-
叶绿素是植物叶片中吸收和传递光能的主要色素分子,直接影响植物叶片光合作用的效能;叶绿素含量水平也是反映植物营养状况和生长发育进程的重要指标[1-3]。现有研究表明:植物叶片在可见光区(400~700 nm),红边区(680~760 nm)和近红外光区(780~1 300 nm)光谱反射率与叶片光合色素含量有较高的相关性[4]。伍南等[5]研究表明:可用NDVI等所建高光谱特征参数估测病虫害胁迫下的杉木Cunninghamia lanceolata叶绿素含量。Sims等[6]提出了修正型光谱指数mSR705和mND705用于估算具有不同表面反射率的树木叶片中的叶绿素含量。冯伟等[7]研究表明:红边位置(λred)与叶片色素含量之间具有稳定而密切的相关性。时启龙等[8]研究发现酸雨胁迫下的亚热带树种光谱反射率红边位置与其叶绿素含量变化规律基本一致。目前,有关酸雨对植物叶绿素含量的影响研究结果不同。樊后保等[9]发现pH值<3.5的酸雨能降低女贞 Ligustrum lucidum的叶绿素含量,但是对叶绿素a/b影响不显著;Shan等[10]研究赤松Pinus densiflora时发现低浓度的酸雨增加了叶绿素总量,但叶绿素a/b没有受到显著影响。大量研究表明,一定浓度的化感物质会降低植物叶片的叶绿素含量,从而影响植物的生长[11];Bhatt等[12]发现青冈栎Quercus glauca和白橡Quercus leucotrichophora根际土浸提液显著降低了小麦Triticum aestivum,油菜Brassica campestris和扁豆Quercus glauca的叶片色素含量;Padhy等[13]研究得出桉树Eucalyptus globulus凋落物对穇子Eleusine coracana色素的合成产生抑制作用。然而,有关酸雨与化感物质复合作用的研究鲜有报道。柳杉Cryptomeria fortunei为常绿针叶乔木,是中国特有种。目前,对柳杉的研究主要集中于病虫害防治[14]和群落结构[15]等方面。马原[16]研究得出柳杉幼苗对酸雨较敏感,本课题组前期开展了凋落物化感物质对柳杉种子萌发的研究[17]。为了进一步探讨酸雨和凋落物交叉组合对柳杉幼苗生长的影响,本文研究了酸雨、凋落物和酸雨与凋落物复合处理对柳杉体内色素含量和反射光谱特性的影响,并对柳杉幼苗体内色素含量与其反射光谱特性间的关系进行了相关性分析。
HTML
-
供试材料为3年生柳杉实生苗(由江西林业种苗公司提供),株高30~40 cm。2012年4月栽置于盛有培养土的花盆中(直径35 cm,高26 cm),1株·盆-1。盆栽苗置于温室中,常规管理,缓苗2个月后进行实验。柳杉凋落物采自天目山柳杉林下,自然风干保存备用。根据浙江临安当地酸雨浓度及酸性降水中粒子组成,按[V(硫酸)]∶[V(硝酸)]=4∶1的比例配制母液,用水稀释成pH 4.0酸雨溶液。
-
选取长势一致的盆栽柳杉进行处理,设对照组(ck)、pH 4.0酸雨处理组(Tr1),60 g凋落物处理组(Tr2,通过预实验得出60 g凋落物处理柳杉幼苗时,柳杉幼苗生长与对照具有显著差异)和pH 4.0酸雨与60 g凋落物复合处理(Tr3)等4个处理组,重复6盆·处理-1,共24盆。通过实地样方调查,天目山柳杉林凋落物为400~800 g·m-2,为模拟柳杉自然生长环境,本实验将60 g凋落物均匀铺在盆中。根据试验地全年降水量1 628.6 mm,柳杉幼苗喷淋酸雨100 mL·株-1·次-1,平均喷淋2次·周-1。实验处理时间为2012年6-10月。
-
将0.1 g剪碎的新鲜的柳杉幼苗叶剪碎后置于具塞试管中,加体积分数为80%丙酮5 mL,室温下遮光萃取至样品完全变白后,分别在470,646和663 nm处测定其吸光度值D(λ),重复6次·样品-1。然后,按Lichtenthaler的计算公式[18] 分别计算叶绿素a,叶绿素b和类胡萝卜素质量分数。
-
采用UniSpec-SC型单通道光谱分析仪(PP-System,US)测定柳杉针叶在310~1 130 nm处的反射光谱数据,采样间隔1 nm,分辨率1 nm。Unispec-SC单通道光谱分析仪内置1个卤素灯,测定时,将分支光纤的一端连接到卤素灯的输出端口,另一端连接到检测器的输入端口,光纤探头端固定在UNI500标准夹中。测定柳杉3株·次-1,重复6次·株-1,取其平均值作为该样品的光谱反射率。测量过程中及时进行标准白板校正,用Multispec 5.1数据处理软件读取反射光谱原始数据。
-
光谱数据微分处理:将柳杉叶片反射光谱通过式(1)进行一阶微分处理得到微分光谱:
(1)其中:λi为波段i处的波长值;Rλi为波长λi处的光谱反射率值;△λ为波长λ(i-1)到λi的差值,由光谱采样间隔决定。
“三边”参数计算方法:分别在490~530,560~640和680~750 nm范围内确定蓝边、黄边和红边位置、幅值和面积。红边位置λred为红光范围内一阶微分光谱最大值所对应的波长,红边幅值Dλred为一阶微分光谱的最大值,红边面积Sred为一阶微分光谱线所包围的面积。黄边(黄边位置λyellow,黄边幅值Dλyellow,黄边面积Syellow)和蓝边(蓝边位置λblue,蓝边幅值Dλblue,蓝边面积Sblue)参数与红边参数意义类似。可直接利用相关公式计算得到的反射光谱参数[6, 19-30](表 1)。
反射光谱参数 定义 文献来源 反射率倒数(RR) 1R700 [19] 反射光谱指数680(SR680) R800/R680 [20] 反射光谱指数705(SR7ffi) R750/R705 [21] 改良的归一化差值指数(mSR705 (R750-R 445 ) / (R705-R 445 ) [21] 色素比值指数a(PSSRa) R800/R680 [22] 色素比值指数b(PSSRb) R800/R635 [22] 反射光谱比值指数a(RARSa) R675/R703 [23] 反射光谱比值指数b(RARSb) R650/R703 [23] 归一化指数(NDVI) (R803-R680/(R803+R680) [24] 红度归一化指数(rBDVI) (R750-R705)/ (R750+R705) [21] 改良的红边比值指数(mBD705) (R750-R705)/ (R750+R705-2R445) [6] 绿度归一化指数(gNDN) (R750-R550) /(R750+R550) [25] 色素归一化差值指数a(PSNDa) (R800-R675)/(R800+R675) [5] 色素归一化差值指数b(PSNDb) (R803-R650/(R803+R650) [5] 反射光谱比值指数c(RARSc) R760/R503 [23] 色素比值指数c(PSSRc) R800/R503 [5] 结构不敏感色素指数(SIPI) (R803-R445/(R803-R680) [26] 植被衰减指数(PSRI) (R680-R500) /R750 [27] 类胡萝卜素指数1(CRI1) 1m [28] 类胡萝卜素指数2(CRI2) 1 /R510-R700 [28] 改良类胡萝卜指数(mCRI) (1/R510-1/R550)-R780 [29] 光化学反射指数(PRI) (R531-R570/(R531+R570) [30] 说明% R代表反射,下标代表光谱波段或波长。 Table 1. Reflectance spectrum parameters
-
试验数据采用SPSS 13.0统计软件进行统计分析,用Matlab 7.1软件对光谱信息进行去噪处理,并提取出与叶绿素有关的特征波段。利用OriginPro 8.0软件绘图。
1.1. 实验材料
1.2. 实验设计
1.3. 研究方法
1.3.1. 叶片色素质量分数测定
1.3.2. 光谱数据采集
1.3.3. 光谱分析方法
1.4. 数据处理
-
酸雨(Tr1),凋落物(Tr2)和酸雨与凋落物复合(Tr3)对柳杉幼苗叶绿素a,叶绿素b和叶绿素a/b比值表现出不同程度的降低(表 2)。Tr1,Tr2和Tr3处理使柳杉幼苗叶绿素a质量分数呈极显著的降低(P<0.01),与对照相比分别降低了11.6%,26.1%和39.1%;Tr1处理的柳杉幼苗叶绿素b质量分数与对照达到显著差异(P<0.05),而Tr2和Tr3处理比对照降低了14.8%和22.2%(P<0.01);Tr1,Tr2和Tr3处理的柳杉幼苗叶绿素总量比对照分别降低了0.09,0.21和0.32倍;对照叶绿素a/b比值分别是Tr1,Tr2和Tr3处理的1.07,1.15和1.21倍,呈极显著性差异(P<0.01);不同处理柳杉幼苗叶片的类胡萝卜素质量分数无显著差异。
处理 叶绿素 a /(mg.g-1) 叶绿素 b /(mg.g-1) 类胡萝卜素/(mg.g-1) 叶绿素总量/(mg.g-1) 叶绿素a/b ck 1.38 ± 0.03dD 0.54 ± 0.02dC 0.30 ± 0.01bAQ 1.23 ± 0.05dD 2.56 ± 0.02cC Tr1 1.22 ± 0.02cC 0.51 ± 0.04cC 0.31 ± 0.02cB 1.12 ± 0.06cC 2.39 ± 0.03bB Tr2 1.02 ± 0.02bQ 0.46 ± 0.03bQ 0.29 ± 0.00aA 0.97 ± 0.04bB 2.17 ± 0.03aA Tr3 0.85 ± 0.04aA 0.42 ± 0.03aA 0.29 ± 0.02aA 0.84 ± 0.07 aA 2.02 ± 0.02aA 说明:表中数据为平均数±标准误,小写字母不同表示0.05水平上的差异性(P<0.05),大写字母不同表示0.01水平上的差异性(P<0.01)。 Table 2. Changes of pigment contents of Cryptomeria fortunei under diiferent treatments
-
不同处理柳杉幼苗光谱反射率曲线的整体变化趋势一致(图 1),其中反射光谱在波长496和675 nm处各有1个吸收低谷,而在近红外区达到最大值且几乎不变。不同处理柳杉光谱反射率在绿光区(525~605 nm)和近红外区(750~1 000 nm)呈显著性差异(P<0.05)。其中在550 nm处,Tr1,Tr2和Tr3处理柳杉光谱反射率具有极显著差异(P<0.01),与对照相比光谱反射率分别增加了11.4%,20.1%和28.5%;在近红外区光谱反射率大小依次为Tr3>Tr1>对照>Tr2。
-
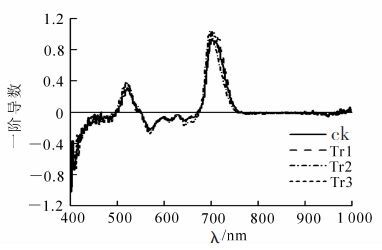
由于背景噪声对反射光谱的影响很大,因此在实际分析光谱数据时,为了降低背景噪声以提高各参数的准确性,需要对原始数据进行微分变换。对不同处理柳杉幼苗的反射光谱数据进行一阶导数处理,结果见图 2和表 3。Tr1和Tr3处理极显著增加了红边面积Sred (P<0.01);Tr1,Tr2和Tr3处理均极显著的增加了蓝边幅值Dλblue,蓝边面积Sblue和红边幅值Dλred(P<0.01),最大值出现在Tr3处理中。不同处理极显著降低了黄边幅值Dλyellow和黄边面积Syellow(P<0.01),最小值也出现在Tr3处理中。红边位置λred位于绿色植物反射光谱红光范围(680~760 nm)内,主要与叶绿素质量分数有关,是绿色植物光谱最明显的特征之一。本研究表明:不同处理柳杉幼苗在680~760 nm波段内都只有1个峰值,红边位置λred出现“蓝移”现象。此外,Tr2和Tr3处理柳杉幼苗与对照的蓝边位置λblue无显著差异;Tr1和Tr2处理下,柳杉幼苗黄边位置λyellow也无显著差异。
处理 蓝边位置 蓝边幅值 蓝边面积 黄边位置 黄边幅值 黄边面积 红边位置 红边幅值 红边面积 ck 520.3±1.15aA 0.31±0.04aA 5.35±1.00aA 629.0±1.00bB -0.037±0.006cC -8.67±0.74dD 702.0±0.33bB 0.94±0.10aA 37.37±3.09bB Tr1 522.5±1.73bB 0.34±0.03bB 6.31±0.84bB 631.0±1.41bB -0.050±0.003aA -9.76±0.77cC 701.0±1.41aA 1.19±0.04cC 46.33±3.06cC Tr2 519.3±0.58aA 0.38±0.02cC 8.17±1.00cC 631.7±0.58bB -0.046±0.005bB -1022±050bB 700.0±1.73aA 1.05±0.04bB 36.45±4.30aA Tr3 520.5±1.00aA 0.56±0.06dD 11.65±1.28dD 627.0±3.56aA -0.051±0.007aA -13.40±0.88aA 699.8±1.89aA 1.35±0.09dD 50.06±2.14dD Table 3. Three edge parameters of Cryptomeria fortunei under different treatments
-
光谱参数是绿色植物的光谱反射特征,是反映植物生长状况的最常用光谱变量。不同处理下,柳杉幼苗各反射光谱参数表现出不同的变化(表 4)。Tr1,Tr2和Tr3处理的反射光谱参数包括反射率倒数,改良的归一化差值指数,反射光谱比值指数a,红度归一化指数和改良的红边比值指数均比对照极显著降低(P<0.01),如反射率倒数分别比对照减小了14.3%,19.6%和25.0%;Tr1和Tr3处理极显著降低反射光谱比值指数b(P<0.01)。Tr2处理极显著降低了反射光谱指数680,归一化指数,色素归一化差值指数a,色素归一化差值指数b和反射光谱比值指数c(P<0.01);而Tr1和Tr3处理极显著增加了反射光谱指数680,归一化指数,色素归一化差值指数a,色素归一化差值指数b,反射光谱比值指数c,结构不敏感色素指数,植被衰减指数和改良类胡萝卜指数(P<0.01),但是反射光谱指数705无显著变化。反射率倒数,反射光谱比值指数a和反射光谱比值指数b等12个光谱参数的最大值或者最小值出现在Tr3处理,如反射光谱比值指数a比对照,Tr1和Tr2分别降低了36.0%,20.5%和25.5%。
处理 反射率倒数 反射光谱指数 680 反射光谱指数 705 改良的归一化 差值指数 反射光谱比 值指数a 反射光谱比值 指数b 归一化指数 红度归一化 指数 改良的红边比 值指数 色素归一化差 值指数a 色素归一化差 值指数b 反射光谱比值 指数 结构不敏感色 素指数 植被衰减指数 改良类胡萝卜 指数 光化学反射 指数 ck 0.056±0.003 bB 5.831±0.149 bB 1.998±0.027 bB 4.519±0.822 cC 0.417±0.026 cC 0.484±0.032 cC 0.706±0.026 bB 0.333±0.008 dD 0.633±0.047 dD 0.714±0.034 bB 0.675±0.028 bB 3.815±0.323 bB 0.778±0.025 aA -0.094±0.012 aA 1.223 ±0.243 aA 0.041±0.006 cC Tr1 0.048±0.001 aA 7.127±0.60 cC 1.977±0.121 bB 3.328±0.628 bB 0.341±0.036 bB 0.438±0.031 bB 0.753±0.023 cC 0.327±0.018 cC 0.530±0.061 cC 0.760±0.022 cC 0.701±0.024 cC 4.602±0.212 cC 0.829±0.016 cB -0.080±0.009 bB 1.657±0.192 bB 0.011±0.002 aA Tr2 0.045±0.002 aA 5.278±0.304 aA 1.660±0.061 aA 2.651±0.451 aA 0.364±0.031 bB 0.470±0.009 cC 0.679±0.006 aA 0.246±0.008 aA 0.441±0.042 aA 0.691±0.014 aA 0.616±0.012 aA 3.566±0.181 aA 0.792±0.012 bA -0.093±0.006 aA 1.210±0.087 aA 0.022±0.002 bB Tr3 0.044±0.004 aA 8.449±0.831 dD 1.921±0.160 bB 2.709±0.46 aA 0.271 ±0.028 aA 0.361±0.028 aA 0.787±0.021 dD 0.314±0.039 bB 0.454±0.057 bB 0.800±0.021 dD 0.740±0.038 dD 5.509±0.349 dD 0.867±0.021 dC -0.066±0.008 cC 2.237±0.132 cC 0.046±0.003 dD Table 4. Changes of reflectance spectrum parameters of C. fortune) under different treatments
-
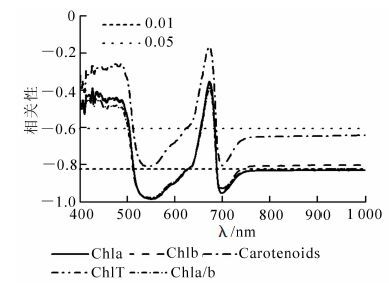
不同处理柳杉幼苗光谱反射率与色素质量分数的相关性分析表明(图 3),无论可见光区还是近红外区,柳杉光谱反射率与色素质量分数均呈负相关,且叶绿素质量分数和叶绿素a/b与514~629 nm及690~1 000 nm的光谱反射率达极显著相关(P<0.01),在550和700 nm处相关系数最大绝对值大于0.92;类胡萝卜素与光谱反射率在540和701 nm处绝对值为0.81和0.79,达到极显著相关(P<0.01)。

Figure 3. Correlation between pigment contents and reflectance spectra of Cryptomeria fortunei under different treatments
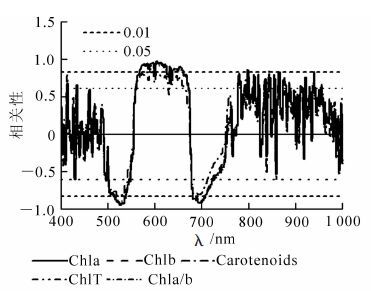
如图 4所示:不同处理柳杉幼苗色素质量分数和叶绿素a/b与其光谱反射率的一阶微分光谱在500~533 nm,538~674 nm,682~725 nm等处均达显著相关(P<0.05)。在567~633 nm和637~650 nm波段达极显著正相关(P<0.01),其中567~633 nm波段相关系数最大,单波段603 nm处与叶绿素a、叶绿素b,类胡萝卜素和叶绿素a/b的相关系数分别为0.98,0.97,0.83和0.90;而在514~536 nm和680~706 nm显著相关波段呈极显著负相关(P<0.01),其中514~536 nm相关系数的绝对值最大,单波段532 nm处的相关系数绝对值最大,与叶绿素a,叶绿素b,类胡萝卜素和叶绿素a/b的相关系数分别为0.92,0.92,0.82和0.92。
-
由反射光谱参数与色素质量分数相关性表明(表 5),改良的归一化差值指数和改良的红边比值指与叶绿素a,叶绿素b质量分数和叶绿素a/b的相关性均达到显著正水平(P<0.05);反射光谱指数680,归一化指数,色素归一化差值指数a,反射光谱比值指数c和改良类胡萝卜指数与叶绿素a,叶绿素b质量分数和叶绿素a/b的相关性均达到显著负水平(P<0.05)。反射光谱参数反射率倒数,反射光谱比值指数a,反射光谱比值指数b,黄边面积,黄边幅值和红边位置与叶绿素a,叶绿素b质量分数和叶绿素a/b之间均达到极显著正相关(P<0.01),而结构不敏感色素指数、蓝边幅值、蓝边面积、红边幅值和红边面积达到极显著负相关(P<0.01),其中反射率倒数、蓝边幅值、蓝边面积、黄边幅值、黄边面积、红边幅值和红边面积与叶绿素质量分数之间的相关性高于或接近0.8。反射光谱指数705,红度归一化指数,色素归一化差值指数b,光化学反射指数,蓝边位置和黄边位置与叶绿素a,叶绿素b质量分数和叶绿素a/b相关性均不显著。此外,蓝边、黄边和红边(除蓝边与黄边位置)参数总体上优于波段组合参数与色素的相关性。
光谱参数 叶绿素a 叶绿素b 类胡萝卜素 叶绿素总量 叶绿素a/b 反射率倒数 0.925** 0.924** 0.787** 0.925** 0.887** 反射光谱指数680 0.588* 0.559* 0.511* 0.579* 0.564* 反射光谱指数A05 0.006 0.051 0.128 0.016 0.042 改良的归一化差值指数 0.614* 0.633** 0.559* 0.616* 0.551* 反射光谱比值指数a 0.722** 0.710** 0.678** 0.715** 0.669** 反射光谱比值指数b 0.665** 0.654** 0.679** 0.659** 0.615** 归一化指数 0.594* 0.561* 0.505* 0.584* 0.576* 红度归一化指数 0.016 0.059 0.126 0.025 0.032 改良的红边比值指数 0.618* 0.630** 0.590* 0.617* 0.551* 色素归一化差值指数a 0.609** 0.577* 0.527* 0.599* 0.588* 色素归一化差值指数b 0.492 0.459 0.448 0.482 0.482 反射光谱比值指数c 0.560* 0.532* 0.489 0.551* 0.532* 结构不敏感色素指数 0.668** 0.639** 0.551* 0.656** 0.638** 植被衰减指数 0.507* 0.487 0.467 0.498* 0.464 改良类胡萝卜指数 0.612* 0.591* 0.541* 0.605* 574* 光化学反射指数 0.151 0.155 0.035 0.154 0.113 蓝边位置 0.01D 0.043 0.002 0.017 0.014 蓝边幅值 0.910** 0.904** 0.825** 0.908** 0.889** 蓝边面积 0.880** 0.875** 0.795** 0.877** 0.854** 黄边位置 0.339 0.298 0.435 0.322 0.355 黄边幅值 0.879** 0.882** 0.738** 0.882** 0.866** 黄边面积 0.957** 0.952** 0.831** 0.957** 0.943** 红边位置 0.772** 0.780** 0.702** 0.773** 0.732** 红边幅值 0.871** 0.855** 0.739** 0.868** 0.849** 红边面积 0.814** 0.788** 0.648** 0.809** 0.809** 说明*表示差异显著(P<0.05),**表示差异极显著(P<0.01),n=16。 Table 5. Correlation between reflectance spectrum parameters and pigment contents of Cryptomeria fortunei under different treatments
2.1. 不同处理柳杉幼苗叶绿素质量分数差异分析
2.2. 不同处理柳杉幼苗反射光谱特征
2.3. 不同处理柳杉幼苗反射光谱的三边特征
2.4. 不同处理柳杉幼苗光谱参数的变化
2.5. 光谱反射率和一阶微分光谱与色素质量分数的相关性
2.6. 反射光谱参数与色素质量分数的相关性
-
叶绿素是植物体内的主要光合色素,其质量分数往往是植物营养胁迫、光合能力和衰老进程等生理状态的良好指示剂[1, 31]。大量研究表明:酸雨和化感作用会导致植物叶绿素质量分数下降[11-12]。pH≤3.5的酸雨会降低龙眼Dimorcarpus longana色素质量分数和叶绿素a/b[32],而小麦Triticum aestivuml叶绿素质量分数和叶绿素 a/b比值会随着酸的积累显著降低[33]。本研究结果表明:酸雨单一因子处理显著降低柳杉叶绿素a,叶绿素b质量分数和叶绿素a/b,说明是酸雨胁迫使柳杉叶片叶绿素生物合成减弱,分解速度加快所致[32-34]。马原[16]研究得出酸雨会破坏柳杉叶绿体,导致叶绿素质量分数下降,与本研究结果一致。凋落物单一因子处理极显著降低了的柳杉幼苗叶绿素a,叶绿素b和叶绿素a/b,说明柳杉凋落物化感作用促进了叶绿素a的降解[35]。酸雨和凋落物复合作用下叶绿素a,叶绿素b质量分数和叶绿素a/b下降幅度大于两者单一处理,其原因可能是酸雨胁迫下植物的化感物质溶解加速,使柳杉凋落物化感物质的种类和数量增加[36]。
当植被受胁迫时,光谱特性会发生相应的变化[37]。已有研究报道酸雨会使水稻Oryza sativa叶片可见光区和中红外区反射率升高,一阶和二阶微分光谱发生蓝移[38],病虫害胁迫的植被红边位置会发生“蓝移现象”[39]。本研究中,用酸雨和凋落物处理的柳杉叶片可见光波段内的光谱反射率发生“蓝移”现象,且在550 nm附近呈现出叶绿素的强反射峰,与前人的研究结果[31]相一致。此外,不同处理极显著降低了柳杉反射光谱参数反射率倒数,改良的归一化差值指数和反射光谱比值指数a,红度归一化指数和改良的红边比值指数(P<0.01),其中酸雨和凋落物复合处理的光谱参数最小,与柳杉幼苗的叶绿素a,叶绿素b质量分数和叶绿素a/b降低且酸雨和凋落物复合处理值最小相吻合,说明两者复合处理对柳杉的胁迫作用大于两者任一因子单独处理。
大量研究表明植物光合色素质量分数与光谱原始反射率、微分导数和光谱参数有较高的相关性[40]。病害下棉花Gossypium spp.叶绿素质量分数与归一化指数,结构不敏感色素指数,光化学反射指数等光谱参数显著相关[41],玉米Zea mays叶片中色素质量分数也与光谱参数色素比值指数a,红边位置,红边幅值和红边面积等表现出极显著的相关性[4]。本研究发现,不同处理柳杉叶片色素质量分数和叶绿素a/b与原始反射率在514~629 nm及近红外区达到极显著负相关(P<0.01),且与一阶微分光谱在424~486,552~682,698~ 755和762~772 nm波段达到极显著相关(P<0.01),说明可以通过光谱反射率和微分光谱对酸雨和凋落物胁迫下柳杉色素含量进行估测。不同处理柳杉叶片叶绿素a,叶绿素b质量分数和叶绿素a/b与改良的归一化差值指数,改良的红边比值指数和反射光谱指数680等7个光谱参数极显著相关(P<0.05),且与反射率倒数,结构不敏感色素指数和红边位置等11个光谱参数呈极显著相关(P<0.01)。其中,反射率倒数,蓝边幅值,蓝边面积,黄边面积,黄边幅值,红边幅值和红边面积与叶绿素a、叶绿素b质量分数和叶绿素a/b相关性均接近或大于0.80(P<0.01),这表明以上7个反射光谱参数更适用于监测酸雨和凋落物处理下柳杉的光合色素质量分数变化。
综上所述,在酸雨的作用下使柳杉凋落物的化感作用增强,可能是由于酸雨增强柳杉凋落物化感物质的释放。反射率倒数,蓝边幅值,黄边面积,黄边幅值,蓝边面积,红边幅值和红边面积等光谱参数与叶绿素a,叶绿素b和叶绿素a/b相关性最大,可以作为估测柳杉色素质量分数的光谱参数。













 DownLoad:
DownLoad: