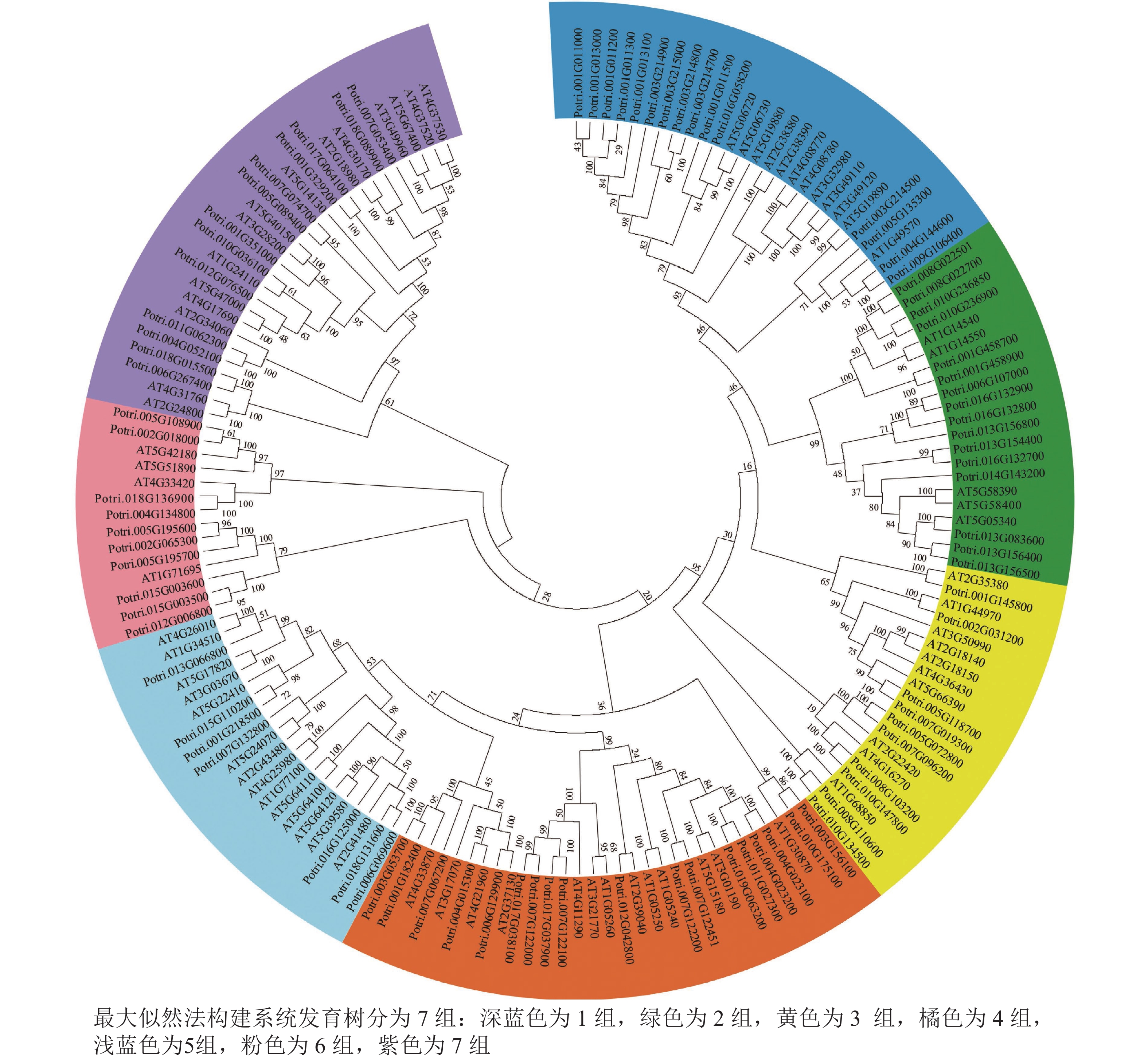
使用拟南芥和毛果杨PRX家族的同源蛋白序列,构建系统发育树(图1)并进行保守结构域分析(图2)发现:该家族基因高度保守。对PRX基因家族启动子进行顺式作用元件分析(图3)发现:该基因家族成员含有响应生长素(IAA)、脱落酸(ABA)、茉莉酸甲酯(MeJA)等激素的功能元件以及响应低温、干旱、盐害等非生物胁迫的功能元件。因此,PRX家族为植物生长发育和参与抵御环境胁迫中的重要家族。毛果杨Potri.004G052100 (84K杨同源基因命名为PagPRX19)与拟南芥AtPRX19 (At2G34060)的同源关系较近。研究表明:AtPRX19参与调控拟南芥的抗逆性[9, 16],推测与其同源的杨树基因PRX19也具有此功能。此外,PRX19启动子区域含有与盐胁迫密切相关的IAA、ABA和MeJA等激素相关顺式作用元件,参与植物耐盐调控。选取PagPRX19基因分析其在84K杨中的表达模式(图4),结果显示:PagPRX19基因在各个组织均有表达,在木质部表达量最高。
-
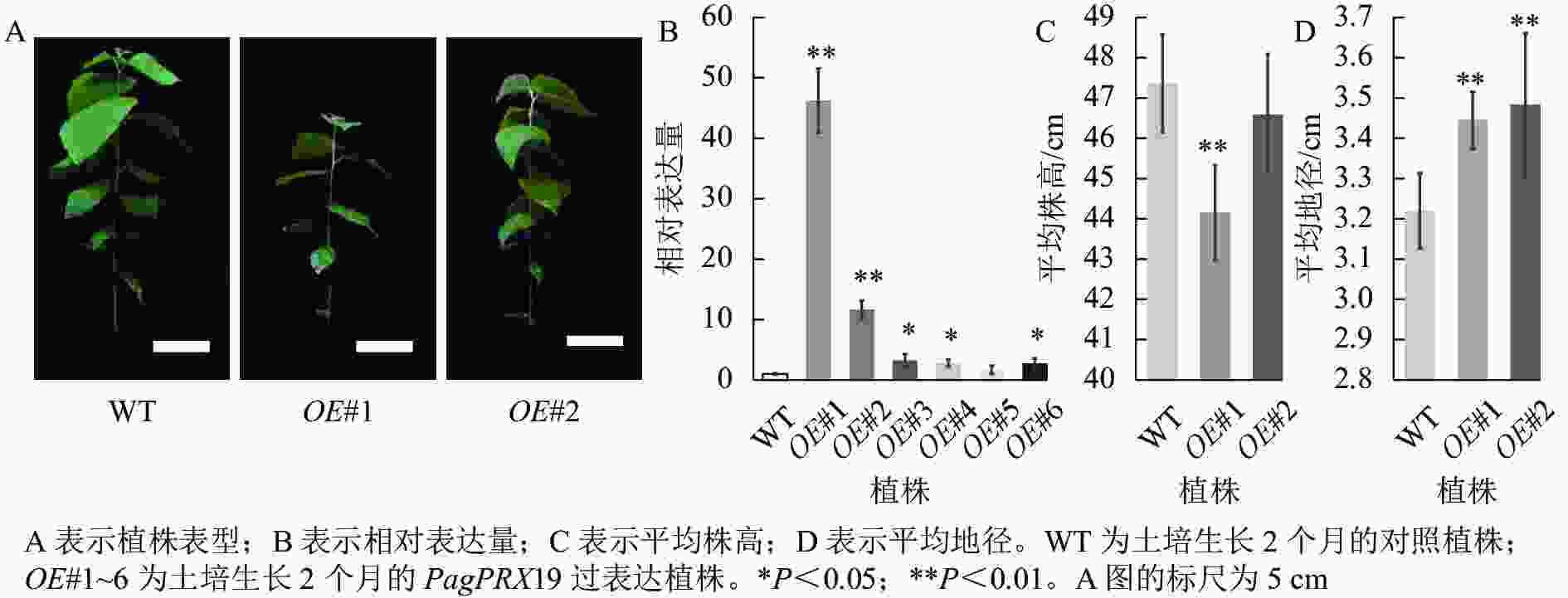
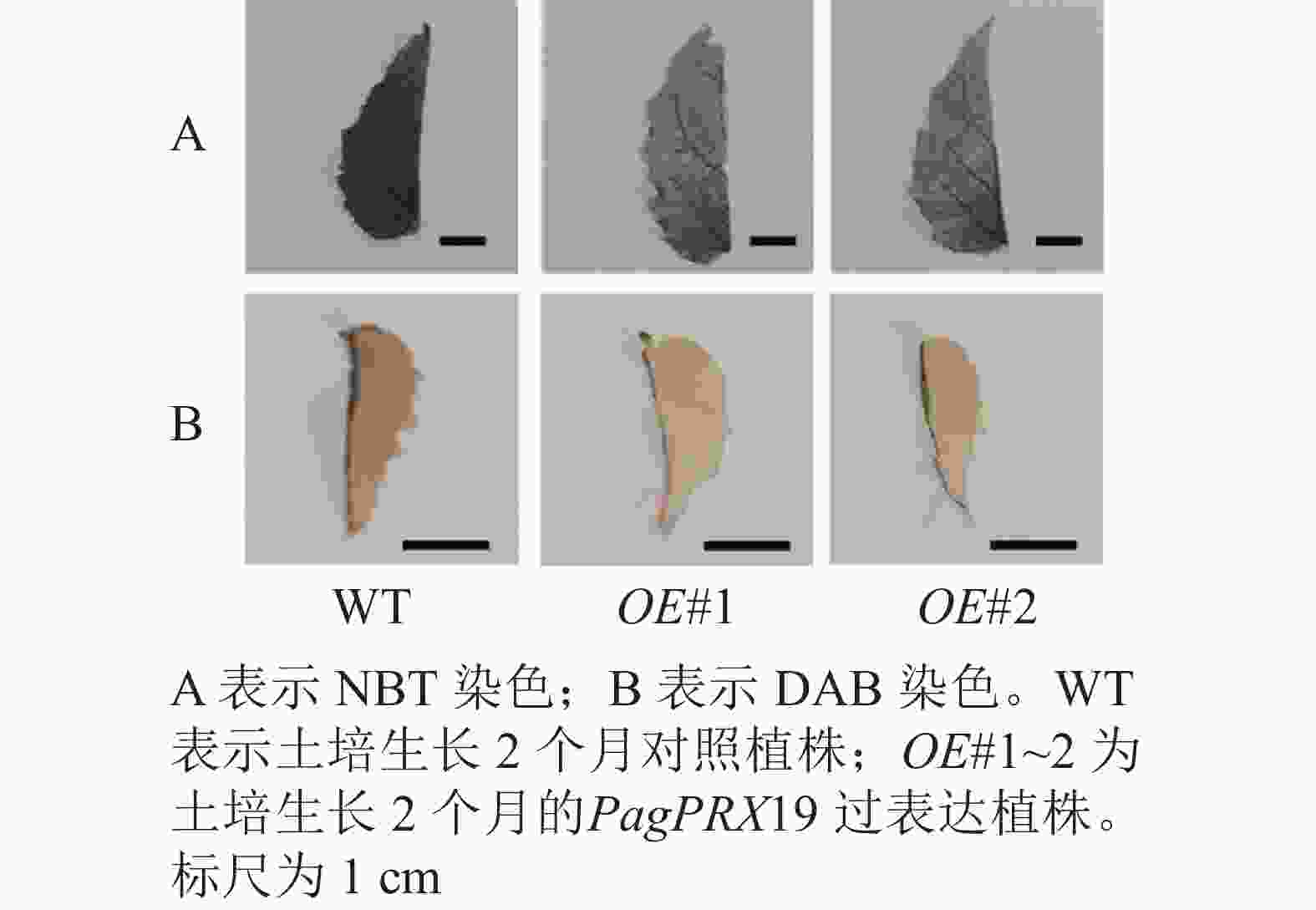
对转基因技术获得的6株植株进行阳性苗鉴定,通过RT-qPCR分析不同阳性株系PagPRX19的表达量,选取了相对表达量分别为对照46和12倍的2个株系OE#1、OE#2进行表型分析(图5)。分别选取6株生长2个月的土培苗统计株高和地径发现:株系OE#1的株高(44.15 cm)与对照(47.36 cm)相比极显著下降(P<0.01),而株系OE#2的株高(46.58 cm)与对照相比无显著差异。株系OE#1的地径(3.42 cm)和OE#2的地径(3.49 cm)与对照(3.22 cm)相比极显著增加(P<0.01)。取植株第3片叶进行DAB和NBT化学染色(图6)发现:与对照相比,PagPRX19过表达植株叶片的ROS水平显著减少。对植株第7节间茎段组织切片进行DAB和NBT化学染色(图7)发现:与对照相比,过表达植株茎段的ROS水平降低,说明过表达PagPRX19导致植株ROS降低。
-
在100 mmol·L−1NaCl生根培养基培养条件下(图8),对照植株组培苗叶片大面积脱落,植株呈黄褐色,干枯严重甚至死亡。而过表达叶片失绿和皱缩情况与对照相比较轻,且无叶片脱落,说明过表达植株较对照具有更高的耐盐性。在100 mmol·L−1NaCl溶液灌溉处理下,对照植株叶片大面积脱落,皱缩明显,严重失绿。过表达株系OE#2叶片轻度失绿,叶缘处皱缩不明显。株系OE#1仅叶缘处轻微皱缩,与正常生长的处理差异不明显,与对照相比,过表达株系有更高的耐盐性,其中表达量较高的株系OE#1较OE#2耐盐性更强。

图 8 盐胁迫下对照和转基因植株的表型特征
Figure 8. Phenotypic characteristics of non- and transgenic poplars under salt stress
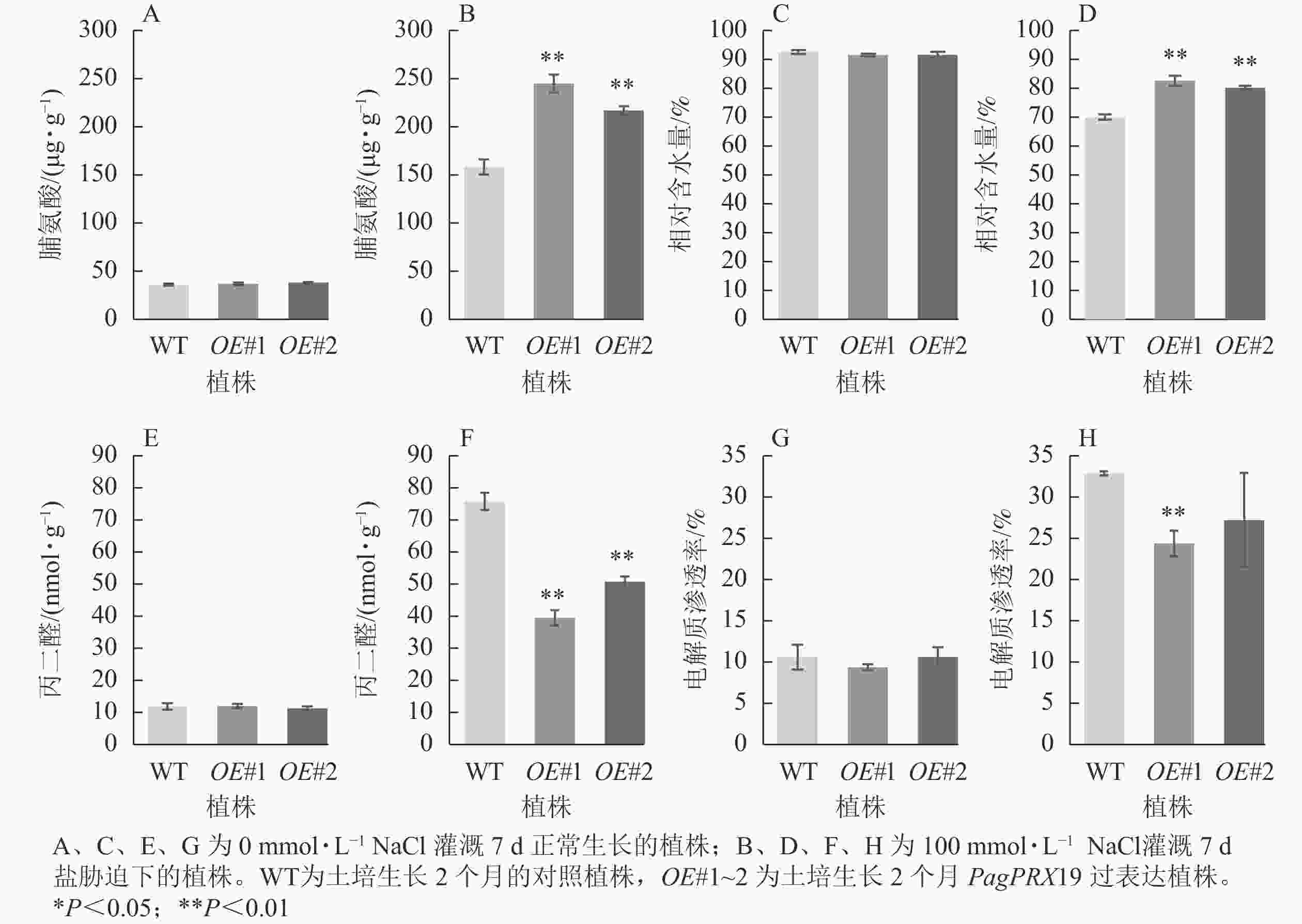
从图9可见:正常生长时,对照与过表达植株脯氨酸质量分数、相对含水量、丙二醛质量摩尔浓度、电解质渗透率无显著差异。盐胁迫下,相较于对照植株的脯氨酸质量分数(158.26 μg·g−1),过表达植株脯氨酸质量分数极显著增加(OE#1为244.94 μg·g−1,OE#2为217.02 μg·g−1)(P<0.01)。与对照叶片相对含水量(70.05%)相比,过表达植株叶片相对含水量极显著高于对照(OE#1为82.68%,OE#2为80.25%)(P< 0.01)。与对照丙二醛质量摩尔浓度(75.76 nmol·g−1)相比,过表达植株丙二醛质量摩尔浓度(OE#1为39.44 nmol·g−1,OE#2为50.76 nmol·g−1)较低且差异极显著(P<0.01)。与对照电解质渗透率(32.75%)相比,过表达植株电解质渗透率(OE#1为24.24%,OE#2为27.09%)较低,且株系OE#1与对照差异极显著(P<0.01)。
-
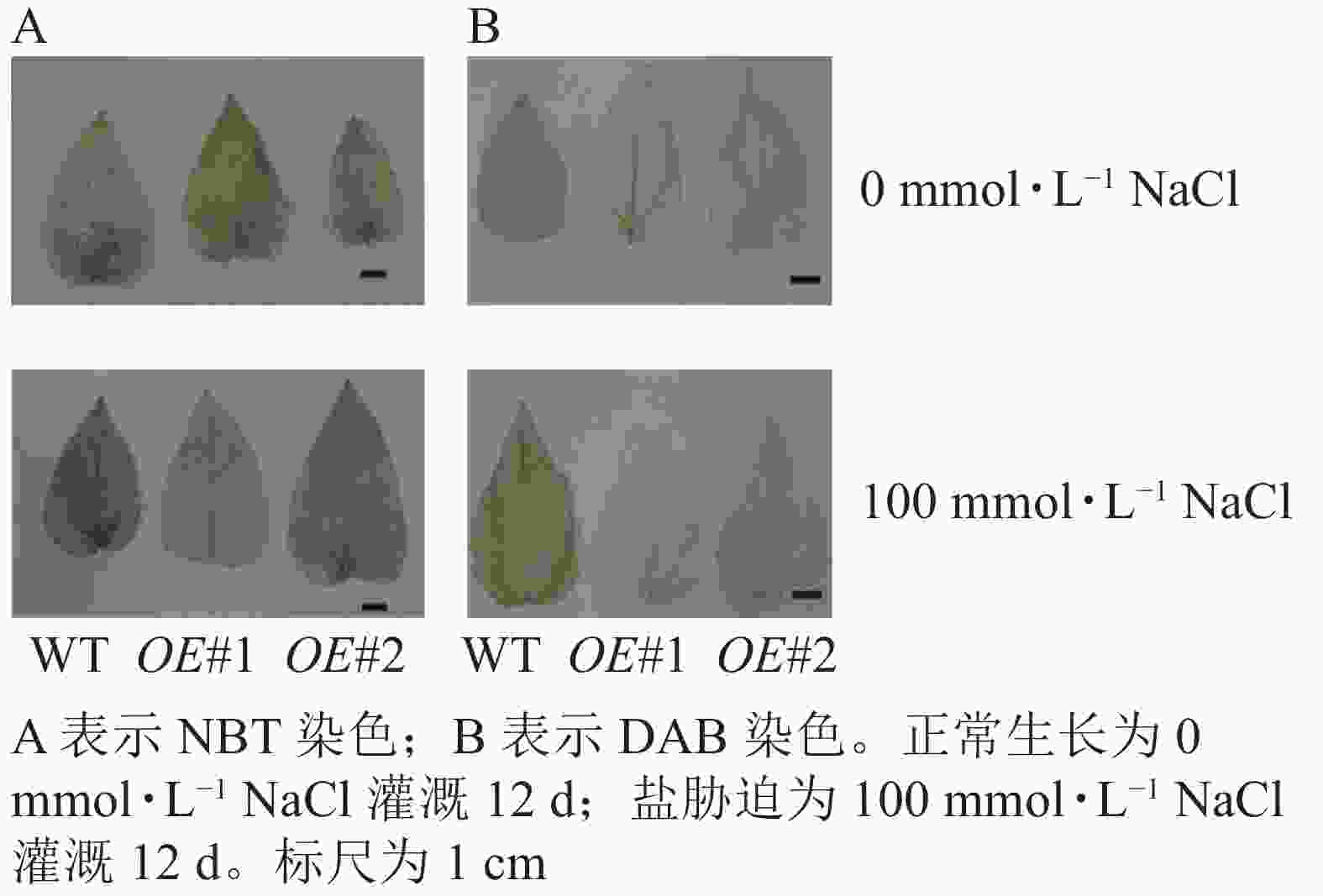
对盐胁迫处理后的土培苗叶片进行NBT和DAB染色(图10)发现:与对照相比,过表达植株叶片的ROS信号少。对盐胁迫处理后的组培苗根部进行DAB和NBT染色(图11)后发现:与对照相比,PagPRX19过表达植株根部的ROS信号少。说明在盐胁迫下,由于过表达PagPRX19导致植株ROS水平下降。
-
过氧化酶PRX可催化H2O2,降低ROS水平,减轻胁迫对细胞的伤害[3]。因此,通过抗氧化酶清除盐胁迫产生的ROS,以抵御胁迫带来的细胞损伤,提高林木的抗性,可作为抗逆分子育种手段。本研究通过过表达过氧化酶基因,改变84K杨内源ROS水平,从而探究ROS水平对杨树耐盐性的影响。
生物信息学分析发现:PagPRX19存在多个与IAA、ABA、或MeJA相关的顺式作用元件,这些激素与盐胁迫密切相关,参与植物耐盐调控。基因表达模式分析发现:PagPRX19在植株各个组织都有表达。因此选取该基因作为研究对象,创制过表达植株。与对照植株相比,过表达植株叶片和茎段ROS水平降低。过基因植株H2O2降低,O2 .−也随之降低。可能是过表达加速了H2O2的清除,植物自身调控SOD酶加速分解O2 .−产生H2O2,从而导致了O2 .−减少[9]。对获得的转基因植株进行盐胁迫处理,在100 mmol·L−1NaCl处理下,对照植株出现了胁迫症状,但过表达植株症状轻或没有出现症状。过表达植株可能通过降低盐胁迫下植株氧化胁迫程度,增强耐盐性。ROS水平检测结果验证了这一推论,盐胁迫下,过表达植株与对照相比,ROS水平仍保持较低水平。表明过表达PagPRX19可降低盐胁迫下ROS的伤害,从而增强了转基因植株的抗性。
研究表明:盐胁迫下脯氨酸质量分数增加[17],葡萄Vitis vinifera砧木在一定程度内的耐盐性越强,脯氨酸等有机渗透物质就越多[18]。本研究表明:盐胁迫下,过表达PagPRX19植株脯氨酸质量分数高于对照植株,使得植株的耐盐能力增强。此外,盐胁迫造成植物叶片含水量下降,植物生理活动会受到影响[19]。丙二醛和电解质渗透率反映了细胞膜损坏程度[20]。盐胁迫下,植株细胞膜被破坏,细胞膜内物质外渗,电解质渗透率升高,丙二醛增加[21−22],而耐盐品种一般表现为叶片相对含水量高、电解质渗透率和丙二醛低的特点[19, 23]。本研究中,盐胁迫下PagPRX19过表达植株叶片相对含水量比对照高,丙二醛、电解质渗透率降低,表明转基因植株细胞膜完整性较好,耐盐性增强。
-
过表达过氧化物酶基因PagPRX19可降低84K杨植株内源ROS水平,且在盐胁迫下过表达植株ROS仍保持低水平,细胞膜的破坏程度降低、叶片的持水能力增强。表明在杨树中过表达PagPRX19,能促进盐胁迫下植株内源ROS的清除,从而减缓盐胁迫造成的氧化胁迫,提高植株的耐盐性。
Effects of peroxidase gene PagPRX19 on salt tolerance of poplar ‘84K’
-
摘要:
目的 盐害作为影响植物生长发育的非生物胁迫因子,严重威胁林木生长。在受到盐胁迫时,植物内源活性氧(ROS)水平增加,造成氧化胁迫,影响植株正常生长发育。因此,可通过增强过氧化物酶PRX家族成员表达水平,改变ROS水平,以增强杨树Populus耐盐能力,揭示PRX成员参与调控杨树盐胁迫响应的机制。 方法 以银腺杨‘84K’ Populus alba × P. glandulosa ‘84K’为材料,生物信息学分析选取PRX家族成员PagPRX19进行克隆并构建过表达载体,农杆菌Agrobacterium tumefaciens介导叶盘转化法获得过表达植株。以银腺杨‘84K’ PagPRX19过表达植株生长45 d的组培苗和生长2个月的土培苗为实验材料,进行盐胁迫处理,以非转基因植株为对照。观察植株表型,检测脯氨酸、丙二醛、电解质渗透率等生理指标并进行分析。 结果 ①克隆了PagPRX19基因,构建过表达载体,获得转基因阳性植株。经分子鉴定选取2个过表达株系OE#1和OE#2为实验材料做后续分析。②与对照相比,过表达植株株高下降,地径增加。③盐胁迫处理下,过表达植株相较于对照表现为叶片皱缩以及植株生长受到抑制程度低,组培苗的盐胁迫处理表现为相似结果。④转基因植株的ROS水平降低,而且在盐胁迫下过表达植株叶片和根的ROS仍保持较对照低的水平。盐胁迫下过表达植株较对照脯氨酸增加,叶片持水能力增强,丙二醛和电解质渗透率降低。从生理方面显示转基因植株具有较高的耐盐能力。 结论 过表达PagPRX19可降低盐胁迫下杨树转基因植株的ROS水平,缓解氧化胁迫,增强了植株耐盐性。图11参23 -
关键词:
- 银腺杨‘84K’ /
- 过氧化物酶 /
- 内源活性氧(ROS) /
- 盐胁迫 /
- 耐盐性
Abstract:Objective Salt damage, as an abiotic stress factor affecting plant growth and development, seriously threatens tree growth. Under salt stress, the level of endogenous reactive oxygen species (ROS) in plants increases, resulting in oxidative stress and affecting the normal growth and development of plants. By enhancing the expression level of PRX family members and changing ROS levels, the salt tolerance ability of poplar can be enhanced, and the mechanism of PRX members involved in regulating salt stress response can be revealed. Method Poplar ‘84K’ (Populus alba × P. glandulosa ‘84K’) was used as material, and PagPRX19, a member of PRX family, was selected for cloning and construction of overexpression vector by bioinformatics analysis. Overexpressed plants were obtained by Agrobacterium tumefaciens mediated leaf disk transformation. The tissue culture seedlings of non-transgenetic plants (the control) and PagPRX19 overexpressed plants growing for 45 days and the soil culture seedlings growing for 2 months were used as experimental materials for salt stress treatment. The plant phenotype was observed and the physiological indexes such as proline, malondialdehyde and electrolyte permeability were detected and analyzed. Result (1) PagPRX19 gene was cloned, overexpression vector was constructed, and transgenic positive plants were obtained. After molecular identification, 2 overexpressed lines OE#1 and OE#2 were used for further analysis. (2) Preliminary phenotypic observation showed that PagPRX19 overexpressed plants decreased in height and increased in ground diameter, compared with the control. (3) Compared with the control, the overexpressed plants showed lower leaf shrinkage and plant growth inhibition under salt stress. Tissue culture seedlings showed similar results under salt stress. (4) ROS level of transgenic plants decreased, and ROS level of leaves and roots of overexpressed plants remained lower than that of the control under salt stress. The content of proline in overexpressed plants increased, the water holding capacity of leaves increased, and the contents of malondialdehyde and electrolyte permeability were lower than those of wild type. The transgenic plants showed high salt tolerance from the physiological aspect. Conclusion Overexpression of PagPRX19 can reduce ROS level of transgenic poplar ‘84K’ plants under salt stress, alleviate oxidative stress, and enhance salt tolerance. [Ch, 11 fig. 23 ref.] -
-
[1] 蔡晓锋, 胡体旭, 叶杰, 等. 植物盐胁迫抗性的分子机制研究进展[J]. 华中农业大学学报, 2015, 34(3): 134 − 141. CAI Xiaofeng, HU Tixu, YE Jie, et al. Molecular mechanisms of salinity tolerance in plants [J]. J Huazhong Agric Univ, 2015, 34(3): 134 − 141. [2] SHAH A N, TANVEER M, ABBAS A, et al. Targeting salt stress coping mechanisms for stress tolerance in Brassica: a research perspective [J]. Plant Physiol Biochem, 2021, 158: 53 − 64. [3] YANF Yongqing, YOU Yan. Unraveling salt stress signaling in plants [J]. Integrative Plant Biol, 2018, 60(9): 796 − 804. [4] JOSE A M, MARIA O, AGUSTINA B V, et al. Plant responses to salt stress: adaptive mechanisms[J/OL]. Agronomy, 2017, 7(1): 18[2022-05-18]. doi: 10.3390/agronomy7010018. [5] MOHAMMAD A A, NISHA S T, TITTAL M, et al. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions [J]. Physiol Mol Biol Plants, 2017, 23(1): 731 − 744. [6] ZHANG Huilong, CHEN Deng, YAO Jun, et al. Populus euphratica JRL mediates ABA response, ionic and ROS homeostasis in Arabidopsis under salt stress [J/OL]. Mol Sci, 2019, 20(4): 815[2022-05-20]. doi: 10.3390/ijms20040815. [7] LI Lihong, YI Huilan. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants [J]. Plant Physiol Biochem, 2012, 58: 46 − 53. [8] SU Peisen, YAN Jun, LI Wen, et al. A member of wheat class Ⅲ peroxidase gene family, TaPRX-2A, enhanced the tolerance of salt stress[J/OL]. BMC Plant Biol, 2020, 20(1): 392[2022-05-21]. doi: 10.1186/s12870-020-02602-1. [9] HÄFFNER E, KONIETZZKI S, DIIEDERICHSEN E. Keeping control: the role of senescence and development in plant pathogenesis and defense [J]. Plants, 2015, 4(3): 449 − 488. [10] MORITA S, KAMINAKA H, TAKEHIRO M, et al. Induction of rice cytosolic ascorbate peroxidase mRNA by oxidative stress; the involvement of hydrogen peroxide in oxidative stress signalling [J]. Plant Cell Physiol, 1999, 40(4): 417 − 422. [11] WANG Yu, WANG Qianqian, ZHAO Yang, et al. Systematic analysis of maize class Ⅲ peroxidase gene family reveals a conserved subfamily involved in abiotic stress response [J]. Gene, 2015, 566(1): 95 − 108. [12] ALMAGRO L, GOMEZ ROS LV, BELCHI-NAVARRO S, et al. Class Ⅲ peroxidases in plant defence reactions [J]. J Exp Bot, 2009, 60(2): 377 − 390. [13] THAKUR A K, KUMAR P, PARMAR N, et al. Achievements and prospects of genetic engineering in poplar: a review [J]. New For, 2021, 52(2): 889 − 920. [14] 江成. 杨树钙离子依赖核酸酶在木质部分化中的作用机制研究[D]. 北京: 中国林业科学研究院, 2018. JIANG Cheng. The Role of Ca+ Dependent DNase During the Xylem Differentiation in Poplar[D]. Beijing: Chinese Academy of Forestry, 2018. [15] 姚俊广, 耿娅, 刘依静, 等. S-腺苷甲硫氨酸脱羧酶基因对银腺杨84K抗旱性的影响[J]. 林业科学, 2022, 58(2): 125 − 132. YAO Junguang, GENG Ya, LIU Yijing, et al. Effects of S-adenosylmethionine decarboxylase gene on drought tolerance of Populus alba × P. glandulosa ‘84K’ [J]. Sci Silv Sin, 2022, 58(2): 125 − 132. [16] GANGULY D R, CRISP P A, EICHTEN S R, et al. The Arabidopsis DNA methylome is stable under transgenerational drought stress [J]. Plant Physiol, 2017, 175(4): 889 − 920. [17] MARCIN N, MARIA S. The relationship between the antioxidant system and proline metabolism in the leaves of cucumber plants acclimated to salt stress[J/OL]. Cells, 2021, 10(3): 609[2022-05-15]. doi: 10.3390/cells10030609. [18] 鲁倩君, 刘迎, 赵宝龙, 等. 葡萄砧木耐盐碱性研究进展[J]. 中外葡萄与葡萄酒, 2022(4): 75 − 80. LU Qianjun, LIU Ying, ZHAO Baolong, et al. Research progress on salt-alkaline tolerace of grape rootstock [J]. Sino-Overseas Grapevine Wine, 2022(4): 75 − 80. [19] MEHARI T G, HOU Y, XU Y, et al. Overexpression of cotton GhNAC072 gene enhances drought and salt stress tolerance in transgenic Arabidopsis [J]. BMC Genomics, 2022, 23(1): 64 − 69. [20] 黄婷, 麻冬梅, 王文静, 等. 2种紫花苜蓿耐盐生理特性的初步研究[J]. 水土保持学报, 2020, 34(2): 216 − 221. HUANG Ting, MA Dongmei, WANG Wenjing, et al. Preliminary study on physiological characteristics of salt tolerance of two genotypes of alfalfa [J]. J Soil Water Conserv, 2020, 34(2): 216 − 221. [21] 董亚茹, 聂玉霞, 李云芝, 等. 瞬时过表达MnERF2基因对桑树耐盐性的影响[J]. 山东农业科学, 2022, 54(4): 9 − 16. DONG Yaru, NIE Yuxia, LI Yunzhi, et al. Effects of MNERF2 gene on salt tolerance in transient overexpression mulberry [J]. Shandong Agric Sci, 2022, 54(4): 9 − 16. [22] 陈奋奇, 方鹏, 白明兴, 等. 外源脯氨酸缓解玉米幼苗盐胁迫的效应[J]. 草业科学, 2022, 39(4): 747 − 755. CHEN Fenqi, FANG Peng, BAI Mingxing, et al. Mitigation of salt stress in maize seedlings by exogenous proline application [J]. Pratacult Sci, 2022, 39(4): 747 − 755. [23] 张涛, 马肖静, 朱新红, 等. NaCl胁迫对不同耐盐性辣椒幼苗生理生化指标的影响[J]. 山东农业科学, 2021, 53(12): 38 − 43. ZHANG Tao, MA Xiaojing, ZHU Xinhong, et al. Effects of NaCl stress on physiological and biochemical indexes of Capsicum annuum L. seedlings with different salt tolerance [J]. Shandong Agric Sci, 2021, 53(12): 38 − 43. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20220387






 下载:
下载: