-
自然界中许多微生物可以与宿主植物之间形成复杂的共生关系,促进宿主植物的生长和发育[1]。反之,宿主植物也为这些微生物的定殖和生长提供丰富的碳源。相比叶片、茎干等地上器官,植物根系虽然深埋土壤中,但却被认为是植物与微生物交流互作最为频繁的部位,也是地球生态系统中最为复杂的组分之一[2]。在过去的几十年里,植物-根际微生物间的相互作用研究主要在植物与微生物相互作用分子机制、根际微生物功能、优良微生物资源发掘及产品开发等方面[3−6]。油菜Brassica napus、花生Arachis hypogaea、大豆Glycine max等油料作物是人类食用植物油和动物蛋白饲料的主要来源,也是世界上最重要的大宗农产品之一[7]。一方面,随着近年来经济增长,国内对食用和工业用油料的需求急剧增加。然而,由于耕地面积限制、全球气候变化、国际贸易冲突等多重因素的影响,国内油料产需缺口仍在不断扩大,对外需求日渐攀升,自给率不足40%[8]。另一方面,油料作物种植中,仍存在着化肥使用过多,有机肥使用率不高等问题,进一步导致了土壤的退化板结、根际微生态失衡、病虫害加剧等后果,一定程度上制约了油料作物产业的可持续发展[9]。近年来,有研究发现,油菜、大豆等油料作物的籽粒质量和含油量与根系微生物的组成和活性密切相关[10−12]。这些研究为利用根际微生物来调控油料作物的生长、促进油料作物产量和出油率的提升提供了新的思路。本研究从油料作物的根际微生物组成及影响因素、两者之间的相互作用关系,以及农业生产中的应用3个层面,综述了近年来国内外的相关研究进展,以期为今后探究油料作物根际土壤环境与微生物关系,以及开发优良微生物菌剂应用于油料作物生产提供参考。
-
在油料作物生长过程中,不断产生的枝叶凋落物及根系分泌物会吸引各种土壤微生物聚集在其根系周围,通过特定的竞争、筛选与重组,逐渐形成特有的根际微生物群落。①由于不同作物有着不同的根系形态和环境条件,其根际土壤微生物的组成有很大差异。同时相同油料作物的不同品种,由于对不同环境适应能力差异,其根系生长状态不同,因此其根际微生物组成也存在差异。如对4个不同品种花生的根际细菌群落丰度研究[13]发现:在正常条件下,4个花生品种的土壤微生物群落主要由放线菌门Actinobacteria、变形菌门Proteobacteria、绿弯菌门Chloroflexi、酸杆菌门Acidobacteria和蓝菌门Cyanobacteria的细菌组成。而在盐胁迫条件下,相比2个非耐盐品种,2个耐盐品种花生的蓝菌门和变形菌门细菌的丰度显著增加,而酸杆菌门细菌丰度却明显减少。②油料作物的根际微生物组成同时受到其宿主植物发育时期的影响。在大豆中,苗期的根际微生物丰富度与多样性普遍高于其成熟期。而在成熟期,放线菌属Actinomycetes细菌数量增加,变形菌属Proteus和酸杆菌属Acidobacterium细菌数量却相应减少[14]。在油菜中,变形菌门细菌在苗期的相对丰度显著高于花期和角果成熟期,而酸杆菌门、蓝细菌门、厚壁菌门Firmicutes细菌的丰度,在花期要显著高于苗期和角果成熟期[15] 。③油料作物根际微生物组成还受到土壤环境因子的影响。如在油菜中,影响油菜根际微生物群落的主要因素有土壤铵态氮(${\rm{NH}}_4^+ $)、可溶性磷、总氮、总碳、总氮、蔗糖酶和有机质,而海拔和硝态氮(${\rm{NO}}_3^- $)的影响次之,土壤pH对油菜根际土壤群落影响最小[16]。④由于不同耕作方式下,油料植物根系生长发育以及土壤生态条件都不相同,因此油料作物根际微生物的组成还受到其耕作方式的影响。如在连作条件下,花生、大豆、芝麻Sesamum indicum等油料作物的根际真菌数量大幅度增加,而根际亚硝酸细菌和硝酸细菌等有益微生物的生长则受到抑制[17]。而在轮作条件下,假单胞菌Pseudomonas spp.、白地霉菌Geotrichum candidum等根际微生物的比例明显高于连作,同时病原微生物如踝节菌Talaromyces spp.、黑曲霉菌Aspergillus niger、粉红黏帚菌Clonostachys rosea和沙雷氏菌Serratia spp.等明显低于连作条件[18]。
综上所述,油料作物的品种、生育期、土壤环境和耕作方式等都会影响油料作物根际微生物的组成,且上述因素相互制约,共同作用,塑造了油料作物的根际微生物群落结构特征。
-
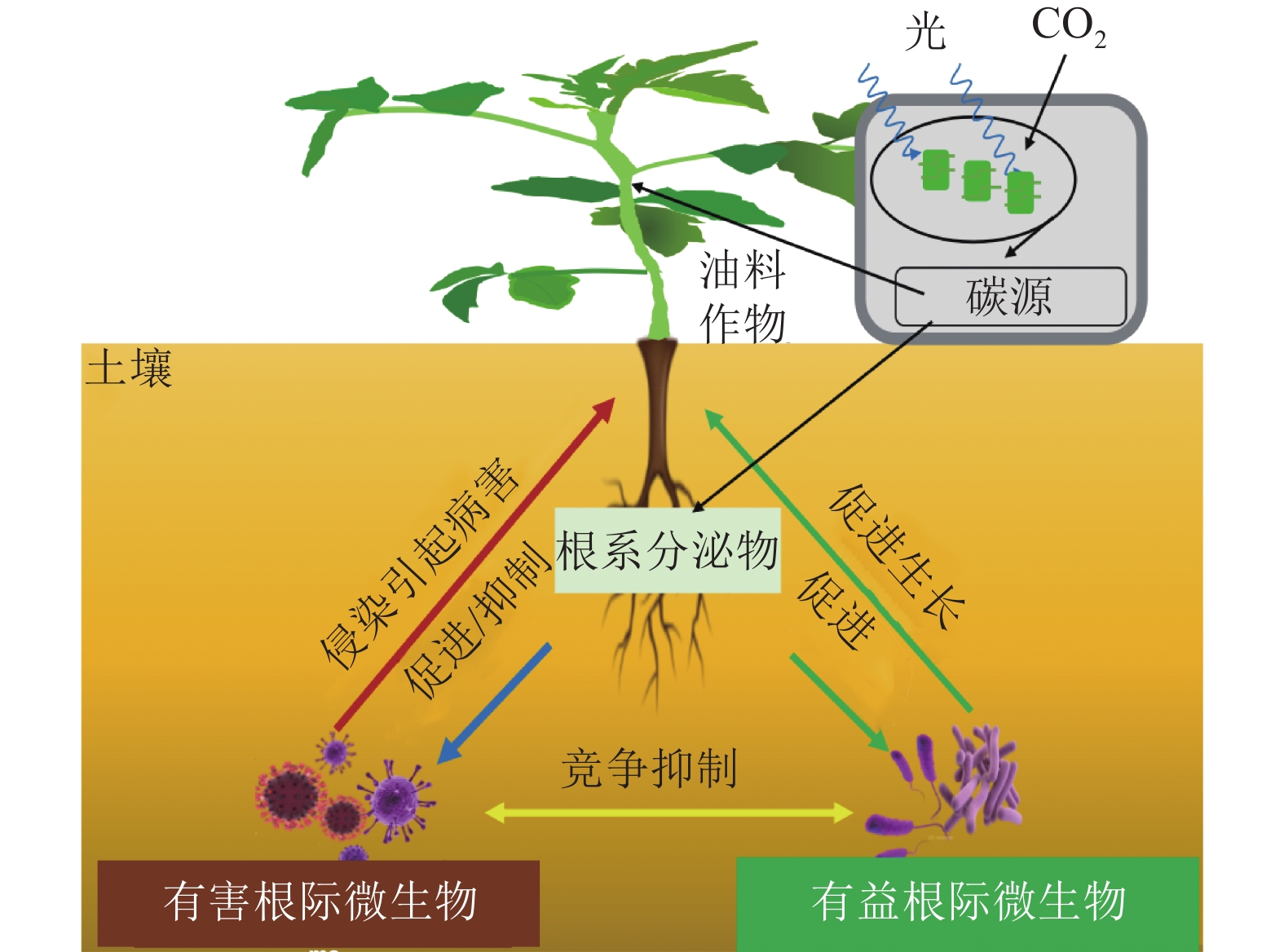
自然界中,植物与其根际微生物之间有复杂的相互作用关系,共同维持了一种微妙的生态平衡(图1)。一方面,植物的凋落物、细胞及其溶解产物、根系分泌物,可以作为碳源吸引有益微生物定殖。已有研究报道:油料作物根系分泌物含有各种氨基酸、糖类、脂肪酸、维生素、生长因子、激素和有机酸等,这些分泌物不仅为微生物提供了丰富的碳源,同时也可作为信号分子来影响微生物的种群大小和群落分布[19]。DARRAH[20]测定了微生物沿着植物根系可溶性碳梯度的分布和丰度情况,发现根际微生物的分布与根部的可溶性碳浓度分布直接相关, 说明微生物生物量的积累依赖于根系分泌物的释放。有研究[21]表明:花生、大豆根系分泌的类黄酮物质可吸引根瘤菌定殖,同时诱导根瘤菌的结瘤基因表达,形成根瘤进行共生固氮。此外,花生、大豆、芝麻、向日葵Helianthus annuus等油料作物的根系还可以分泌独金脚内酯,诱导丛枝菌根真菌球囊菌门Glomeromycota产生菌丝分枝,形成共生关系,促进植物对有机磷和其他矿物质的吸收[22]。油菜的根系分泌物包含了柠檬酸、苹果酸、琥珀酸、草酸、α-酮戊二酸、乳酸和酒石酸等有机酸,以及阿拉伯糖、半乳糖、葡萄糖、乳糖、山梨醇、山梨糖、蔗糖、甘露醇、木糖和果糖等糖类,进一步分析发现:除α-酮戊二酸、木糖和阿拉伯糖以外,上述根系分泌物都对根系巨大芽孢杆菌具有趋化性,且苹果酸和山梨醇的趋化性最强[23]。另一方面, 一些研究也表明:油料作物根系分泌物反而会给一些病原微生物提供养分,导致有益土壤微生物的生长会受到抑制,引发土传病害。如在连作条件下,大豆根际分泌物(糖、氨基酸、有机酸)是根腐病发生的重要诱因[24]。此外, 2, 4-二叔丁基苯酚和香草酸等大豆根系分泌物,对根际微生物具有明显的化感作用,具体表现为低浓度促进微生物生长、高浓度则抑制微生物生长[25]。
-
有益根际微生物是指一类通常定殖在植物根系表面、根内或游离于植物根际,起到促进植物营养物质的利用、参与植物抗病免疫、提高植物抗逆能力等作用的细菌和真菌 [26]。在油料作物中,有益根际微生物可以通过提高养分可利用性促进作物的生长(表1)。氮素是植物生长最重要的元素之一,豆科油料作物(花生和大豆)可以利用根瘤菌Rhizobium共生固氮,即根瘤菌自身的固氮酶将空气中的氮气转化成植物可以吸收利用的${\rm{NH}}_4^+ $,促进植物生长[27]。磷是植物生长不可或缺的营养元素。土壤中大部分的磷元素都以难溶的无机磷形态存在,不能被植物吸收利用,而广泛存在于根际的解磷细菌可以将无机磷转化为植物可以吸收的可溶性磷[28]。目前,根际细菌中研究较多的解磷细菌主要有芽孢杆菌属Bacillus、假单胞菌属和埃希氏菌属Escherichia等[29]。在油菜中,接种解磷细菌可以显著提升地上部分质量,同时土壤有机质、全磷和速效磷含量都显著增加[30]。铁是植物和微生物生长的必需元素,地球表面铁元素多以三价铁化合物存在,但生物有效性铁离子(Fe3+)在环境中含量却很低。研究发现:固氮菌属Azotobacter、根瘤菌属Rhizobium和肠杆菌属Enterobacter的一些微生物可以分泌铁载体(低分子量的铁结合蛋白)来螯合Fe3+,帮助植物吸收铁元素[31]。在油菜中,接种可分泌铁载体的荧光假单胞菌株,不仅促进了油菜生长,也提高了油菜对菌核病的抗性[32]。
表 1 有益根际微生物对油料作物的影响
Table 1. Effects of beneficial rhizosphere microorganisms on oil crops
对油料作物影响 油料作物 土壤微生物 影响机制 参考文献 促进油料作物营养物质利用 花生、大豆 根瘤菌属Rhizobium 共生固氮 [27] 油菜 芽孢杆菌属Bacillus, 假单胞菌属
Pseudomonas, 埃希氏菌属Escherichia磷转化 [29−30] 花生、大豆、油菜 固氮菌Azotobacter spp., 根瘤菌Rhizobium
spp., 肠杆菌Enterobacter spp.螯合三价铁 [31−32] 提高油料作物抗病能力 油菜 假单胞菌Pseudomonas spp., 枯草芽孢杆菌
Bacillus subtilis分泌抗菌化合物 [33, 39] 花生、油菜、大豆 枯草芽孢杆菌Bacillus subtilis, 伯克霍尔德
菌Burkholderia cepacia, 链霉菌
Streptomyces spp., 假单胞菌
Pseudomonas spp., 黏质沙雷氏菌
Serratia marcescens产生铁载体 [41] 油菜 枯草芽孢杆菌Bacillus subtilis 诱导植物抗性 [40] 花生、大豆、油菜 假单胞杆菌Pseudomonas, 黏质
沙雷氏菌Serratia marcescens竞争生态位 [37] 提高油料作物抵抗
非生物逆境胁迫花生、大豆 假单胞菌属Pseudomonas, 芽孢杆菌属
Bacillus, 根瘤菌属Rhizobium产生小分子渗透物质 [44−45] 花生、大豆 根瘤菌属Rhizobium 诱导植物抗氧化物质合成 [46] 油菜 多黏类芽孢杆菌Paenibacillus polymyxa 调节抗逆基因表达 [47, 51] 油菜 阴沟肠杆菌Enterobacter cloacae, 肠棒杆菌
Enterobacter spp. 克雷伯菌Klebsiella spp.分泌激素 [50] 提高油脂积累 油菜、大豆 荧光假单胞菌Pseudomonas fluorescens [10] -
有益微生物还被发现可以抑制植物病害发生(表1)。现已明确的有益微生物生物防病机制,主要分为分泌抗菌化合物、产生铁载体、诱导植物系统抗性以及与根际病原菌竞争生态位等[33−36]。这些机制要么单独发挥作用,要么具有协同效应。如枯草芽孢杆菌Bacillus subtilis、伯克霍尔德菌Burkholderia cepacia、链霉菌Streptomyces spp.、假单胞菌Pseudomonas spp.和沙雷氏菌Serratia marcescens等根际细菌,可以有效抑制植物病原体的定植和暴发,是良好的生物防治剂[37]。在大豆、芝麻、向日葵等油料作物中,炭腐病是一种广泛存在的土传真菌性病害,其致病菌为菜豆壳球孢菌Macrophomina phaseoli,为腐生营养型,生命力强,化学杀菌剂很难灭除,而假单胞菌和芽孢杆菌产生的抗真菌代谢产物硝吡咯菌素和藤黄绿脓菌素等物质,却可以有效抑制菜豆壳球孢菌的生长,从而防治炭腐病[38−39]。在油菜中,核盘菌Sclerotinia sclerotiorum是引发菌核病的主要病原体。研究发现:油菜内生菌枯草芽孢杆菌可以在温室和大田条件下诱导油菜抗性,达到对油菜菌核病的防治效果[40]。此外,研究还发现:铁载体介导的菌群互作是影响土传病害发生的重要因素[41],这为生物防治土传病害的有益菌群构建提供了新的视角。
-
有益根际微生物还可以增加油料作物对非生物逆境胁迫的抗性(表1)。全球气候变化引发的干旱、高温、盐碱、低温等非生物逆境,对油料作物的产量和品质影响巨大[42]。在生产实践中,除了品种改良,利用根际微生物来改善油料作物的非生物胁迫抗性,是经济且高效的手段。研究发现:在非生物逆境环境中,根际微生物可以通过产生小分子渗透调节物质、诱导植物合成抗氧化物质、调节抗逆基因表达等促进植物的抗逆性[43]。根际接种AK-1假单胞菌和 SJ-5芽孢杆菌菌株,会明显增强大豆的耐盐能力,其脯氨酸合成相关基因GmP5CS表达上调,脯氨酸含量明显上升[44]。类似的,在根瘤菌中过表达海藻糖合成基因otsA,菌体内的海藻糖浓度明显升高,将其接种大豆后,大豆的耐旱能力得到了显著提高,干旱下产量较对照提升了38%[45]。研究还发现:接种根瘤菌会诱导油料作物抗氧化酶系统(如超氧化物歧化酶、过氧化物酶、过氧化氢酶等)活性,提高植物的抗逆性 [46]。此外,一些根际细菌通过上调或下调植物的干旱响应基因(如抗坏血酸过氧化物酶、S-腺苷甲硫氨酸合成酶和热激蛋白)来赋予植物耐旱性[47]。如转录组分析发现:与对照相比,接种拟杆菌属的多黏类芽孢杆菌Paenibacillus polymyxa,宿主植物干旱响应基因Erd15的表达水平明显提高[48]。在逆境胁迫下,植物会分泌各类激素来应对胁迫。一些根系微生物也可以通过分泌植物激素来调节宿主植物的抗逆反应[49]。如有研究发现:80%的根际细菌都可以产生吲哚乙酸(IAA),来调节宿主植物生长。在盐胁迫下,油菜根系微生物耐盐阴沟肠杆菌Enterobacter cloacae HSNJ4分泌的IAA含量明显增加,而乙烯含量下降,提升了油菜的耐盐性[50]。
-
油脂积累作为油料作物生长发育的重要组成部分备受关注(表1),利用油脂代谢途径的分子机制,进行基因改造是近年来调控油料作物油脂积累的主要手段 [51],虽然此方法对油料作物油脂积累的提升具有明显效果,但转基因突变体作物可能会对生态环境安全以及人体造成潜在的威胁[52]。利用根际微生物与油料作物共生提高油脂积累作为一种新的绿色安全方法越来越受到重视。 JIMENEZ等[10]利用根际有益微生物荧光假单胞菌Pseudomonas fluorescens LBUM677接种油菜和大豆,以不接种为对照,发现不仅显著提高了油菜与大豆种子的质量和数量,同时显著提高了种子的含油量,并且对油脂组分分析发现接种荧光假单胞菌LBUM677的油菜和大豆所产生的棕榈酸(C16∶0)、油酸(C18∶1)、亚油酸(C18∶2)、α-亚麻酸(C18∶3)和硬脂酸(C18∶0)等主要脂肪酸含量均显著增加。虽然根际微生物用于增加油料作物总含油量和各脂肪酸组分含量的作用机制目前尚不清楚,但部分研究[53−54]认为:可能是由于所接根部微生物能够产生1-氨基环丙烷-1-羧酸(ACC)脱氨酶、吲哚乙酸、铁载体并且具有溶解磷酸盐的能力等,各类机制互相作用,相互影响,从而调节了油料作物油脂的积累。
-
花生、油菜、大豆等油料作物容易受到土壤病原微生物的侵染,暴发土传病[55]。土传病是指土壤中的病原微生物在外界条件适宜时,侵染植物的根部或茎部而发生的病害,因其很难根治又称植物癌症。研究发现:土传病的病原体主要是真菌、细菌和线虫,它们侵染后会导致植物出现纹枯、枯萎、立枯、猝倒、根腐、软腐、根肿和丛根等病症[56]。茄腐镰刀菌Haematococca spp.、尖孢镰刀菌Fusarium oxysporum和丝核菌Rhizoctonia solani等多种真菌混合侵染,是大豆根腐病的主要病原体,会导致大豆减产5%~10%,严重时减产达60%[57]。芸薹根肿菌Plasmodiophora brassicae引发的根肿病被称为“油菜癌症”。因为芸薹根肿菌生命力顽强,感染后的油菜根部肿大甚至腐烂,植株矮小,叶片变黄甚至枯萎,严重时整株枯死,减产60%以上,甚至绝收[58]。由镰刀菌属Fusarium spp.细菌引起的花生根腐病是花生常见病害。花生苗期与成熟期均可感染该病,苗期染病的植株,主根变黑,地上部矮小,下部叶片先变黄、萎蔫、枯死,并逐渐向上部叶片扩展蔓延。而成熟期染病的植株,根部形成略凹陷的长条形褐色病斑, 植株地上部逐渐黄化、萎蔫和枯死[59]。综上,土壤中的病原性真菌和细菌对油料作物的危害是巨大的,因此,应通过培育抗病品种[60]、喷施药剂[61]、生物防控[62]和调整栽培[17−18]等方式,积极管理油料作物的根部病原微生物群落,避免根部有害微生物对油料作物产量造成不利影响。
-
根际微生物与宿主油料作物之间相互影响,不同的根际微生物之间也会产生相互作用。如根瘤菌和丛枝菌根真菌都可以与花生、大豆共生,接种丛枝菌根真菌的植株的根瘤数明显多于不接种丛枝菌根真菌的植株,接种根瘤菌的植株根部丛枝菌根真菌侵染显著高于不接种根瘤菌的植株。与此同时,丛枝菌根真菌与根瘤菌的混合接种比单接种可以得到更好的抗病效果,两者同时接种有协同增效作用[63]。另一方面,根际微生物相互作用也会对油料作物产生负面影响,病原细菌和真菌的过度增殖会导致油料作物染病,它们会和根部有益微生物群落竞争,使油料作物不能有效利用营养元素,从而影响油料作物的生长和发育[64]。要提高油料作物生产效率,更好地理解其根际微生物之间的相互作用是必不可少的,有必要进行更多的研究来阐明其中复杂的机制。
-
基于对油料作物与根际微生物之间相互作用的认知,越来越多的研究聚焦于如何利用微生物来调节油料作物的产量与品质。目前,有 3 种调控模式被广泛应用,分别是调控根际微生物、调控宿主植物、调节耕种方式。
调控根际微生物,一方面是要在土壤中筛选与发掘更多的优良菌株[65],另一方面,是要通过分子手段定向改造根际微生物,使其具有特定优良性状[66]。对于改造的工程菌,其实际施用效果还需进行评价。现阶段使用的根际菌剂产品,其保质期和田间药效期均较短,而产品储存条件要求较高,产品的作物普适性较差,效果不稳定,且成本高 [67]。针对上述问题,对应的研究仍需继续进行。
调控宿主植物是通过诱变或基因工程等手段来改造油料作物,调控其根系分泌物来改变根际的营养成分、pH等环境因素,从而直接或间接影响根际土壤微生物的组成与数量[68]。但在油料作物新品种选育与推广中,转基因作物中的外源基因是否会对土壤微生物生态系统产生不利影响仍需要进行安全评估。已有的研究结果显示:大部分植物转基因事件对于其根际微生物影响并不显著[69−71]。但也有研究表明:转基因作物会影响根际某些微生物的丰度,或影响某个发育期根际微生物的多样性[72]。因此,仍需要监测转基因油料作物一定时间内的根际微生物动态变化过程,为优良品系的应用与推广提供指导。
调节耕种方式是利用不同的耕种方式来影响油料作物根际微生物的丰度与分布。免耕农业系统中的土壤微生物生物量与多样性明显高于传统耕作系统,且在免耕条件下微生物的活力在不同土层有明显的差异, 土壤表层微生物活力最强,而在传统耕作中微生物分布则比较均匀[73]。相比于单作、连作等耕作方式,轮作可明显改善油料作物根际微生物的活性与丰度,使有益细菌种群增加,改善土壤微生态环境,同时可抑制土传病害的发生[74]。此外,将豆科Leguminosae油料作物与禾本科Poaceae作物进行间作,根际分泌物明显增多,不仅提高了根际有益微生物的丰度,还促进了作物健康生长[75]。因此,在实际生产中,可利用不同耕作模式,来调节根际微生物的活性和种群结构,促进油料作物生长。
-
近年来,全球植物油产需缺口不断增大,在不与粮食争地的前提下,提高单位面积的油料作物产量已成为当务之急。但是,目前油料作物增产仍依赖使用大量化肥和农药,这一定程度上制约了油料作物产业的可持续发展。因此,探索并利用根际微生物与花生、大豆、油菜等宿主油料作物之间的相互作用机制,提高产量和改善品质,对发展高效、生态、绿色的现代农业具有重要意义。以下几个方向值得进一步深入研究:①在机制层面,应重点关注与研究菌根相关土壤中氮素同化途径与分配机制、乙烯合成调控等植物与根际微生物共生机制、以及植物抗性基因对根际微生物群落组成的影响;②在技术层面,整合各种组学方法、表观遗传学和宏基因组等技术,研究植物-微生物-土壤系统的耦合动态机制已成为趋势;③在生产应用层面,需要进一步综合开发、验证和应用更多种类的根际微生物,并将其整合到改良作物的生物技术体系中,与作物育种、绿色栽培、高效植保、品质提升进行紧密结合,从而综合提高油料作物的产量、抗病性、品质和经济效益。
Recent research progress in rhizospheric microbes of oil crops
-
摘要: 花生Arachis hypogaea、油菜Brassica napus、大豆Glycine max等油料作物是中国食用和工业用油脂的重要来源,其根系微生物的活性和相互作用,会直接影响油料的产量和品质。近年来,有关油料作物根际微生物的研究已逐渐成为国内外的研究热点。本研究从油料作物微生物群落组成及其影响因素、相互作用机制和农业生产应用3个层面,综述了近年来国内外油料作物根际微生物的相关研究进展。结果发现:①影响油料作物根际微生物组成主要因素有油料作物的品种、生育期、土壤环境和耕作方式等;②油料作物与根际微生物相互作用的研究主要在利用有益根际微生物对油料作物抗逆性、产量以及品质的提升方面;③目前在农业生产中,主要通过调控根际微生物、调控宿主油料作物以及调节耕种方式来促进油料作物生长。通过对油料作物与根际微生物相互作用的研究,为今后探究油料作物与根际微生物之间的相互作用关系,以及拓展微生物菌剂在油料作物中的应用提供科学参考。图1表1参75Abstract: Peanut (Arachis hypogaea), rape (Brassica napus) and soybean (Glycine max) are important sources for edible and industrial oil in China. The yield and quality of oil could be affected by the activities of the rhizospheric microbes and the interactions between them. In recent years, the rhizospheric microbes of oil crops are receiving more and more attentions around the world. The research progress in microbes community composition and influencing factors, interaction mechanisms and the agricultural applications of the root microbes in oil crops were summarized. Results show: (1) The main factors affecting the composition of rhizosphere microorganisms in oil crops include: cultivar, growth period, soil environment, and cultivation methods of oil crops; (2) The research on the interaction between oil crops and rhizosphere microorganisms mainly focuses on utilizing beneficial rhizosphere microorganisms to improve the stress resistance, yield, and quality of oil crops; (3) At present, in agricultural production, the growth of oil crops is mainly promoted by regulating rhizosphere microorganisms, regulating host oil crops, and regulating cultivation methods. By studying the interaction between oil crops and rhizosphere microorganisms, scientific references are provided for exploring the interaction relationship between oil crops and rhizosphere microorganisms in the future, as well as expanding the application of microbial agents in oil crops. [Ch, 1 fig. 1 tab. 75 ref.]
-
Key words:
- oil crop /
- rhizospheric microbe /
- interaction /
- stress resistance /
- cellular signal
-
表 1 有益根际微生物对油料作物的影响
Table 1. Effects of beneficial rhizosphere microorganisms on oil crops
对油料作物影响 油料作物 土壤微生物 影响机制 参考文献 促进油料作物营养物质利用 花生、大豆 根瘤菌属Rhizobium 共生固氮 [27] 油菜 芽孢杆菌属Bacillus, 假单胞菌属
Pseudomonas, 埃希氏菌属Escherichia磷转化 [29−30] 花生、大豆、油菜 固氮菌Azotobacter spp., 根瘤菌Rhizobium
spp., 肠杆菌Enterobacter spp.螯合三价铁 [31−32] 提高油料作物抗病能力 油菜 假单胞菌Pseudomonas spp., 枯草芽孢杆菌
Bacillus subtilis分泌抗菌化合物 [33, 39] 花生、油菜、大豆 枯草芽孢杆菌Bacillus subtilis, 伯克霍尔德
菌Burkholderia cepacia, 链霉菌
Streptomyces spp., 假单胞菌
Pseudomonas spp., 黏质沙雷氏菌
Serratia marcescens产生铁载体 [41] 油菜 枯草芽孢杆菌Bacillus subtilis 诱导植物抗性 [40] 花生、大豆、油菜 假单胞杆菌Pseudomonas, 黏质
沙雷氏菌Serratia marcescens竞争生态位 [37] 提高油料作物抵抗
非生物逆境胁迫花生、大豆 假单胞菌属Pseudomonas, 芽孢杆菌属
Bacillus, 根瘤菌属Rhizobium产生小分子渗透物质 [44−45] 花生、大豆 根瘤菌属Rhizobium 诱导植物抗氧化物质合成 [46] 油菜 多黏类芽孢杆菌Paenibacillus polymyxa 调节抗逆基因表达 [47, 51] 油菜 阴沟肠杆菌Enterobacter cloacae, 肠棒杆菌
Enterobacter spp. 克雷伯菌Klebsiella spp.分泌激素 [50] 提高油脂积累 油菜、大豆 荧光假单胞菌Pseudomonas fluorescens [10] -
[1] TRIVEDI P, LEACH J E, TRINGE S G, et al. Plant-microbiome interactions: from community assembly to plant health [J]. Nature Reviews Microbiology, 2020, 18(11): 607 − 621. [2] RAAIJMAKERS J M, PAULITZ T C, STEINBERG C, et al. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms [J]. Plant and Soil, 2008, 321(1/2): 341 − 361. [3] DONG Wentao, ZHU Yayun, CHANG Huizhong, et al. An SHR-SCR module specifies legume cortical cell fate to enable nodulation [J]. Nature, 2021, 589(7843): 586 − 590. [4] LIU Yin, HU Wen, HUANG Qing, et al. Plastic mulch debris in rhizosphere: interactions with soil-microbe-plant systems[J/OL]. The Science of the Total Environment, 2022, 807: 151435[2023-02-01]. doi: 10.1016/j.scitotenv.2021.151435. [5] de SOUSA L P, GUERREIRO-FILHO O, MONDEGO J M C. The rhizosphere microbiomes of five species of coffee trees [J/OL]. Microbiology Spectrum, 2022, 10(2): e0044422[2023-02-01]. doi: 10.1128/spectrum.00444-22. [6] WANG Liyang, RENGEL Z, ZHANG Kai, et al. Ensuring future food security and resource sustainability: insights into the rhizosphere [J/OL]. iScience, 2022, 25(4): 104168[2023-02-01]. doi: 10.1016/j.isci.2022.104168. [7] 吴传云, 王超, 冯健, 等. 我国油料作物产业发展现状与机械化生产对策建议[J]. 农机科技推广, 2021(3): 18 − 19, 26. WU Chuanyun, WANG Chao, FENG Jian, et al. The current development status of China’s oil crop industry and suggestions for mechanized production [J]. Agriculture Machinery Technology, 2021(3): 18 − 19, 26. [8] 罗屹. 中国油料作物收获和储存损失测算及其资源环境影响评估[J]. 中国油料作物学报, 2022, 44(2): 249 − 256. LUO Yi. On farm harvest and storage losses of oil crops and the impact on resources and environment in China [J]. Chinese Journal of Oil Crop Sciences, 2022, 44(2): 249 − 256. [9] 徐晓侠. 我国农作物施肥存在的问题及对策[J]. 农业开发与装备, 2020(7): 72, 74. XU Xiaoxia. Problems and countermeasures of crop fertilization in China [J]. Agricultural Development &Equipments, 2020(7): 72, 74. [10] JIMNEZ J A, NOVINSCAK A, FILION M. Pseudomonas fluorescens LBUM677 differentially increases plant biomass, total oil content and lipid composition in three oilseed crops [J]. Journal of Applied Microbiology, 2020, 128(4): 1119 − 1127. [11] SHWETA B, MAHESHWARI D K, DUBEY R C, et al. Beneficial effects of fluorescent pseudomonads on seed germination, growth promotion, and suppression of charcoal rot in groundnut (Arachis hypogea L. ) [J]. Journal of Microbiology and Biotechnology, 2008, 18(9): 1578 − 1583. [12] AFZAL A, BANO A, FATIMA M. Higher soybean yield by inoculation with N-fixing and P-solubilizing bacteria [J]. Agronomy for Sustainable Development, 2010, 30(2): 487 − 495. [13] XU Yang, ZHANG Zhimeng, DING Hong, et al. Comprehensive effects of salt stress and peanut cultivars on the rhizosphere bacterial community diversity of peanut [J/OL]. Archives of Microbiology, 2021, 204(1): 15[2023-02-01]. doi: 10.1007/s00203-021-02619-6. [14] WANG Lin, LI Zhiying, LIU Ruirui, et al. Bacterial diversity in soybean rhizosphere soil at seedling and mature stages [J]. Polish Journal of Microbiology, 2019, 68(2): 281 − 284. [15] 杜坤, 王婷, 杨阳, 等. 转mEPSPS基因甘蓝型油菜对根际土壤细菌群落结构和多样性的影响[J]. 中国油料作物学报, 2021, 43(1): 131 − 140. DU Kun, WANG Ting, YANG Yang, et al. Effect of transgenic Brassica napus with mEPSPS gene on diversity and structure of rhizosphere microbial communities [J]. Chinese Journal of Oil Crop Sciences, 2021, 43(1): 131 − 140. [16] 杜娟, 聂丽妍, 魏丽萍, 等. 西藏林芝市油菜根际细菌群落特征分析[J]. 高原农业, 2022, 6(1): 16 − 28. DU Juan, NIE Liyan, WEI Liping, et al. Characterization of inter-root bacterial community of oilseed rape in Nyingchi, Tibet [J]. Journal of Plateau Agriculture, 2022, 6(1): 16 − 28. [17] 汪瑞清, 肖运萍, 魏林根, 等. 油料作物连作障碍形成机理与生态修复措施研究进展[J]. 农学学报, 2015, 5(6): 29 − 33. WANG Ruiqing, XIAO Yunping, WEI Lin’gen, et al. The research progress of formation mechanism and ecological restoration measures on continuous cropping obstacles of oil crops [J]. Journal of Agriculture, 2015, 5(6): 29 − 33. [18] 姚小东, 李孝刚, 丁昌峰, 等. 连作和轮作模式下花生土壤微生物群落不同微域分布特征[J]. 土壤学报, 2019, 56(4): 975 − 985. YAO Xiaodong, LI Xiaogang, DING Changfeng, et al. Microzone distribution characteristics of soil microbial community with peanut cropping system, monocropping or rotation [J]. Acta Pedologica Sinica, 2019, 56(4): 975 − 985. [19] 张豆豆, 梁新华, 王俊. 植物根系分泌物研究综述[J]. 中国农学通报, 2014, 30(35): 314 − 320. ZHANG Doudou, LIANG Xinhua, WANG Jun. A review of plant root exudates [J]. Chinese Agricultural Science Bulletin, 2014, 30(35): 314 − 320. [20] DARRAH P R. Models of the rhizosphere [J]. Plant and Soil, 1991, 133: 187 − 199. [21] DONG Wei, SONG Yuguang. The significance of flavonoids in the process of biological nitrogen fixation [J/OL]. International Journal of Molecular Sciences, 2020, 21(16): 59265[2023-02-01]. doi: 10.3390/ijms21165926. [22] GOMEZ-ROLDAN V, FERMAS S, BREWER P B, et al. Strigolactone inhibition of shoot branching [J]. Nature, 2008, 455(7210): 189 − 194. [23] 胡小加, 谢立华, 余常兵, 等. 巨大芽孢杆菌A6对油菜根系分泌物所含有机酸和糖类的趋化性[J]. 中国油料作物学报, 2011, 33(4): 416 − 419. HU Xiaojia, XIE Lihua, YU Changbing et al. Chemotaxis of Bacillus megaterium strain A6 towards organic acid and saccharide from roots exudates of rapeseed [J]. Chinese Journal of Oil Crop Sciences, 2011, 33(4): 416 − 419. [24] 韩丽梅, 鞠会艳, 王旭明. 大豆连作土壤有机化合物对大豆根腐病菌生长的影响[J]. 大豆科学, 2004(1): 36 − 40. HAN Limei, JU Huiyan, WANG Xuming. Influence of the organic compounds in continuous cropping soybean on pathogenic of root rot [J]. Soybean Science, 2004(1): 36 − 40. [25] 曲晓华, 赵晓燕, 马杰, 等. 大豆根系分泌物中特定物质对土壤微生物活性的影响[J]. 福建农业学报, 2015, 30(3): 298 − 302. QU Xiaohua, ZHAO Xiaoyan, MA Jie, et al. Effect of soybean root exudates on microbial biomass and activity in soil [J]. Fujian Journal of Agricultural Sciences, 2015, 30(3): 298 − 302. [26] BHATTACHARYYA P N, JHA D K. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture [J]. World Journal of Microbiology &Biotechnology, 2012, 28(4): 1327 − 1350. [27] HE Chuynmei, GAO Hui, WANG Haijiao, et al. GSK3-mediated stress signaling inhibits legume-rhizobium symbiosis by phosphorylating GmNSP1 in soybean [J]. Molecular Plant, 2021, 14(3): 488 − 502. [28] ZENG Qingwei, DING Xiaolei, WANG Jiangchuan, et al. Insight into soil nitrogen and phosphorus availability and agricultural sustainability by plant growth-promoting rhizobacteria [J]. Environmental Science and Pollution Research International, 2022, 29(30): 45089 − 45106. [29] BARGAZ A, ELHAISSOUFI W, KHOURCHI S, et al. Benefits of phosphate solubilizing bacteria on belowground crop performance for improved crop acquisition of phosphorus [J/OL]. Microbiological Research, 2021, 252, 126842[2023-02-01]. doi: 10.1016/j.micres.2021.126842. [30] 贺立虎, 李娟丽. 解磷菌对油菜品质及土壤理化性质的影响[J]. 陕西农业科学, 2018, 64(8): 47 − 50. HE Lihu, LI Juanli. Effects of phosphate solubilizing bacteria on rape quality and soil physicochemical properties [J]. Shaanxi Journal of Agricultural Sciences, 2018, 64(8): 47 − 50. [31] FLORES-F LIX J D, SILVA L R, RIVERA L P, et al. Plants probiotics as a tool to produce highly functional fruits: the case of phyllo bacterium and vitamin C in strawberries [J/OL]. PLoS One, 2015, 10(4): e0122281[2023-02-03]. doi: 10.1371/journal.pone.0122281. [32] 王平, 李慧, 邱译萱, 等. 荧光假单胞菌株P13分泌铁载体抑制油菜菌核病菌[J]. 上海师范大学学报(自然科学版), 2010, 39(2): 200 − 203. WANG Ping, LI Hui, QIU Yixuan, et al. Siderophores produced by Pseudomonas fluorescens P13 against Sclerotinia sclerotiorum [J]. Journal of Shanghai Normal University (Natural Sciences), 2010, 39(2): 200 − 203. [33] JÜRGEN K H L, KOLNAAR R, RAVENSBERG W J. Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy [J/OL]. Frontiers in Plant Science, 2019, 10: 845[2023-02-03]. doi: 10.3389/fpls.2019.00845. [34] PIRTTIL A M , TABAS H M P , BARUAH N, et al. Biofertilizers and biocontrol agents for agriculture: how to identify and develop new potent microbial strains and traits [J/OL]. Microorganisms, 2021, 9(4): 871[2023-02-01]. doi: 10.3390/microorganisms9040817. [35] MARTINEZ C, AVIS T J, SIMARD J N, et al. The role of antibiosis in the antagonism of different bacteria towards Helminthosporium solani, the causal agent of potato silver scurf [J]. Phytoprotection, 2006, 87(2): 69 − 75. [36] PARANI K, SAHA B K. Prospects of using phosphate solubilizing Pseudomonas as bio fertilizer [J/OL]. European Journal of Biological Sciences, 2012, 4(2): 63117[2023-02-01]. doi: 10.5829/idosi.ejbs.2012.4.2.63117. [37] MARK G, MORRISSEY J P, HIGGINS P, et al. Molecular-based strategies to exploit Pseudomonas biocontrol strains for environmental biotechnology applications [J]. FEMS Microbiology Ecology, 2006, 56(2): 167 − 177. [38] DAVE K, GOTHALWAL R, SINGH M, et al. Facets of rhizospheric microflora in biocontrol of phytopathogen Macrophomina phaseolina in oil crop soybean [J]. Archives of Microbiology, 2021, 203: 405 − 412. [39] LE N M, YARYURA P M, MONTECCHIA M S, et al. Antifungal activity of selected indigenous Pseudomonas and Bacillus from the soybean rhizosphere [J/OL]. International Journal of Microbiology, 2009: 572049[2023-02-01]. doi: 10.1155/2009/572049. [40] 高小宁. 植物内生细菌菌株Em7对油菜菌核病的防治研究 [D]. 杨凌: 西北农林科技大学, 2012. GAO Xiaoning. Study on the Biocontrol Efficacy of Endophytic Bacterium Em7 Isolate against Sclerotinia sclerotiorum on Oil Seed Rape[D]. Yangling: Northwest A&F University, 2012. [41] GU Shaohua, WEI Zhong, SHAO Zhengying, et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes [J]. Nature Microbiology, 2020, 5(8): 1002 − 1010. [42] FAROOQ M, HUSSAIN M, USMAN M, et al. Impact of abiotic stresses on grain composition and quality in food legumes [J]. Journal of Agricultural and Food Chemistry, 2018, 66(34): 8887 − 8897. [43] VEJAN P, ABDULLAH R, KHADIRAN T, et al. Role of plant growth promoting Rhizobacteria in agricultural sustainability-a review [J/OL]. Molecules, 2016, 21(5): 573[2023-02-01]. doi:10.3390/molecules21050573. [44] KUMARI S, VAISHNAV A, JAIN S, et al. Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycine max L. Merrill) [J]. Journal of Plant Growth Regulation, 2015, 34(3): 558 − 573. [45] SUÁREZ R, WONG A, RAMIREZ M, et al. Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia [J]. Molecular Plant-Microbe Interactions, 2008, 21(7): 958 − 966. [46] ABDELKRIM S, JEBARA S H, JEBARA M. Antioxidant systems responses and the compatible solutes as contributing factors to lead accumulation and tolerance in Lathyrus sativus inoculated by plant growth promoting Rhizobacteria [J]. Ecotoxicology and Environmental Safety, 2018, 166: 427 − 436. [47] IGIEHON N O, BABALOLA O O. Below-ground-above-ground plant-microbial interactions: focusing on soybean, Rhizobacteria and mycorrhizal fungi [J]. Open Microbiology Journal, 2018, 12: 261 − 279. [48] TIMMUSK S, WAGNER E G. The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses [J]. Molecular Plant-Microbe Interactions, 1999, 12(11): 951 − 959. [49] LU Tao, KE Mingjing, LAVOIE M, et al. Rhizosphere microorganisms can influence the timing of plant flowering [J/OL]. Microbiome, 2018, 6(1): 231[2023-02-01]. doi:10.1186/s40168-018-0615-0. [50] LI Huashan, LEI Peng, PANG Xiao, et al. Enhanced tolerance to salt stress in canola (Brassica napus L. ) seedlings inoculated with the halotolerant enterobacter cloacae HSNJ4 [J]. Applied Soil Ecology, 2017, 119: 26 − 34. [51] LIU Fang, XIA Yuping, WU Lei, et al. Enhanced seed oil content by overexpressing genes related to triacylglyceride synthesis [J]. Gene, 2015, 557(2): 163 − 171. [52] BRUNE P D, CULLER A H, RIDLEY W P, et al. Safety of GM crops: compositional analysis [J]. Journal of Agricultural and Food Chemistry, 2013, 61(35): 8243 − 8247. [53] MAJEED A, ABBASI K, HAMEED S, et al. Pseudomonas sp. AF-54 containing multiple plant beneficial traits acts as growth enhancer of Helianthus annuus L. under reduced fertilizer input [J]. Microbiological Research, 2018, 216: 56 − 69. [54] GOUDA S, KERRY R G, DAS G, et al. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture [J]. Microbiological Research, 2018, 206: 131 − 140. [55] ZHANG Wei, ZHANG Bowen, DENG Jiefu, et al. The resistance of peanut to soil-borne pathogens improved by rhizosphere probiotics under calcium treatment [J/OL]. BMC Microbiology, 2021, 21(1): 299[2023-02-01]. doi: 10.1186/s12866-021-02355-3. [56] 杨珍, 戴传超, 王兴祥, 等. 作物土传真菌病害发生的根际微生物机制研究进展[J]. 土壤学报, 2019, 56(1): 12 − 22. YANG Zhen, DAI Chuanchao, WANG Xingxiang, et al. Advance in research on rhizosphere microbial mechanisms of crop soil-borne fungal diseases [J]. Acta Pedologica Sinica, 2019, 56(1): 12 − 22. [57] 成瑢, 董铮, 李魏, 等. 大豆根腐病研究进展[J]. 中国农学通报, 2016, 32(8): 58 − 62. CHENG Rong, DONG Zheng, LI Wei, et al. Research progress of soybean root rot [J]. Chinese Agricultural Science Bulletin, 2016, 32(8): 58 − 62. [58] WALLENHAMMAR A C, OMER Z S, EDIN E, et al. Influence of soil-borne inoculum of Plasmodiophora brassicae measured by qPCR on disease severity of clubroot-resistant cultivars of winter oilseed rape (Brassica napus L. ) [J/OL]. Pathogens, 2021, 10(4): 433[2023-02-01]. doi: 10.3390/pathogens10040433. [59] 邢小萍, 袁虹霞, 孙炳剑, 等. 花生根部主要土传真菌病害的发生与防治[J]. 园艺与种苗, 2010, 30(6): 441 − 444. XING Xiaoping, YUAN Hongxia, SONG Bingjian, et al. Occurrence regularity and control of main peanut root soil-born fungal disease [J]. Horticulture &Seed, 2010, 30(6): 441 − 444. [60] 黄新琦, 蔡祖聪. 土壤微生物与作物土传病害控制[J]. 中国科学院院刊, 2017, 32(6): 593 − 600. HUANG Xinqi, CAI Zucong. Soil microbes and control of soil-borne diseases [J]. Bulletin of Chinese Academy of Sciences, 2017, 32(6): 593 − 600. [61] 高游慧, 郑泽慧, 张越, 等. 根际微生态防治作物土传真菌病害的机制研究进展[J]. 中国农业大学学报, 2021, 26(6): 100 − 113. GAO Youhui, ZHENG Zehui, ZHANG Yue, et al. Mechanism of rhizosphere micro-ecology in controlling soil-borne fungal diseases: a review [J]. Journal of China Agricultural University, 2021, 26(6): 100 − 113. [62] MAZZOLA M, FREILICH S. Prospects for biological soilborne disease control: application of indigenous versus synthetic microbiomes [J]. Phytopathology, 2017, 107(3): 256 − 263. [63] SINGH C S. Arbuscular mycorrhiza (AM) in association with Rhizobium sp. improves nodulation, N2 fixation, and N utilization of pigeon pea (Cajanus cajan), as assessed with a N15 technique, in pots [J]. Microbiological Research, 1996, 151(1): 87 − 92. [64] PARKIN T B, GRAHAM J A. Effects of crop rotation on root-associated microbial communities of oilseed rape (Brassica napus) [J]. Applied and Environmental Microbiology, 1990, 56(2): 349 − 354. [65] 刘鹏, 田颖哲, 钟永嘉, 等. 酸性土壤上花生高效根瘤菌的分离及应用[J]. 中国农业科学, 2019, 52(19): 3393 − 3403. LIU Peng, TIAN Yingzhe, ZHONG Yongjia, et al. Isolation and application of effective rhizobium strains in peanut on acidic soils [J]. Scientia Agricultura Sinica, 2019, 52(19): 3393 − 3403. [66] SUGAWARA M, CYTRYN E J, SADOWSKY M J. Functional role of Bradyrhizobium japonicum trehalose biosynthesis and metabolism genes during physiological stress and nodulation [J]. Appllied and Environmental Microbiology, 2010, 76(4): 1071 − 1081. [67] 邹德勋, 徐凤花, 潘俊波, 等. 抗生菌剂在缓解大豆重迎茬根际微生态障碍中的作用[J]. 大豆科学, 2007, 26(2): 280 − 283. ZOU Dexun, XU Fenghua, PAN Junbo, et al. Fuction of antimicrobial agent on delaying rhizosphere microbe obstacle of continuous and one year intermittent cropping soybean [J]. Soybean Science, 2007, 26(2): 280 − 283. [68] GÉVAUDANT F, DUBY G, von STEDINGK E, et al. Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance [J]. Plant Physiology, 2007, 144(4): 1763 − 1776. [69] LUO Junyu, ZHANG Shuai, ZHU Xiangzhen, et al. Effects of soil salinity on rhizosphere soil microbes in transgenic Bt cotton fields [J]. Journal of Integrative Agriculture, 2017, 16(7): 1624 − 1633. [70] KAHLON J G, JACOBSEN H J, CAHILL J F, et al. Antifungal genes expressed in transgenic pea (Pisum sativum L. ) do not affect root colonization of arbuscular mycorrhizae fungi [J]. Mycorrhiza, 2017, 27(7): 683 − 694. [71] BIDONDO L F, ALMASIA N, BAZZINI A, et al. The overexpression of antifungal genes enhances resistance to rhizoctonia solani in transgenic potato plants without affecting arbuscular mycorrhizal symbiosis [J/OL]. Crop Protection, 2019, 124: 104837[2023-02-01]. doi: 10.1016/j.cropro.2019.05.031. [72] van WYK D, ADELEKE R, RHODE O, et al. Ecological guild and enzyme activities of rhizosphere soil microbial communities associated with Bt-maize cultivation under field conditions in north west province of South Africa [J]. Journal of Basic Microbiology, 2017, 57(9): 781 − 792. [73] LUPWAYI N Z, ARSHAD M, RICE W A, et al. Bacterial diversity in water-stable aggregates of soils under conventional and zero tillage management [J]. Applied Soil Ecology, 2001, 16(3): 251 − 261. [74] 李春杰, 许艳丽, 陈海山, 等. 耕作方式对连作大豆生长发育及产量的影响[J]. 中国油料作物学报, 2008, 30(4): 455 − 459. LI Chunjie, XU Yanli, CHEN Haishan, et al. Effects of tillage patterns on development and yield of continuous cropping soybean [J]. Chinese Journal of Oil Crop Sciences, 2008, 30(4): 455 − 459. [75] 黄涛, 冯远娇, 王建武. 禾本科‖豆科间作对土壤微生物影响的研究进展[J]. 生态科学, 2022, 41(3): 229 − 236. HUANG Tao, FENG Yuanjiao, WANG Jianwu. A review on the effects of cereal‖ legume intercropping on soil microorganisms [J]. Ecological Science, 2022, 41(3): 229 − 236. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20230139






 下载:
下载: