-
百合Lilium spp.是百合科Liliaceae百合属Lilium多年生草本球根植物,被誉为“球根花卉之王”,具重要观赏及食药用价值[1]。百合鳞茎发育是影响百合质量的关键过程,在百合生命周期中伴随着源-库关系的转换[2];采用离体手段开展百合小鳞茎发育生物学研究,具可控、高效、材料一致性强等优势[3]。百合鳞茎的主要储藏物质为淀粉,占干物质量的60%以上,包括直链淀粉和支链淀粉,淀粉代谢被认为参与调控百合鳞茎发育[4]。淀粉合成酶(starch synthase, SS)是淀粉生物合成过程中的关键酶之一,主要有颗粒结合淀粉合成酶(granule-bound starch synthase, GBSS)和可溶性淀粉合成酶(soluble starch synthase, SSS)2种,分别参与直链和支链淀粉的合成。其中,GBSS能通过α-1, 4-D-糖苷键将腺苷二磷酸葡萄糖中的葡萄糖残基添加到葡聚糖的非还原端,延长葡聚糖的直链,是直链淀粉合成过程中的关键酶[5];常见GBSSⅠ(30~70 kD)和GBSSⅡ(70~100 kD)等2种同工酶。目前,已从拟南芥Arabidopsis thaliana[6]、马铃薯Solanum tuberosum[7]等植物中克隆得到GBSS基因,且发现GBSSI的缺失会造成直链淀粉的合成受阻,导致植物体内直链淀粉缺失[8]。前期研究发现:离体鳞茎促进型处理(低浓度多效唑,5×10-4 mmol·L-1),GBSS酶活性显著增强[9]。然而,针对百合GBSS基因的克隆及表达特性研究仍较匮乏[10],制约了百合鳞茎发育机制的进一步深入探讨。本研究以东方百合‘索邦’Lilium oriental ‘Sorbonne’离体苗为材料,通过形态观察和淀粉含量测定,判断其库-源关系转换,准确划分小鳞茎膨大过程发育时期;结合前期转录组测序数据,利用cDNA末端快速扩增技术(RACE)克隆‘索邦’颗粒结合淀粉合成酶基因(LohGBSS),并通过定量即时聚合酶链式反应(qRT-PCR)技术分析该基因在不同组织及发育时期的表达模式,为研究LohGBSS在离体百合淀粉合成及鳞茎发育过程中的调控机制和百合淀粉品质育种奠定理论基础。
-
供试材料为浙江大学园林研究所组培室的东方百合‘索邦’组培材料。无菌培养室光强为60 μmol·m-2·s-1,温度为(25±2)℃。
-
根据本实验室建立的鳞茎发育生物学研究体系获得足量单芽[3],并作一定改动(成球培养基为50 mL,试验期可提供充足养分)。将芽转入含MS(70 g·L-1蔗糖+8 g·L-1琼脂)[11]的成球培养基中,转入后0、15、25、35、45、55和75 d观察并拍照,统计小鳞茎直径和鲜质量,计算0~15(P1)、15~25(P2)、25~35(P3)、35~45(P4)、45~55(P5)和55~75 d(P6)的鳞茎膨大速率。
-
对应形态学观测时间点,随机选取生长状况良好的小鳞茎,切掉叶片和根部,将剩余部位切碎混匀后称量0.3 g,液氮速冻30 min后转入-80 ℃冰箱中保存,采用蒽酮法测定淀粉质量分数[1],重复3次取平均值。计算P1、P2、P3、P4、P5和P6各期的淀粉累积率。
-
采用改良十六烷基三甲基溴化铵法(CTAB法)提取‘索邦’鳞茎总RNA[1],通过blastx数据库对基因进行同源性比对,从前期转录组数据中[1]得到LohGBSS cDNA序列片段,采用Beacon Designer 7软件设计3′端和5′端RACE端引物。使用Clontech SMARTerⓇ RACE 5′/3′试剂盒(Takara公司)合成第1链cDNA,克隆得到LohGBSS cDNA 5′和3′端序列(参照试剂盒)。对RACE产物进行回收、加Poly尾、纯化,连接至pGEM-T载体上,转化大肠埃希菌Escherichia coli DH5α的感受态细胞,筛选阳性克隆,送至上海生工生物工程股份有限公司测序。
-
利用实时荧光定量PCR技术对LohGBSS在不同组织(叶、茎段、鳞茎、根)及不同时期(0、15、45、60和75 d)的表达特性进行分析。根据LohGBSS的cDNA序列,设计特异引物,上游引物:5′-AGGAAGGATTCACTGGATT-3′,下游引物:5′-TTGGACATAGGAGCGATT-3′。以GAPDH为内参基因,上游引物:5′-GAATGGCAAGCTAACTGGAATG-3′,下游引物:5′-CAGCCTTGATCTGATCGTAAGT-3′。利用CFX ConnectTM荧光定量PCR检测系统(Bio-Rad公司)分析LohGBSS的表达特性。PCR反应条件为:95 ℃,2 min;95 ℃,5 s;60 ℃,30 s;39个循环。用比较Ct值法计算相对表达量。重复3次取平均值。
-
鳞茎膨大速率(g·株-1·d-1)=m鲜(1+n)-m鲜n / t,淀粉积累率=[w淀粉(1+n)-w淀粉n]/w淀粉n,其中:n=(1,2,3,4,5,6)[1],即为相邻取样点,t为相邻2次取样间隔天数(d)。运用WPS Excel 2018及SPSS 17.0软件进行统计学分析。
-
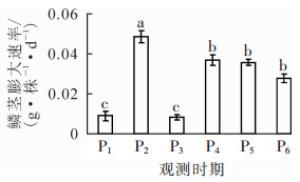
小鳞茎转入成球培养基后,培养基提供主要养分,离体植株持续发育。15 d时即可明显观察到根的形成,根数及根长不断增加;叶片在转接后5~6 d抽出,叶面积扩大;培养至45 d,叶片出现黄化(图 1)。小鳞茎直径在实验期间快速增长,至75 d时达1.93 cm(表 1),小鳞茎鲜质量具相似变化趋势。以2次观测间隔为阶段,计算鳞茎膨大速率可知:P2(15~25 d)时最高,为0.048 g·株-1·d-1(图 2)。

图 1 ‘索邦'离体小鳞茎发育情况
Figure 1. Growth status of 'Sorbonne' bulblets during different developmental stages
表 1 ‘索邦'离体小鳞茎发育形态指标
Table 1. Developmental morphological indexes of 'Sorbonne' bulblets
培养时长/d 根数/条 根长/cm 叶片数/片 株高/cm 小鱗茎鲜质量/g 小鱗茎直径/cm 0 3.00±0.00b 0.10±0.01d 0.00±0.00d 1.00±0.03e 0.04±0.01d 0.42±0.02e 15 3.67±0.45b 0.57±0.04d 2.67±0.33c 2.78±0.22d 0.14±0.03d 0.53±0.06e 25 11.33±0.86a 1.59±0.08c 3.33±0.33bc 4.20±0.07c 0.70±0.04c 0.85±0.07d 35 10.33±0.67a 1.94±0.10c 4.67±0.67ab 5.00±0.09b 0.80±0.40bc 0.99±0.19cd 45 13.00±1.08a 2.61±0.16b 5.00±0.57a 5.02±0.10b 1.28±0.18b 1.21±0.12c 55 13.66±0.67a 2.66±0.17ab 5.01±0.47a 5.94±0.17a 1.82±0.36a 1.57±0.14b 75 15.00±1.00a 3.22±0.42a 5.67±0.33a 6.16±0.23a 2.08±0.17a 1.93±0.12a 说明:同列不同字母表示差异显著(P < 0.05) -
在小鳞茎发育期间,淀粉质量分数总体呈递增规律,但在15~45 d时变化不大,甚至略有下降(图 3A);鳞茎膨大与淀粉质量分数呈正相关,相关系数为0.90。淀粉积累速率在P1(0~15 d)阶段最高,为9.29,表明在小鳞茎膨大初期,淀粉需快速积累以供鳞茎形态建成,P2(15~25 d)阶段迅速降低,之后变化趋于平稳,但在P4(25~45 d)阶段淀粉积累率出现负值,说明淀粉被消耗,可能用于地上部及鳞茎的库源平衡,随后又出现明显积累(图 3B)。
-
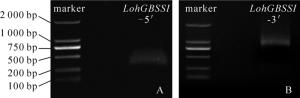
采用5′RACE技术扩增得到1条长度为283 bp(图 4A)的基因序列,采用3′RACE技术扩增得到1条长度为769 bp(图 4B)的基因序列。将所得的5′和3′端序列与LohGBSS cDNA中间序列拼接,得到全长为1 913 bp的LohGBSS cDNA序列,包含1个1 665 bp的开放阅读框(ORF),共编码554个氨基酸(图 5)。
-
将百合LohGBSS与其他植物GBSS基因的氨基酸序列在MEGA 6软件中进行比对分析,并用Neighbor-Joining算法构建系统进化树,结果发现:百合LohGBSS和兰州百合、玉米Zea mays等亲缘关系较近(图 6)。
将百合LohGBSS基因编码的氨基酸序列在NCBI Blastn中比对,发现该序列与兰州百合(AJG44453.1)的同源性最高,达92.00%,与海枣Phoenix dactylifera(XP_008775302.1)的同源性达69.00%,与大豆Glycine max(XP_003556431.1)的同源性达67.00%。用DNAMAN软件将LohGBSS氨基酸序列与GenBank中其他植物的GBSS序列进行比对,发现与兰州百合、马铃薯、玉米的GBSS氨基酸序列一致性分别达86.36%、61.80%、59.55%(图 7)。
通过ProtParam软件()分析发现:LohGBSS基因编码的多肽分子量约为60.89 kD,推测的等电点为6.08,带负电荷的氨基酸残基数(天冬氨酸和谷氨酸)为65个,带正电荷的氨基酸残基数(精氨酸和赖氨酸)为61个,总平均疏水性为-0.112,为亲水性蛋白,不稳定系数为28.12,表明该蛋白是稳定的。通过TMHMM 2.0软件对LohGBSS氨基酸序列的跨膜区进行分析,结果显示:554个氨基酸全部位于膜外,表明该蛋白均不具有跨膜区,不是跨膜蛋白。利用SMART软件对百合LohGBSS编码的GBSS蛋白结构域进行分析(图 8A),发现其具有1个淀粉合成酶催化域(Glyco_transf_5)和2个糖基转移酶结构域(Glyco_transf_1和Glyco_transf_1_4)。

图 8 百合GBSS蛋白功能域分析和单体三级结构预测
Figure 8. Functional domains analysis and predicted monomer 3D structure of GBSS in lily
利用SWISS-MODEL预测LohGBSS蛋白的三级结构(图 8B),结果显示:其与同为单子叶植物的水稻Oryza sativa GBSSⅠ催化域晶体结构的一致性达69.29%,与大麦Hordeum vulgare GBSSI催化域结构的一致性达44.82%。推测该基因可能是百合GBSSI基因,将其命名为LohGBSSI。
-
对LohGBSSI在‘索邦’不同组织中的表达进行分析(图 9A),发现60 d时LohGBSSI在鳞茎中的表达量最高,在叶中的表达量高于茎段,在根中的表达量最低。差异显著性分析发现:LohGBSSI在叶和鳞茎中的表达较茎段和根差异显著,但两者无显著性差异,在茎段和根中的表达也无明显差异。对鳞茎中LohGBSSI在‘索邦’不同发育时期的表达进行分析(图 9B),发现LohGBSSI在15 d时表达量最高,0~15 d增加幅度较大,其后开始减少,45 d之后变化较小,差异不显著。
-
培养基中添加的蔗糖可保证小鳞茎膨大所需养分[1]。前期研究发现,若仅在转接初期添加碳源,可获得的最大小鳞茎直径及鲜质量分别为8.81 mm及256 mg;转接75 d时,由于“碳饥饿”的发生,鳞茎最终鲜质量仅为160 mg[4]。本研究发现:通过使用充足的培养基,最大鳞茎直径可达1.93 cm,为前者的2.19倍,鲜质量可达2.02 g,为前者的7.89倍。表明加倍碳源可获得2倍以上的鳞茎膨大效益。
离体条件,小鳞茎在发育过程中常是“源-库复合体”的状态,会随着发育过程不断转换,主要取决于培养基中的碳供应[2]。在小鳞茎膨大初期(0~15 d),能量物质主要来自于培养基,转接芽快速响应并形成小鳞茎,此时初生小鳞茎仅担任“库”的功能,集中体现在淀粉合成相关基因如LohGBSSI在15 d时高表达(图 9A),且此时淀粉质量分数亦有显著积累(图 3A),表明淀粉积累为芽鳞片化(即小鳞茎形成)所必需。随着叶片和根的不断形成,在小鳞茎快速膨大期(15~55 d),叶片光合作用产生的同化物与培养基中的蔗糖共同供应小鳞茎及植株的生长发育,此时的小鳞茎既担任“库”的功能,也与叶片之间形成双向运输,担任“源”的功能,是“源-库复合体”的状态。具体而言,25~45 d淀粉积累率出现负值(图 3B),淀粉被消耗,地上部叶片数增加(图 1);相应地,25~35 d时小鳞茎膨大速率跌至最低点(图 2B),可以推测此时同化物大多转运至叶片,保证叶片的营养生长,小鳞茎主要担负“源”的功能。45 d后小鳞茎叶片面积几乎无变化,小鳞茎内部淀粉含量又开始迅速积累(图 3),膨大速率加快。45 d小鳞茎内部LohGBSSI基因的相对表达量较15 d时显著降低,但仍处于较高水平,这也与小鳞茎淀粉快速积累的结果相符(图 3)。因此,根据小鳞茎发育过程中淀粉积累速率及膨大速率,及其源-库关系转换,可将小鳞茎的膨大过程分为:0~15 d,小鳞茎膨大初期,此时主要为鳞茎形态建成;15~55 d,小鳞茎快速膨大期,以源-库复合体的形式保证鳞茎膨大;55~75 d,小鳞茎膨大后期,鳞茎内含物的进一步充实。百合离体大球在移栽后存活率高,且生长更快[1],应重点调控小鳞茎快速膨大期,关注其源-库复合体的特殊状态,这与吴沙沙[2]在‘索邦’地栽球的研究结果类似。
GBSSI主要负责植物直链淀粉的合成,过表达或抑制GBSSI基因的表达,会导致淀粉中直链淀粉的含量上升或降低[12]。关于GBSS的分子水平研究主要以水稻、小麦、马铃薯等作物的淀粉品质育种为主。有研究表明:GBSSI既影响大麦籽粒淀粉支链淀粉浓度,还和支链淀粉的链长有关[13]。在木薯Manihot esculenta中,CRISPR-Cas 9介导的直链淀粉生物合成相关的2个基因的靶向突变,即蛋白靶向淀粉(PTST1)或颗粒结合淀粉合成酶(GBSS),可以降低或消除根淀粉中的直链淀粉含量[14]。GBSS的活性对马铃薯中直链淀粉与支链淀粉比例、淀粉链长及结构特性有很大影响[15]。
本研究用整齐一致的‘索邦’离体材料克隆得到LohGBSS(GenBank登录号:MF101407.1)。LohGBSS cDNA全长为1 913 bp,开放阅读框长1 665 bp,编码554个氨基酸,编码的蛋白分子量约60.89 kD,与兰州百合GBSSI的同源性较高,达92%,与其他物种GBSSI的同源性达65%~69%,故推测该基因可能是百合GBSSI基因,将其命名为LohGBSSI。LohGBSSI编码的蛋白具有1个以ADP-葡萄糖为葡萄糖供体的淀粉合成酶催化域和2个将糖分从活跃供体转移至特定受体的糖基转移酶结构域。三级结构预测表明:其与水稻GBSSⅠ催化域晶体结构十分相似,表明该蛋白具有淀粉合成功能。
在木薯中,GBSSI基因在茎的表达量最高,在块根发育过程中仅在形成期和成熟期表达[16];在黄芪Astragalus membranaceus中,GBSSI基因在毛状根及根中的表达量高于茎和叶[17]。而LohGBSSI在‘索邦’的叶、茎段、鳞茎和根中均有表达,虽在鳞茎中的表达量最高,但与叶片无显著差异(图 9),推测其可能在百合鳞茎和叶的直链淀粉合成中起重要作用;同时,不同发育阶段的表达谱表明:其在小鳞茎发育的整个阶段均有表达,尤其在鳞茎形态建成中发挥作用。这与木薯中的研究一致[16]。
综上所述,本研究在测定东方百合‘索邦’形态和生理指标的基础上,发现离体百合鳞茎形成和发育过程中,鳞茎直径及鲜质量不断增加,淀粉质量分数总体呈现递增趋势,且根据鳞茎膨大速率及淀粉积累率,及其源-库关系转换,可将离体小鳞茎发育过程分为:小鳞茎膨大初期(0~15 d),小鳞茎快速膨大期(15~55 d)及小鳞茎膨大后期(55~75 d);同时,淀粉在发育初期即需快速合成以保证鳞片化进程。进一步地,从鳞茎中克隆得到淀粉合成关键酶基因LohGBSSI的cDNA全长序列,得到其编码氨基酸的理化性质和蛋白结构,通过序列比较和生物信息学软件分析,推测该基因属于GBSSI基因家族,LohGBSSI基因表达量与淀粉质量分数相关。通过实时荧光定量PCR法,分析LohGBSSI在‘索邦’不同组织的表达情况,发现LohGBSSI基因在鳞茎和叶中表达显著高于茎段和根,表明直链淀粉合成的最主要部位为鳞茎;不同发育时期鳞茎内LohGBSSI的表达在15 d时最高,暗示鳞茎形成初期直链淀粉合成基因对于鳞茎形态建成具重要作用。本研究为后续解析淀粉合成关键酶基因GBSS在鳞茎发育中的功能奠定了基础,也为百合进行淀粉相关基因修饰提供了依据。
In vitro bulblet development and analysis of starch synthase gene(LohGBSSI) from Lilium 'Sorbonne'
-
摘要:
目的 淀粉是百合Lilium spp.鳞茎的主要组成成分,淀粉代谢对于百合鳞茎发育至关重要,对其开展深入研究有助于解决鳞茎繁育慢等产业化难题。 方法 以主栽品种东方百合‘索邦’Lilium ‘Sorbonne’离体鳞茎为材料,在观测不同发育时期形态和淀粉积累的基础上,利用cDNA末端快速扩增技术(RACE),克隆淀粉合成代谢关键酶颗粒结合型淀粉合成酶基因LohGBSSI,并对其开展不同组织及发育期基因表达谱分析。 结果 ① 根据形态及源-库转换,离体百合鳞茎形成和发育过程可分为小鳞茎膨大初期(0~15 d),小鳞茎快速膨大期(15~55 d)及小鳞茎膨大后期(55~75 d)。②‘索邦’颗粒结合型淀粉合成酶基因LohGBSSI(GenBank登录号:MF101407.1)全长1 913 bp,开放阅读框(ORF)长为1 665 bp,编码554个氨基酸。氨基酸序列与兰州百合Lilium davidii var.unicolor GBSSI同源性较高(92%),推测该基因可能属于GBSSI基因家族。③LohGBSSI基因在鳞茎和叶中表达显著高于茎段和根,表明直链淀粉合成的最主要部位为源-库关键器官;不同发育时期鳞茎内LohGBSSI的表达在15 d时最高,暗示鳞茎形成初期需淀粉快速合成以便形态建成。 结论 为后续解析淀粉合成关键酶基因GBSS在鳞茎发育中的功能奠定了基础,也为百合进行淀粉相关基因修饰提供了依据。 Abstract:Objective To promote the breeding speed of Lilium spp. bulbs, a study is conducted of the metabolism of starch, a major component of lily bulbs. Method Taking the bulbs in vitro of oriental hybrids Lilium 'Sorbonne', the most popular variety as the research subject, observations are made of the morphological changes and starch accumulation during different development stages. Thereafter, the granule bound starch synthase gene LohGBSSI was cloned with RACE employed, and the gene expression profiles of different tissues and development stages were analyzed. Result (1) During the formation and development process of lily bulblets cultured in vitro, in terms of the morphological traits and source-sink transition, there are mainly three stages namely the initial swelling stage, the rapid swelling stage and later swelling stage. (2) The total length of LohGBSSI (GenBank accession number:MF101407.1) is 1 913 bp, and the open reading frame is 1 665 bp, encoding 554 amino acids, which was highly homologous to GBSSI of Lilium davidii var. unicolor (92%), suggesting that this gene might belong with the GBSSI gene family. (3) The expression of LohGBSSI gene in bulbs and leaves was significantly higher than that in stem segments and roots, indicating that the most important site for amylose synthesis was the main source-sink organs. Furthermore, the LohGBSSI expression was highest at 15 d in bulblet, suggesting that rapid starch synthesis is a necessity for the early bulblet morphogenesis. Conclusion This study has laid a foundation for further functional analysis of GBSS in bulb development and also provided a basis for future lily starch relevant gene modification. -
Key words:
- botany /
- Lilium 'Sorbonne' /
- bulblet development /
- GBSS /
- cloning /
- different expression
-
表 1 ‘索邦'离体小鳞茎发育形态指标
Table 1. Developmental morphological indexes of 'Sorbonne' bulblets
培养时长/d 根数/条 根长/cm 叶片数/片 株高/cm 小鱗茎鲜质量/g 小鱗茎直径/cm 0 3.00±0.00b 0.10±0.01d 0.00±0.00d 1.00±0.03e 0.04±0.01d 0.42±0.02e 15 3.67±0.45b 0.57±0.04d 2.67±0.33c 2.78±0.22d 0.14±0.03d 0.53±0.06e 25 11.33±0.86a 1.59±0.08c 3.33±0.33bc 4.20±0.07c 0.70±0.04c 0.85±0.07d 35 10.33±0.67a 1.94±0.10c 4.67±0.67ab 5.00±0.09b 0.80±0.40bc 0.99±0.19cd 45 13.00±1.08a 2.61±0.16b 5.00±0.57a 5.02±0.10b 1.28±0.18b 1.21±0.12c 55 13.66±0.67a 2.66±0.17ab 5.01±0.47a 5.94±0.17a 1.82±0.36a 1.57±0.14b 75 15.00±1.00a 3.22±0.42a 5.67±0.33a 6.16±0.23a 2.08±0.17a 1.93±0.12a 说明:同列不同字母表示差异显著(P < 0.05) -
[1] 吴昀.基于离体模式体系的百合小鳞茎发生发育机制研究[D].杭州: 浙江大学, 2016. WU Yun. The Mechanisms of Lily Bulblet Initiation and Development Based on The In Vitro Model System[D]. Hangzhou: Zhejiang University, 2016. [2] 吴沙沙.东方百合索邦鳞茎源库转换规律研究[D].北京: 北京林业大学, 2012. WU Shasha. Rules of Source: Sink Exchange of the Bulb of Oriental Lily 'Sorbonne'[D]. Beijing: Beijing Forestry University, 2012. [3] WU Yun, LI Yue, MA Yidi, et al. Hormone and antioxidant responses of Lilium Oriental hybrids 'Sorbonne' bulblets to humic acid treatments in vitro[J]. J Hortic Sci Biotechnol, 2017, 92(2):155-167. [4] WU Yun, XIA Yiping, ZHANG Jiaping, et al. Low humic acids promote in vitro lily bulblet enlargement by enhancing roots growth and carbohydrate metabolism[J]. J Zhejiang Univ Sci B, 2016, 17(11):892-904. [5] LI Z, RAHMAN S, KOSAR-HASHEMI B, et al. Cloning and characterization of a gene encoding wheat starch synthase Ⅰ[J]. Theor Appl Genet, 1999, 98(8):1208-1216. [6] CHENG Jun, KHAN M A, QIU Wenming, et al. Diversification of genes encoding granule-bound starch synthase in monocots and dicots is marked by multiple genome-wide duplication events[J]. PLoS One, 2012, 7(1):e30088. doi:10.1371/journal.pone. 0030088. [7] BANSAL A, KUMARI V, TANEJA D, et al. Molecular cloning and characterization of granule-bound starch synthase Ⅰ (GBSSI) alleles from potato and sequence analysis for detection of cis-regulatory motifs[J]. Plant Cell Tissue Organ Cult, 2012, 109(2):247-261. [8] AHUJA G, JAISWAL S, HUCL P, et al. Wheat genome specific granule-bound starch synthase Ⅰ differentially influence grain starch synthesis[J]. Carbohydr Polym, 2014, 114:87-94. [9] WU Yun, SUN Minyi, ZHANG Jiaping, et al. Differential effects of paclobutrazol on the bulblet growth of oriental lily cultured in vitro:growth behavior, carbohydrate metabolism, and antioxidant capacity[J]. J Plant Growth Regul, 2019, 38(2):359-372. [10] 张进忠, 孙嘉曼, 李朝生, 等.百合鳞茎发育过程中淀粉合成相关酶基因的克隆及表达分析[J].广西植物, 2019, 39(4):446-452. ZHANG Jinzhong, SUN Jiaman, LI Chaosheng, et al. Cloning of starch synthase-related enzyme gene and its expression analysis in process of bulblet develop of Lilium[J]. Guihaia, 2019, 39(4):446-452. [11] MURASHIGE T, SKOOG F. A revised medium for rapid growth and bio assays with tobacco tissue cultures[J]. Physiol Plant, 1962, 15(3):473-497. [12] OTANI M, HAMADA T, KATAYAMA K, et al. Inhibition of the gene expression for granule-bound starch synthase Ⅰ by RNA interference in sweet potato plants[J]. Plant Cell Rep, 2007, 26(10):1801-1807. [13] ASARE E K, BÅGA M, ROSSNAGEL B G, et al. Polymorphism in the barley granule bound starch synthase 1(GBSSI) gene associated with grain starch variant amylose concentration[J]. J Agric Food Chem, 2012, 60(40):10082-10092. [14] BULL S E, SEUNG D, CHANEZ C, et al. Accelerated ex situ breeding of GBSS-and PTST1-edited cassava for modified starch[J]. Sci Adv, 2018, 4(9):eaat6086. doi:10.1126/sciadv.aat6086. [15] JOBLING S A, WESTCOTT R J, TAYAL A, et al. Production of a freeze-thaw-stable potato starch by antisense inhibition of three starch synthase genes[J]. Nat Biotechnol, 2002, 20(3):295-299. [16] 许娟, 罗兴录, 赵德征.木薯淀粉合成相关酶基因的克隆及表达分析[J].生物技术通报, 2012(11):101-109. XU Juan, LUO Xinglu, ZHAO Dezheng. Cloning and expression of starch synthesis related enzyme gene in common cassava[J]. Biotechnol Bull, 2012(11):101-109. [17] 彭佶松, 赵淑娟, 吴晓俊, 等.黄芪毛状根GBSSI基因cDNA克隆及其结构分析[J].植物学报, 2000, 42(9):940-945. PENG Jisong, ZHAO Shujuan, WU Xiaojun, et al. cDNA cloning and structural analysis of granule bound starch synthase gene of hairy roots of Astragalus membranaceus[J]. Acta Bot Sin, 2000, 42(9):940-945. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2020.02.002






 下载:
下载: